Abstract
Argan (Argania spinosa (L.) Skeels) is an endangered and endemic agroforestry species of Morocco highly appreciated for its nutraceutical properties. Herein, the antioxidant activity, total phenolic content and fatty acids were evaluated in different extracts obtained from callus culture, seeds and leaves of four argan genotypes: G25, G36, G41 and G84. Callus induction, proliferation and morphology varied depending on genotype and explant type. The highest callus induction rate (97.5%) was observed in the cotyledon explants of genotype G84. The radical scavenging activity values ranged from 76.5 to 98.1%. The essential oils extracted from calli induced from seedling-derived leaves exhibited a slightly higher radical scavenging activity (91.7%) than those extracted from field-grown leaves (90–91.1%). The total phenolic content ranged from 0.72 mg/g dry weight gallic acid equivalent in the methanolic extracts of G84 callus obtained from seedling-derived leaves to 198.26 mg/g dry weight gallic acid equivalent in the essential oils of G84 seeds. The fatty acid composition varied significantly among the different samples. The essential oils extracted from seeds and callus obtained from cotyledon explants have high contents in oleic and linoleic acids (26–37.9% and 25–36.8%, respectively), while the major fatty acid found in the essential oils of leaves and callus obtained from seedling-derived leaves was eicosenoic acid (18.8–45.4%). The present study showed that argan callus culture could be envisaged for sustainable and continuous production of bioactive compounds, and that each extract analyzed had unique and distinct characteristics.
Key message
The essential oils and other extracts obtained from argan callus induced in vitro were characterized and were compared with those obtained from seeds and field-grown leaves
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Argan (Argania spinosa (L.) Skeels) is an endangered and endemic tree of Morocco (Koufan et al. 2018). It is an agroforestry species that belongs to the Sapotaceae family (Guillaume et al. 2019; Koufan et al. 2018). The argan tree is natively grown in a limited Atlantic coastal area of Morocco characterized by specific microclimatic conditions that allow its survival (Guillaume et al. 2019). Today, this species can be found in other countries such as Algeria, Spain, Kuwait and Mexico (Falasca et al. 2018).
Argan plays important ecological roles such as preventing desertification, preserving biodiversity and improving soil and water quality (Moukrim et al. 2019). However, its most important attribute is its socioeconomic impact. In fact, in Morocco, argan oil extraction and processing activities contribute to the income of around one-fifth of the local population of its cultivation area (Metougui et al. 2017). Besides, argan oil is highly appreciated for its nutraceutical properties. Indeed, this oil is rich in bioactive compounds that have protective effects against many diseases (Charrouf and Guillaume 2008; Khallouki et al. 2003). These attributes, along with the limited geographical area of cultivation, make argan oil one of the most expensive edible oils in the market (Lybbert et al. 2011).
While many studies have been previously carried out to characterize the edible oil of argan (e.g. Charrouf and Guillaume 2008; El Kharrassi et al. 2018), there is a lack of information concerning the characterization of its essential oils and other extracts. For example, the methanolic and ethanolic ones. Essential oils are a group of secondary metabolites known for their antimicrobial and antioxidant activities since they contain phenolic functional groups (Swamy et al. 2016; Vergis et al. 2013). They have been widely used in pharmaceutic, cosmetic and food industries, and recently, their use was suggested in plant micropropagation since they exhibited remarkable activities against tissue browning and endophytic bacteria of date palm (Meziani et al. 2019). Essential oils can be extracted from different parts and organs of plants, including callus induced in vitro (Flamini et al. 2002; Jawdat et al. 2016). Interestingly, extracting essential oils from callus was suggested as an efficient and renewable approach to produce bioactive compounds from plants (Razavizadeh and Komatsu 2018). Similarly to essential oils, the methanolic and ethanolic extracts of plants also contain bioactive compounds that have interesting biological activities and can be used in pharmaceutical and food industries (Lin et al. 2018; Mayouf et al. 2019). Some of these compounds are polyphenols and fatty acids. Polyphenols are bioactive molecules and a class of plant secondary metabolites known for their antioxidant activity and their role in preventing many degenerative diseases (Abbas et al. 2017; Hättenschwiler and Vitousek 2000; Scalbert et al. 2005). Fatty acids are also bioactive molecules that play important roles in promoting human health (Fauser et al. 2011; Wang et al. 2012). They are known for mediating important biological functions such as cell signaling regulation, and gene activation and expression. Fatty acids are also essential for normal growth and development, and were reported to reduce the risk of coronary heart disease, to decrease the incidence of hypertension and to improve cognitive functions in old age (Ibarguren et al. 2014).
Studies on the characterization of essential oils, methanolic and ethanolic extracts of argan and their biological activities are very scarce. El Kabouss et al. (2002) analyzed the composition of the essential oil of argan leaves and found that it contains mainly sesquiterpene alcohols and hydrocarbons. In addition, the antibacterial activity of this oil was demonstrated. Harhar et al. (2010) reported that the main component of the essential oil of argan fruit pulp is camphor, suggesting its use as insecticide, whereas Haloui and Meniai (2017) determined the fatty acid composition of the essential oil from argan seeds. On the other hand, Dakiche et al. (2016) evaluated the total polyphenol and flavonoid contents in the methanolic extracts of argan leaves and reported their antioxidant and antibacterial activities as well as their cytotoxic properties against PC3 human prostate cancer cells. Samane et al. (2006) indicated that the methanolic extracts of the almonds and cake of argan could be used against some serious chronic diseases while Guinda et al. (2011) determined the composition in triterpenic compounds of the ethanolic extracts of argan leaves and fruits.
