Abstract
Hypericum perforatum cell suspensions were evaluated for their growth, phenylpropanoid and naphtodianthrone productions, and antioxidant activity after treatments with fungal elicitors Fusarium oxysporum, Phoma exigua and Botrytis cinerea. Elicited cells displayed a reduced biomass production, a rapid stimulation of secondary metabolites production and a modification of cell redox state compared to control. Cells responded strongly towards the applied elicitors through the enhanced production of naphtodianthrones. Hypericin and pseudohypericin production was significantly increased (up to fourfold) in the early growth phase and remained stable all along the post-elicitation period. Significant increase in contents of total phenolics, total flavonoids and total anthocyanins was observed during the entire period of cultivation, while total flavanols were enhanced at the end of post-elicitation. The enzymatic activities of phenylalanine ammonia lyase and chalcone isomerase were remarkably elevated in elicited cells confirming a strong activation of phenylpropanoid/flavonoid pathways. The fingerprint profile of Fourier transform infrared spectroscopy spectra from the cell walls showed a little variation in lignin accumulation between elicited and control samples. With regards to the antioxidant state, an early up-regulation of peroxidase activity was observed in elicited cells, whereas non-enzymatic properties and catalase activity were enhanced at the end of post-elicitation. These findings suggest the involvement of an efficient antioxidant defense system in the adaptive response of cells to fungal elicitation. Altogether, these results indicated that H. perforatum elicited cells represent a promising experimental system for scale-up production of naphtodianthrones for medicinal uses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypericum perforatum L., commonly known as St. John’s wort is one of the best-studied medicinal plants with well-characterised bioactive constituents. Hypericum extracts contain naphtodianthrones, acyl-phloroglucinols, flavonoids and xanthones with a wide range of pharmacological attributes that are associated with anti-inflammatory, hepatoprotective, antiviral, antimicrobial, antioxidant, antitumoral and wound-healing activity (Nahrstedt and Butterweck 2010). The most significant use of Hypericum pharmaceutical preparations comprises symptomatic treatment of mild-to-moderate depression and recently good perspectives emerged also in the field of major depression (Solomon et al. 2013). H. perforatum has become one of the leading plant-based dietary supplements worldwide and phytopharmaceutical industry is currently supplied by field grown or cultivated plant materials (Murch and Saxena 2006). Hypericins (HYPs) remain the popular marker compounds for routine standardization of the herbal products because of hyperforins instability in the presence of oxygen and light (Nahrstedt and Butterweck 2010). The qualitative and quantitative variations of HYPs are greatly influenced by genetic, physiological, metabolic and environmental conditions (Košuth et al. 2003; Kirakosyan et al. 2004). Therefore, a search for alternative techniques for cultivation of H. perforatum under standardized laboratory conditions will provide development of pharmaceutical preparations with relatively stable naphtodianthrone contents.
Plant cell, tissue and organ culture has been recognized as a promising technology for commercial production of secondary metabolites when natural resources are limited, de novo synthesis is complex or the product has a high commercial value. Furthermore, the deliberate stimulation of defined compounds within carefully regulated in vitro cultures provides an excellent form for in-depth investigation of metabolic pathways under highly controlled micro-environmental conditions (Karuppusamy 2009). Though plant cell cultures could be a potential source of valuable pharmaceuticals, the industrial application of plant cell cultures has been met with limited success and only several high-value natural products such as shikonin, paclitaxel, resveratrol, artemisinin, ginsenosides and ajmalicin have been commercialized (Yue et al. 2014). However, commercial success of this technology is still limited due to low content of desired metabolite, recalcitrant nature and slow growth rate, genotypic variations, chemical instability and uneconomical downstream processing. Numerous strategies have been developed to improve the productivity of plant cell cultures, such as medium optimization, elicitation, precursor feeding, immobilization, in situ product removal, genetic transformation and bioreactor engineering (Murthy et al. 2014). Elicitation is an attractive strategy employed to induce or enhance secondary metabolite production due to addition of trace amounts of elicitors in plant in vitro culture systems. Elicitor may be defined as a substance which, when introduced in small concentrations to a living cell system, initiates or improves the biosynthesis of specific compounds (Namdeo 2007). In many cases, plant cell culture productivity can be enhanced using abiotic and biotic elicitors. Abiotic elicitors are predominantly inorganic salts and physical factors such as ultraviolet light, detergents and metal ions. Numerous biological preparations such as yeast extract, bacterial cell wall components and fungal mycelia extracts are often used as biotic elicitors (Namdeo 2007).