With all these favorable characteristics of essential oils and other extracts derived from argan that confer to them a high economic value, callus culture can be envisaged for sustainable and continuous production of bioactive compounds from this endangered species. Accordingly, the establishment of efficient systems for callus induction and multiplication in argan could be of great benefit for cosmetic and pharmaceutical industries. However, studies on in vitro callogenesis of Argania spinosa, and the evaluation of the biological activity of calli as well as their use as a potential source for metabolite production are very scarce. Lamaoui et al. (2019) evaluated the effects of various plant growth regulator (PGR) combinations on callus induction from different explants of argan and found that 1 mg l−1 1-naphthaleneacetic acid (NAA) and 1 mg l−1 2,4-dichlorophenoxyacetic acid (2,4-D) is the most efficient combination for callogenesis. Besides, it was found that the antioxidant activity of callus is affected by water and salt stresses (Lamaoui et al. 2015, 2019).
The purpose of the present work was to determine and compare the total phenolic content, fatty acids and the antioxidant activity in the ethanolic and methanolic extracts as well as the essential oils of field-grown leaves, seeds, and calli induced in vitro from four selected genotypes of Argania spinosa (L.) Skeels.
Materials and methods
Chemicals
Folin-Ciocalteu’s reagent was purchased from Sigma Aldrich (Lyon, France). Sodium carbonate was purchased from Labkem (Barcelona, Spain). Gallic acid and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Loba Chemie (Mumbai, India). Boron trifluoride (BF3), 2,4-D, NAA, potassium hydroxide (KOH), sodium hydroxide (NaOH), hexane, and ethanol were purchased from Fisher Scientific (Illkirsh, France). Sucrose, indole-3-acetic acid (IAA) and 6-benzyladenine (BA) were purchased from Sigma (Steinheim, Germany). Agar was purchased from Sigma (St. Louis, MO, USA).
Plant material
Leaf and mature fruit samples of argan (Argania spinosa L.) were collected from four genotypes: G25 (G-A2L5), G36 (G-A3L6), G41 (G-A4L1) and G84 (G-A8L4), all grown in the experimental station Melk Zhar (30° 02′ 33.0″ N 9° 33′ 04.0″ W) of the National Institute of Agronomic Research of Morocco (INRA), Regional Center of Agadir (CRRA-Agadir). These genotypes (10 years old) were selected based on their superior agronomic and ecological characteristics. All the leaves and fruits used in the present study were collected in June 2018. The seeds were extracted from the fruits immediately after harvest. All samples (leaves and seeds) were thoroughly washed with sterile distilled water (SDW), dried by lyophilization (Christ Alpha 1–4 LD plus, Germany) for 48 h at − 60 °C, ground to fine powder then stored at 4 °C for later use.
In vitro culture conditions
All culture media were supplemented with 30 g l−1 sucrose and 6 g l−1 agar. The pH of all media was adjusted to 5.7 before autoclaving at 121 °C for 20 min. The cultures were kept at 25 °C and transferred to fresh medium at monthly intervals.
For seed germination and seedling development, a photoperiod of 16 h was applied. For callus induction and multiplication, the cultures were kept under dark conditions. Before utilization, calli were lyophilized for 48 h at − 60 °C, ground to fine powder then stored at 4 °C.
Callus induction
Mature argan fruits were harvested from the previously mentioned genotypes. The fruits were thoroughly washed with tap water then the seeds were extracted and disinfected as follows: they were first thoroughly washed with SDW, then they were disinfected for 10 min in a solution consisting of 50% commercial bleach (5% sodium hypochlorite, ACE, Mohammedia, Morocco) and 50% SDW, followed by three rinses (10 min each) in SDW. Afterwards, two different types of explants were used to induce callogenesis. In the first case, seeds were cultured for one month on agar medium, which consists of 0.6% agar dissolved in distilled water. After germination, the seedlings (Fig. 1a) were transferred to half-strength Murashige and Skoog medium (1/2MS; Murashige and Skoog 1962) supplemented with 1 mg l−1 IAA and 1 mg l−1 BA for two months. The developed young leaves (Fig. 1b) were used as explants. In the second case, zygotic embryos (Fig. 1c) were aseptically excised from the seeds and their cotyledons were used as explants.
Plant material used for callus induction. a Seedlings after 1 month of culture on agar medium. b Shoots of argan seedlings after 2 months of culture on 1/2MS medium supplemented with 1 mg l−1 IAA and 1 mg l−1 BA: leaves were taken from these shoots and used as explants for callus induction. c Argan zygotic embryos from which cotyledon explants were taken
For callus induction and multiplication, the explants were cultured on 1/2MS medium supplemented with 1 mg l−1 NAA and 1 mg l−1 2,4-D as suggested by Lamaoui et al. (2019).
Methanolic extract preparation
Methanolic extracts were prepared according to Yesil-Celiktas et al. (2007). Briefly, 1 g of fine powder of seeds, leaves and calli were mixed with 20 ml methanol in a sonication bath (SB-3200DTDN, Ningbo Hinotek Instrument, Ningbo, China) for 45 min at 20 kHz and 50 °C. The solvent was then evaporated using a rotatory vacuum evaporator (Büchi R-205 with a V-800 vacuum control, Flawil, Switzerland) then the recovered product was stored at − 20 °C for further use.
Ethanolic extract preparation
Ethanolic extracts were prepared according to Robles-Martínez et al. (2016). Briefly, 50 mg of fine powder of seeds, leaves and calli were macerated in 5 ml of absolute ethanol for 2 h at 4 °C and then filtered using Whatman No. 42 filter paper. The samples were adjusted to 5 ml using absolute ethanol then stored at − 20 °C for later use.
Essential oil extraction
Essential oil extraction was performed according to Bernabé-Antonio et al. (2015) with slight modifications. Briefly, 1.5 g of fine powder of seeds, leaves and calli were soxhleted using 250 ml of hexane. The extraction was performed in three cycles of 24 h each at room temperature. The extracts were then dried using rotatory vacuum evaporator then stored at 4 °C until use.
Total phenolic content
For methanolic extracts, the total phenols were determined according to Yesil-Celiktas et al. (2007) with some modifications. Briefly, 14 mg of the methanolic extract was mixed with 2 ml methanol. Afterwards, a 10-µl aliquot was mixed with ultrapure water and 0.5 ml of Folin–Ciocalteu reagent. The solution was shaken vigorously by vortex then left to stand for 5 min. The mixture was neutralized with a solution of 1.5 ml saturated sodium carbonate, shaken vigorously then left to stand again for 1 h at ambient temperature. Absorbance was spectrophotometrically determined at 760 nm.
For ethanolic extracts, 20 µl of each solution was mixed with 1.6 ml of ultrapure water and 0.1 ml of Folin–Ciocalteu reagent. The mixture was left to stand for 8 min at ambient temperature then 0.3 ml of a solution of 20% sodium carbonate was added to it (Robles-Martínez et al. 2016). The reaction mixture was left to stand for 2 h in the dark at ambient temperature and then the absorbance was read spectrophotometrically at 760 nm.
For essential oils, chemical extraction was carried out following the protocol of Seiquer et al. (2015). Subsequently, 10 µl of each sample was mixed with 10 µl of Folin–Ciocalteu reagent. The mixture was left to stand for 3 min then 200 µl of a solution of sodium carbonate (75 g l−1) was added to it. Afterwards, the mixture was adjusted to 250 µl with Milli-Q water, shaken then left to stand for 60 min. The absorbance was read spectrophotometrically at 750 nm (Seiquer et al. 2015).
The total phenolic content was expressed as gallic acid (GAE) equivalents (mg per gram of dry extract) using the following equation, based on the calibration curve: y = 0.0144 x + 0.034, where y is the absorbance.
Fatty acid composition
The fatty acid composition was determined according to Hernandez et al. (2015) with slight modifications. Briefly, 100 µl of the essential oil of each sample (seed, leaf and callus) was mixed with 1 ml of NaOH (2M) in methanol. The mixture was heated in water bath at 80 °C for 20 min with constant stirring, cooled and a solution of 1 ml BF3 (14% in methanol) was added to it. The mixture was warmed again to a temperature of 80 °C for 20 min with constant stirring. The methyl esters were extracted with 1 ml of hexane. The samples were analyzed using GC (YL Instrument, 6500 GC System, Gyeonggi-do, South Korea) equipped with a flame ionization detector (FID) and an HP-Innowax column (30 m * 0.32 mm * 0.25 µm). The chromatographic analysis conditions were as follows: the injector temperature was 220 °C, the oven temperature was 175 °C and the FID temperature was 220 °C.
Antioxidant activity
The antioxidant activity was determined using the free radical scavenging activity DPPH. For methanolic extracts, 500 µg of the stored extract was adjusted to 4 ml using methanol, then a volume of 0.5 ml of methanolic DPPH (1 mM) was added to it (Yesil-Celiktas et al. 2007). The mixture was vortexed for 30 s then left to stand for 30 min at ambient temperature. For ethanolic extracts, 0.5 ml of the extract was mixed with 0.5 ml of absolute ethanol and 1 ml of DPPH ethanol solution (0.1 mM) as described by Robles-Martínez et al. (2016). The mixture was then left to stand at ambient temperature for 30 min. For essential oils, chemical extraction was carried out following the protocol of Seiquer et al. (2015). Subsequently, 50 µl of each sample was mixed with 250 µl of DPPH solution (74 mg l−1 in methanol). The mixture was then incubated for 60 min (Morales and Jiménez-Pérez 2001).
In all cases, the absorbance was spectrophotometrically determined using an Optizen 3220 UV–Visible spectrophotometer (Daejeon, South Korea) at 517 nm. The ability to scavenge DPPH radical was calculated using the following equation: %Radical Scavenging Activity (RSA) = ((ADPPH−ASAMPLE)/ADPPH)*100, where ADPPH is the absorbance of the DPPH solution and ASAMPLE is the absorbance of the sample solution.
Statistical analysis
For callus induction, eight explants (from each plant tissue type) were cultured per petri dish, which was considered as one replicate, and ten replicates were performed. For plant extract and essential oil characterization, three replicates were performed. All results are reported as means ± standard deviation.
All data were subjected to one-way analysis of variance (ANOVA) in a completely randomized design using SPSS v. 21 (IBM-SPSS Inc., Chicago, IL, USA). The means were compared using Student–Newman–Keuls test (P < 0.05), and percentage data were subjected to arcsine transformation before analysis.
Results
Callus induction
Few days after placing seeds on agar medium, the radicle began to emerge, which was the criterion for successful germination. At the end of the first month of culture, large cotyledons and a single leafy shoot were also observed. The germination frequency was 100% in the four genotypes evaluated. After transferring the seedlings to 1/2MS medium supplemented with 1 mg l−1 IAA and 1 mg l−1 BA, shoot elongation and development of new leaves were observed. After 2 months of culture, young leaves were collected from the growing shoots and transferred to callus induction medium.