Several studies have been carried out to investigate the effects of different elicitors on the accumulation of secondary metabolites in Hypericum in vitro cultures. We have previously studied the overproduction of phenylpropanoids and naphtodianthrones in H. perforatum cultures upon treatments with phytohormones (Gadzovska et al. 2005), signal molecules jasmonic acid (JA) and salicylic acid (SA), (Gadzovska et al. 2007, 2013), fungal elicitor Aspergillus flavus (Gadzovska-Simic et al. 2012) and polysaccharides (Gadzovska Simic et al. 2014). Various H. perforatum in vitro cultures have also been tested for their ability to produce naphtodianthrones, acyl-phloroglucinols, flavonoids and xanthones upon treatment with mannan, β-1,3-glucan, pectin, JA, methyl jasmonate, SA, fungal elicitors Phytophthora cinnamoni and Colletotrichum gloeosporioides, as well with bacterial elicitors Agrobacterium tumefaciens and A. rhizogenes (Kirakosyan et al. 2000; Sirvent and Gibson 2002; Walker et al. 2002; Tusevski et al. 2014).
The use of pathogenic and non-pathogenic fungal preparations as elicitors has become one of the most effective strategies to induce phenylpropanoid/flavonoid biosynthetic pathways in plant cells (Dixon et al. 2002; Lattanzio et al. 2006). Necrotrophic pathogens such as Botrytis sp. usually kill the host cells often through secretion of toxins before deriving nutrients from them (Glazebrook 2005). On the other hand, biotrophic pathogens Fusarium sp. or Phoma sp. try to avoid killing the host cells, and derive their nutritional benefits from extensive contact with them and by altering the host metabolism and secretion systems (Leonard and Bushnell 2004; Boerema et al. 2004). An early defense reaction of the plant cell attacked by fungal pathogen includes rapid and transient production of reactive oxygen species (ROS). Plant cells are usually protected against the detrimental effects of ROS by a complex of non-enzymatic and enzymatic antioxidant systems (Gill and Tuteja 2010). It has been demonstrated that the PAL enzyme which catalyses the entry of l-phenylalanine into the phenylpropanoid pathway has reputedly a crucial role in the synthesis of antioxidant/defense-related compounds (Dixon et al. 2002). In this view, Hano et al. (2006) demonstrated that mycelia extracts from the above mentioned fungi induced partitioning of the phenylpropanoid pathway and a rapid stimulation of the monolignol pathway in Linum usitatissimum cultured cells. Lignin deposition leading to the reinforcement of cell walls has been induced in response to microbial attack, thereby acting as a physical barrier against pathogen invasion (Dixon et al. 2002). Further insights into plant-fungal interactions would be desirable to better understand the coordination of phenylpropanoid/flavonoid pathway with naphtodianthrones in H. perforatum cells.
In this study, H. perforatum cell suspensions were treated with fungal mycelia extracts from three types of fungi: Fusarium oxysporum f.sp. lini (non pathogenic), Phoma exigua (pathogenic non necrotrophic) and Botrytis cinerea (pathogenic necrotrophic). Investigations have been focused on the effects of elicitor treatments on: (1) cell biomass production and viability, (2) production of phenylpropanoids (phenolics, flavonoids, flavanols and anthocyanins) and naphtodianthrones [HYP and pseudohypericin (PHYP)], (3) enzyme activities of phenylalanine ammonia lyase (PAL) and chalcone isomerase (CHI), (4) antioxidant state estimated by non-enzymatic properties (NEAOP) and enzymatic activities of peroxidase (POD) and catalase (CAT), and (5) lignin accumulation in cell walls.