After 2 months of culture on callus induction medium, the callus induction rate and callus morphology varied depending on genotype and explant type (seedling-derived leaves and cotyledons of zygotic embryos). The highest callus induction rate (97.5%) was observed in the cotyledons of G84, with significant differences with the other genotypes, which showed 68.7% (G36), 41.2% (G25) and 30% (G41) callus induction rates from cotyledon explants. Regarding seedling-derived leaves, here again, the callus induction rate was higher (75%) in G84 when compared to G36 (63.7%), G25 (30%) and G41 (7.5%; Table 1). On the other hand, callus morphology varied depending on the explant. In fact, calli derived from cotyledon explants (CC) were white and friable (Fig. 2a) while those induced from seedling-derived leaves (CL) had a yellow to brown color and were compact (Fig. 2b).
Callus induction in argan (Argania spinosa L. Skeels): a white and friable calli obtained from cotyledon explants after 2 months of culture on 1/2MS medium supplemented with 1 mg l−1 NAA and 1 mg l−1 2,4-D. b Yellow to brown and compact callus obtained from seedling-derived leaves after 2 months of culture on 1/2MS medium supplemented with 1 mg l−1 NAA and 1 mg l−1 2,4-D. c Cotyledon callus proliferation. d Seedling-derived leaf callus proliferation
While our results clearly demonstrate the effect of argan genotype and explant on callus induction, it is worth noting that callus proliferation also depends on the genotype. In fact, only calli derived from G84 continued to grow after transfer to fresh medium (Fig. 2c, d), while those derived from genotypes G36, G25 and G41 did not show any proliferative potential. Instead, they gradually withered and died. Besides, the G84 calli that were initially white later became yellow (Fig. 2c). Based on these results, only calli obtained from seedling-derived leaf and cotyledon explants of genotype G84 were used for essential oil and extract preparation, and subsequent analyses.
Yield of essential oils
The yield of essential oils varied considerably depending on the plant material used (Table 2). Generally, seeds showed significantly higher yield than leaves and calli. The yield of essential oils from seeds ranged from 737.5 to 774.5 mg/1.5 g dry matter (DM), with no significant difference among the four genotypes. The yield of essential oils from field leaves ranged from 30 mg/1.5 g DM (in G84) to 109.5 mg/1.5 g DM (in G36). Regarding the induced calli, the yield of essential oils was 61.5 mg/1.5 g DM in those induced from seedling-derived leaves and 112.5 mg/1.5 g DM in those induced from cotyledon explants.
Total phenolic content
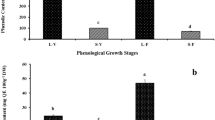
The total phenolic content varied depending on genotype, plant material and the extract used (Table 3). Regarding the methanolic and ethanolic extracts, leaves exhibited higher total phenolic values than seeds and calli. The highest total phenolic content was observed in the ethanolic extracts of leaves of G84 (37.34 mg/g DW GAE). This was followed by the methanolic extracts of G25 leaves with 31.97 mg/g DW GAE. The methanolic and ethanolic extracts of seeds and calli showed lower values, ranging from 0.72 mg/g DW GAE in the methanolic extracts of CL-G84 to 9.14 mg/g DW GAE in the ethanolic extracts of CC-G84. On the other hand, when the total phenolic content was evaluated in essential oils, very high values were observed, ranging from 34.38 mg/g DW GAE in CC-G84 to 198.26 mg/g DW GAE in S-G84. Here again, there was a significant effect of genotype on the phenolic content.
Fatty acid composition
The fatty acid composition varied significantly among the different samples (Table 4). The essential oils extracted from seeds have high contents in oleic and linoleic acids, ranging from 26 (G41) to 34.4% (G25) and from 25 (G84) to 36.8% (G41), respectively. Surprisingly, our findings revealed that the major fatty acid in leaf essential oils is eicosenoic acid, with a content ranging from 18.8 (G36) to 45% (G41 and G84). Moreover, it was found that the essential oils obtained from the leaves of G25 and G41 do not contain oleic acid. Regarding callus essential oils, it was found that those obtained from cotyledon explants are rich in oleic (37.9%) and linoleic (36.5%) acids, whereas those obtained from seedling-derived leaves are rich in eicosenoic acid (45.4%) and do not contain linoleic acid. All these results show that the fatty acid composition in argan essential oils is unique to each genotype, plant material and extract type. Accordingly, each sample may have a specific nutraceutical application.
Antioxidant activity
The RSA varied significantly depending on genotype, plant material and the extract used (Table 5). The highest RSA value (98.1%) was observed in the methanolic extracts from the leaves of G36, with no significant difference with the values obtained with the methanolic extracts of the leaves of G25 (98%), G84 (97.5%) and G41 (97.1%). The methanolic extracts of seeds exhibited lower RSA values, ranging from 76.5% (in G25) to 83.4% (in G84). Regarding the ethanolic extracts prepared from seeds and leaves, the RSA varied from 85.6% in the seeds of G41 to 92.5% in the leaves of G25.
The RSA values of essential oils also varied depending on both the genotype and plant material used. Indeed, the essential oils obtained from leaves showed values between 90 (in G36) and 91.1% (G84), while in those derived from seeds, the RSA ranged from 78.6% (in G36) to 88.1% (in G41).
The extracts obtained from callus culture of G84 showed intermediate results. The lowest RSA value (82%) was observed in the methanolic extracts of cotyledon-derived calli, while the highest RSA value (91.7%) was observed in the essential oils of calli induced from seedling-derived leaves. Based on their antioxidant activity, the essential oils obtained from calli of seedling-derived leaves can be recommended for nutraceutical applications.
Discussion
In the present work, the antioxidant activity, total phenols and fatty acids of methanolic and ethanolic extracts, as well as essential oils derived from calli, leaves and seeds of four argan genotypes were determined and compared. Callus induction was achieved from seedling-derived leaves and cotyledons. Studies on argan callogenesis are scarce. Lamaoui et al. (2019) found that callus formation strongly depends on the explant type and PGRs. These authors reported that the combination of 1 mgl−1 NAA and 1 mgl−1 2,4-D resulted in the highest callus formation rate (95%), and that axillary bud explants exhibited higher callogenesis than leaf, stem and apical bud explants. The PGR combination suggested by Lamaoui et al. (2019) resulted in different callus induction rates, with up to 97.5%. In addition, the findings of the present investigation are consistent with those of Lamaoui et al. (2019). In fact, it was found that callus formation depends on the explant source and was significantly higher in cotyledons than in seedling-derived leaves. There was also a significant effect of genotype on callogenesis. Indeed, G84 showed greater induction and proliferation of callus than the other genotypes. These findings may reflect differences in endogenous plant hormones among different explants and genotypes. In fact, it is well known that the requirement of exogenous PGRs for callogenesis and/or morphogenesis depends on the concentration of endogenous plant hormones, which varies among explants and genotypes (Gaj 2004; Jiménez 2001; Mazri et al. 2017). The effect of genotype on callogenesis was reported in other plant species, including spring barley (Šerhantová et al. 2004), bahiagrass (Akashi et al. 1993) and olive (Mazri et al. 2012). Based on our results, G84 is recommended for metabolite production from callus culture of argan. This genotype has a juvenile period of 5 years, an oil yield of 48–52% and is characterized by abundant foliage, flowering and fruiting.
The findings of the present study showed that callus morphology varies depending on the explant. Indeed, while Lamaoui et al. (2019) reported that the calli obtained from the different explants were yellowish and friable, our findings showed that those induced from cotyledon explants were white and friable, whereas those induced from seedling-derived leaves were yellow to brown and compact. The effect of explant type on callus morphology was also observed in other plant species such as Italian Ryegrass (Takahashi et al. 2004) and Chinese chive (Matsuda and Adachi 1996).
Phenols are a group of chemical compounds that have important biological functions including antimicrobial, antioxidant and neuroprotective activities (De Martino et al. 2009; Kim 2010). Previous studies showed that polyphenols extracted from virgin argan oil have anti-proliferative and pro-apoptotic effects on human prostate cancer cell lines (Bennani et al. 2007), and prevent cardiovascular diseases by protecting low-density lipoprotein from oxidation and increasing reverse cholesterol transport from human THP-1 macrophages (Berrougui et al. 2006). The present study revealed that the total phenolic content varies depending on the genotype and plant material used, but mostly on the extract type, with very high concentrations in essential oils. This is in good agreement with results from the literature. Indeed, Rojas et al. (2005) reported a total phenolic content of 3.1 mg gallic acid per kilogram in argan cosmetic oil, 13.2 mg gallic acid per kilogram in argan alimentary oil and 482.6 mg gallic acid per kilogram in press cake. Cayuela et al. (2008) found that the total phenolic content in argan oils obtained using both semiautomatic and artisanal extraction methods is lower than 10 ppm. El Adib et al. (2015) reported that the total phenolic content varies significantly depending on the plant part (pulp, seed, and leaf extracts). However, it depends also on the genotype and harvesting time.
Fatty acids are a source of metabolic and storage energy, and play important roles in early human development and in metabolism (Innis 2007; Rustan and Drevon 2005). In the present work, it was found that fatty acid composition depends considerably on genotype, plant material and the extract used. Oleic acid (C18:1) and linoleic acid (C18:2) were the major fatty acids in the essential oils extracted from seeds and cotyledon calli. Charrouf and Guillaume (1999), Öntaş et al. (2016), Dakiche et al. (2017) and Ben Mansour et al. (2018) all reported that oleic acid (C18:1) is the major fatty acid in argan oils (roasted, unroasted, laboratory prepared, traditionally extracted, solvent extracted and commercial oils). Unexpectedly, eicosenoic acid (C20:1) was found to be the predominant fatty acid in leaves and callus obtained from seedling-derived leaves. This high level of eicosenoic acid is noteworthy. In fact, this fatty acid was found in breast milk and was reported to have antioxidant and immunomodulatory properties (Henry et al. 2002; Pandey et al. 2017). Based on our results, argan leaves and callus obtained from seedling-derived leaves can be considered as a good source of eicosenoic acid. The fatty acid compositions revealed in this study could be an indicator of which extract will be appropriate for specific nutraceutical applications.
The antioxidant activity of plant extracts has a beneficial effect on human health and is of particular interest to food industry (Amarowicz et al. 1999; Dykes et al. 2003).Generally, few studies have been carried out to examine the antioxidant activity of essential oils and other extracts of argan obtained from field-grown plant material and callus culture. The findings of the present investigation showed that the RSA of argan extracts varies depending on the genotype and plant material. This is in good agreement with the findings of El Adib et al. (2015), who reported that DPPH-scavenging activity varies significantly depending on argan cultivar, the plant part used (leaf, pulp or seed) and harvesting time, with seeds exhibiting the lowest DPPH-scavenging activity. El Monfalouti et al. (2012) also compared the DPPH radical scavenging activity of different argan fruit parts and reported that the RSA was higher in argan fruit pulp when compared with argan shell, kernels and press-cake. Regarding callus induced in vitro, Lamaoui et al. (2019) found that argan calli increase their antioxidant activity linked to oxidative metabolism under salt stress induced by NaCl. Furthermore, it was found that the activity of some important antioxidant enzymes increases in argan calli under water stress induced by PEG 6000 (Lamaoui et al. 2015).
In the present work, no relationship was revealed between the total phenolic content and antioxidant activity. For example, in argan leaves, the total phenolic content was significantly higher in essential oils than in methanolic extracts. However, the methanolic extracts showed higher RSA than essential oils. This is most likely due to the difference in the phenolic composition of the extracts, as well as the difference in other antioxidant components (Ismail et al. 2004). Such results were reported by other researchers who found that, in many plant species, high phenolic content does not correlate with high antioxidant activity (Chahardehi et al. 2009; Chanudom et al. 2014; Ismail et al. 2004; Kähkönen et al. 1999).
Conclusions
We evaluated and compared the antioxidant activity, total phenolic content and fatty acid composition of different extracts from various plant parts of four argan genotypes. Callus induction and proliferation varied significantly depending on genotype and explant type. The RSA values were high in all samples. Besides, it is worth noting that the essential oils of calli induced from seedling-derived leaves have a higher RSA than those obtained from field-grown leaves. The total phenolic content varied depending on genotype, the plant material and the extract used and was very high in essential oils when compared to the other extracts. On the other hand, the fatty acid composition varies greatly among the different samples analyzed. More studies should be carried out on argan callus proliferation and use as a renewable source of sustainable metabolite production. Determination of the detailed phenolic composition of the different argan extracts and evaluation of their biological relevance for possible application in nutraceutical industries are also recommended.
References
Abbas M, Saeed F, Anjum FM, Afzaal M, Tufail T, Bashir MS, Ishtiaq A, Hussain S, Suleria HAR (2017) Natural polyphenols: an overview. Int J Food Prop 20:1689–1699
Amarowicz R, Barl B, Pegg RB (1999) Potential natural antioxidants from Saskatchewan indigenous plants. J Food Lipids 6:317–329
Akashi R, Hashimoto A, Adachi T (1993) Plant regeneration from seed-derived embryogenic callus and cell suspension cultures of bahiagrass (Paspalum notatum). Plant Sci 90:73–80
Ben Mansour R, Ben Slema H, Falleh H, Tounsi M, Kechebar MSA, Ksouri R, Megdiche-Ksouri W (2018) Phytochemical characteristics, antioxidant, and health properties of roasted and unroasted Algerian argan (Argania spinosa) oil. J Food Biochem 42:e12562
Bennani H, Drissi A, Giton F, Kheuang L, Fiet J, Adlouni A (2007) Antiprofilative effect of polyphenols and sterols of virgin argan oil on human prostate cancer cell lines. Cancer Detect Prev 31:64–69
Bernabé-Antonio A, Álvarez L, Salcedo-Pérez E, Toral FALD, Anzaldo-Hernández J, Cruz-Sosa F (2015) Fatty acid profile of intact plants of two different sites and callus cultures derived from seed and leaf explants of Calophyllum brasiliense Cambess: a new resource of non-edible oil. Ind Crop Prod 77:1014–1019
Berrougui H, Cloutier M, Isabelle M, Khalil A (2006) Phenolic-extract from argan oil (Argania spinosa L.) inhibits human low-density lipoprotein (LDL) oxidation and enhances cholesterol efflux from human THP-1 macrophages. Atherosclerosis 184:389–396
Cayuela JA, Rada M, Pérez-Camino MC, Benaissa M, Abdelaziz E, Guinda Á (2008) Characterization of artisanally and semiautomatically extracted argan oils from Morocco. Eur J Lipid Sci Technol 110:1159–1166
Chahardehi AM, Ibrahim D, Sulaiman SF (2009) Antioxidant activity and total phenolic content of some medicinal plants in Urticaceae family. J Appl Biol Sci 2:27–31
Chanudom L, Bhoopong P, Khwanchuea R, Tangpong J (2014) Antioxidant and antimicrobial activities of aqueous & ethanol crude extracts of 13 Thai traditional plants. Int J Curr Microbial Appl Sci 3:549–558
Charrouf Z, Guillaume D (1999) Ethnoeconomical, ethnomedical and phytochemical study of Argania spinosa (L.) Skeels. J Ethnopharmacol 67:7–14
Charrouf Z, Guillaume D (2008) Argan oil: occurrence, composition and impact on human health. Eur J Lipid Sci Technol 110:632–636
Dakiche H, Khali M, Abu-el-Haija AK, Al-Maaytah A, Al-Balas QA (2016) Biological activities and phenolic contents of Argania spinosa L (Sapotaceae) leaf extract. Trop J Pharm Res 15:2563–2570
Dakiche H, Khali M, Boutoumi H (2017) Phytochemical characterization and in vivo anti-inflammatory and wound-healing activities of Argania spinosa (L.) Skeels seed oil. Rec Nat Prod 11:171–184
De Martino L, De Feo V, Fratianni F, Nazzaro F (2009) Chemistry, antioxidant, antibacterial and antifungal activities of volatile oils and their components. Nat Prod Commun 4:1741–1750
Dykes GA, Amarowicz R, Pegg RB (2003) Enhancement of nisin antibacterial activity by a bearberry (Arctostaphylos uva-ursi) leaf extract. Food Microb 20:211–216
El Adib S, Aissi O, Charrouf Z, Ben Jeddi F, Messaoud C (2015) Argania spinosa var. mutica and var. apiculata: variation of fatty-acid composition, phenolic content, and antioxidant and α-amylase-inhibitory activities among varieties, organs, and development stages. Chem Biodivers 12:1322–1338
El Kabouss A, Charrouf Z, Faid M, Garneau FX, Collin G (2002) Chemical composition and antimicrobial activity of the leaf essential oil of Argania spinosa L. Skeels. J Essent Oil Res 14:147–149
El Kharrassi Y, Maata N, Mazri MA, El Kamouni S, Talbi M, El Kebbaj R, Moustaid K, Essamadi AK, Andreoletti P, El Mzouri EH, Cherkaoui-Malki M, Nasser B (2018) Chemical and phytochemical characterizations of argan oil (Argania spinosa L. skeels), olive oil (Olea europaea L. cv. Moroccan picholine), cactus pear (Opuntia megacantha salm-dyck) seed oil and cactus cladode essential oil. J Food Meas Char 12:747–754
El Monfalouti H, Charrouf Z, Belviso S, Ghirardello D, Scursatone B, Guillaume D, Denhez C, Zeppa G (2012) Analysis and antioxidant capacity of the phenolic compounds from argan fruit (Argania spinosa (L.) Skeels). Eur J Lipid Sci Technol 114:446–452
Falasca SL, Pitta-Alvarez S, Ulberich A (2018) The potential growing areas for Argania spinosa (L.) Skeels (Sapotaceae) in Argentinean drylands. Int J Agron. https://doi.org/10.1155/2018/9262659
Fauser JK, Prisciandaro LD, Cummins AG, Howarth GS (2011) Fatty acids as potential adjunctive colorectal chemotherapeutic agents. Cancer Biol Ther 11:724–731
Flamini G, Cioni PL, Morelli I, Macchia M, Ceccarini L (2002) Main agronomic-productive characteristics of two ecotypes of Rosmarinus officinalis L. and chemical composition of their essential oils. J Agric Food Chem 50:3512–3517
Gaj MD (2004) Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabidopsis thaliana (L.) Heynh. Plant Growth Regul 43:27–47
Guillaume D, Pioch D, Charrouf Z (2019) Argan [Argania spinosa (L.) Skeels] oil. In: Ramadan MF (ed) Fruit oils: chemistry and functionality. Springer, Cham, pp 317–352
Guinda A, Rada M, Delgado T, Castellano JM (2011) Pentacyclic triterpenic acids from Argania spinosa. Eur J Lipid Sci Technol 113:231–237
Haloui I, Meniai AH (2017) Supercritical CO2 extraction of essential oil from Algerian Argan (Argania spinosa L.) seeds and yield optimization. Int J Hydrogen Energy 42:12912–12919
Harhar H, Gharby S, Ghanmi M, El Monfalouti H, Guillaume D, Charrouf Z (2010) Composition of the essential oil of Argania spinosa (Sapotaceae) fruit pulp. Nat Prod Commun 5:935–936
Hättenschwiler S, Vitousek PM (2000) The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol Evol 15:238–243
Henry GE, Momin RA, Nair MG, Dewitt DL (2002) Antioxidant and cyclooxygenase activities of fatty acid found in food. J Agric Food Chem 50:2231–2234
Hernandez LR, Mendiola MAR, Castro CA, Gutierrez-Miceli FA (2015) Effect of plant growth regulators on fatty acids composition in Jatropha curcas L. Callus culture. J Oleo Sci 64:325–330
Ibarguren M, López DJ, Escribá PV (2014) The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim Biophys Acta 1838:1518–1528
Innis SM (2007) Fatty acids and early human development. Early Hum Dev 83:761–766
Ismail A, Marjan ZM, Foong CW (2004) Total antioxidant activity and phenolic content in selected vegetables. Food Chem 87:581–586
Jawdat D, Al-Faoury H, Odeh A, Al-Rayan R, Al-Safadi B (2016) Essential oil profiling in callus of some wild and cultivated Daucus genotypes. Ind Crop Prod 94:848–855
Jiménez VM (2001) Regulation of in vitro somatic embryogenesis with emphasis on to the role of endogenous hormones. Rev Brasi Fisio Vegl 13:196–223
Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M (1999) Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem 47:3954–3962
Khallouki F, Younos C, Soulimani R, Oster T, Charrouf Z, Spiegelhalder B, Bartsch H, Owen RW (2003) Consumption of argan oil (Morocco) with its unique profile of fatty acids, tocopherols, squalene, sterols and phenolic compounds should confer valuable cancer chemopreventive effects. Eur J Cancer Prev 12:67–75
Kim YC (2010) Neuroprotective phenolics in medicinal plants. Arch Pharm Res 33:1611–1632
Koufan M, Belkoura I, Alaoui T (2018) The multiplication of the argane tree by microcutting (Argania spinosa L. Skeels). Eur J Biotechnol Biosci 6:47–52
Lamaoui M, Aissam S, Wahbi S, Chakhchar A, Ferradous A, El Moousadik A, Ibnsouda-Koraichi S, Filali-Maltouf A, El Modafar C (2015) Anti-oxidant activity in Argania spinosa callus selected under water stress conditions. J Hortic Sci Biotechnol 90:127–134
Lamaoui M, Chakhchar A, Benlaouane R, El Kharrassi Y, Farissi M, Wahbi S, El Modafar C (2019) Uprising the antioxidant power of Argania spinosa L. callus through abiotic elicitation. Comptes Rendus Biol 342:7–17
Lin KH, Shih MC, Wang P, Yu YP, Lu CP (2018) Effect of different ethanolic concentrations on antioxidant properties and cytoprotective activities of Platostoma palustre Blume. Nat Prod Res 32:2959–2963
Lybbert TJ, Aboudrare A, Chaloud D, Magnan N, Nash M (2011) Booming markets for Moroccan argan oil appear to benefit some rural households while threatening the endemic argan forest. Proc Natl Acad Sci 108:13963–13968
Matsuda Y, Adachi T (1996) Plant regeneration via embryogenesis in commercial cultivars of Chinese chive (Allium tuberosum Rottl.). Plant Sci 119:149–156
Mazri MA, Belkoura I, Pliego-Alfaro F, Belkoura M (2012) Embryogenic capacity of embryo-derived explants from different olive cultivars. Acta Hortic 929:397–403
Mazri MA, Belkoura I, Meziani R, Mokhless B, Nour S (2017) Somatic embryogenesis from bud and leaf explants of date palm (Phoenix dactylifera L.) cv. Najda . 3 Biotech 7:58
Mayouf N, Charef N, Saoudi S, Baghiani A, Khennouf S, Arrar L (2019) Antioxidant and anti-inflammatory effect of Asphodelus microcarpus methanolic extracts. J Ethnopharmacol 239:111914
Metougui ML, Mokhtari M, Maughan PJ, Jellen EN, Benlhabib O (2017) Morphological variability, heritability and correlation studies within an argan tree population (Argania spinosa (L.) Skeels) preserved in situ. Int J Agric For 7:42–51
Meziani R, Mazri MA, Essarioui A, Alem C, Diria G, Gaboun F, El Idrissy H, Laaguidi M, Jaiti F (2019) Towards a new approach of controlling endophytic bacteria associated with date palm explants using essential oils, aqueous and methanolic extracts from medicinal and aromatic plants. Plant Cell Tissue Organ Cult 137:285–295
Morales FJ, Jiménez-Pérez S (2001) Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence. Food Chem 72:119–125
Moukrim S, Lahssini S, Rhazi M, Mharzi Alaoui H, Benabou A, Wahby I, El Madihi M, Arahou M, Rhazi L (2019) Climate change impacts on potential distribution of multipurpose agro-forestry species: Argania spinosa (L.) Skeels as case study. Agrofor Syst 93:1209–1219
Murashige T, Skoog FA (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Phys Planta 15:473–479
Öntaş C, Baba E, Kaplaner E, Küçükaydin S, Öztürk M, Ercan MD (2016) Antibacterial activity of citrus limon peel essential oil and Argania spinosa oil against fish pathogenic bacteria. Kafkas Üniversitesi Veteriner Fakültesi Dergisi 22:741–749
Pandey G, Gupta SS, Bhatia A, Sidhu OP, Rawat AKS, Rao CV (2017) Grilling enhances antidiarrheal activity of Terminalia bellerica Roxb. fruits. J Ethnopharmacol 202:63–66
Razavizadeh R, Komatsu S (2018) Changes in essential oil and physiological parameters of callus and seedlings of Carum copticum L. under in vitro drought stress. J Food Meas Char 12:1581–1592
Robles-Martínez M, Barba-de la Rosa AP, Guéraud F, Negre-Salvayre A, Rossignol M, Santos-Díaz MS (2016) Establishment of callus and cell suspensions of wild and domesticated Opuntia species: study on their potential as a source of metabolite production. Plant Cell Tissue Organ Cult 124:181–189
Rojas LB, Quideau S, Pardon P, Charrouf Z (2005) Colorimetric evaluation of phenolic content and GC-MS characterization of phenolic composition of alimentary and cosmetic argan oil and press cake. J Agric Food Chem 53:9122–9127
Rustan AC, Drevon CA (2005) Fatty acids: structures and properties. In: Encyclopedia of life sciences. Wiley, Hoboken, pp 1–7
Samane S, Noël J, Charrouf Z, Amarouch H, Haddad PS (2006) Insulin-sensitizing and anti-proliferative effects of Argania spinosa seed extracts. eCAM 3:317–327
Scalbert A, Johnson IT, Saltmarsh M (2005) Polyphenols: antioxidants and beyond. Am J Clin Nutr 81(suppl):215S-217S
Seiquer I, Rueda A, Olalla M, Cabrera-Vique C (2015) Assessing the bioavailability of polyphenols and antioxidant properties of extra virgin argan oil by simulated digestion and Caco-2 cell assays. Comparative study with extra virgin olive oil. Food Chem 188:496–503
Šerhantová V, Ehrenbergerová J, Ohnoutková L (2004) Callus induction and regeneration efficiency of spring barley cultivars registered in the Czech Republic. Plant Soil Environ 50:456–462
Swamy MK, Akhtar MS, Sinniah UR (2016) Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evid Based Complement Alternat Med 22:1019
Takahashi W, Komatsu T, Fujimori M, Takamizo T (2004) Screening of regenerable genotypes of Italian ryegrass (Lolium multiflorum Lam.). Plant Prod Sci 7:55–61
Vergis J, Gokulakrishnan P, Agarwal RK, Kuamr A (2013) Essential oils as natural food antimicrobial agents: a review. Crit Rev Food Sci Nutr 55:1320–1323
Wang DC, Sun CH, Liu LY, Sun XH, Jin XW, Song WL, Liu XQ, Wan XL (2012) Serum fatty acid profiles using GC-MS and multivariate statistical analysis: potential biomarkers of Alzheimer’s disease. Neurobiol Aging 33:1057–1066
Yesil-Celiktas O, Nartop P, Gurel A, Bedir E, Vardar-Sukan F (2007) Determination of phenolic content and antioxidant activity of extracts obtained from Rosmarinus officinalis’ calli. J Plant Physiol 164:1536–1542
Author information
Authors and Affiliations
Contributions
MK, IB and MAM conceived the idea and designed research. MK, MAM, AE, HE performed in vitro germination and callus induction experiments. MK, AA and FZ prepared and analyzed argan extracts and essential oils. MK and MAM wrote the manuscript. MAM performed statistical analysis. IB and TA supervised experiments and corrected the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Konstantin V. Kiselev.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koufan, M., Belkoura, I., Mazri, M.A. et al. Determination of antioxidant activity, total phenolics and fatty acids in essential oils and other extracts from callus culture, seeds and leaves of Argania spinosa (L.) Skeels. Plant Cell Tiss Organ Cult 141, 217–227 (2020). https://doi.org/10.1007/s11240-020-01782-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01782-w