Materials and methods
Plant material
Seeds from H. perforatum were collected from wild plants growing in a natural population in the National Park Pelister at about 1394 m asl. Voucher specimen number (060231) of H. perforatum is deposited in the Herbarium at the Faculty of Natural Sciences and Mathematics, University “Ss. Cyril and Methodius”-Skopje, Republic of Macedonia. As for a previous study (Gadzovska et al. 2005), seeds were washed with 70 % ethanol for 30 s, surface sterilized with 1 % NaOCl for 15 min, rinsed 3 times in sterile deionized water and cultured on MS macro and oligoelements (Murashige and Skoog 1962), B5 vitamin solution (Gamborg et al. 1968), supplemented with 3 % sucrose and solidified with 0.7 % agar. No growth regulator was added. The medium was adjusted to pH 5.8 before autoclaving (20 min at 120 °C). In vitro cultures were maintained in a growth chamber at 25 ± 1 °C under a photoperiod of 16 h light, irradiance at 50 μmol m2 s−1 and 50–60 % relative humidity.
Cell suspension cultures
The first pair of leaves were excised from 2-weeks old in vitro germinated seedlings and used as explants to establish callus cultures. They were cultured in Petri dishes on MS/B5 medium supplemented with 1.0 mg L−1 2,4-dichlorophenoxyacetic acid (2,4-D) and 1.0 mg L−1 N6-benzyladenine (BA), 3 % sucrose and 0.7 % agar. Subcultures of calli (1.5–2 g) were carried out every 14 days. Cell suspensions were established from callus cultures after two subcultures. For this, green calli (1.5–2 g) were inoculated in 250 mL Erlenmeyer flasks containing 100 mL liquid MS/B5 medium, supplemented with 1.0 mg L−1 2,4-D, 1.0 mg L−1 BA and 3 % sucrose. The cultures were maintained on a rotary shaker at 100 rpm in the growth chamber. After 2 weeks, the cells released from calli were transferred in 4 volumes of fresh liquid medium and subcultured every 2 weeks.
Elicitor preparation and treatments
Fungal mycelia extracts were prepared according to Hano et al. (2006). Briefly, fungal mycelia were maintained on malt agar medium containing MS macroelements, MW vitamin mixture (Morel and Wetmore 1951), malt extract (20 g L−1), yeast extract (1 g L−1), and glucose (2 g L−1). For elicitor preparation, mycelia were subcultured on MS macro and oligoelements, MW vitamin solution, 3.5 mM morpholinoethane sulfonic acid (MES), sucrose (10 g L−1), and glutamine (250 mg L−1). The medium was adjusted to pH 5.5 before autoclaving (20 min at 120 °C). One-week-old mycelia were rinsed and re-suspended with sterile distilled water to a final concentration of 50 mg mL−1 of fungal mycelium, blended in an Ultra Turrax at full speed for 5 min and then autoclaved for 10 min at 120 °C. Total sugar content of the elicitor preparations was 2.70 ± 0.3 mg mL−1 and no protein were found. One of these fungal elicitors was obtained from a specific flax pathogen: F. oxysporum f.sp. lini, and the others from broad spectrum pathogens: Botrytis cinerea and Phoma exigua. Treatments of H. perforatum cell suspensions with 50 mg mL−1 fungal mycelia extracts were performed 7 days after subculture when cells were in log phase of growth. Cell suspensions cultivated on MS/B5 medium without elicitors were used as a control. After treatment, cell suspensions were photographed with numeric camera coupled to a photonic microscope Olympus BH-2. Cell viability was determined by vital staining with methylene blue as described by Laroche and Gervais (2003). The percentage of dead cells stained with methylene blue was determined and related to cell viability. Treated cell suspensions were then harvested by vacuum filtration on days 1, 4, 7, 14, and 21 of post-elicitation, weighted for growth analysis, frozen in liquid nitrogen or lyophilized and stored at −80 °C, until analysis.
Extraction and quantification of secondary metabolites
Phenolic compounds extraction and quantification were performed as previously reported (Gadzovska et al. 2007, 2013). Briefly, phenolic compounds were extracted from freeze-dried lyophilized and powdered plant material (0.2 g) with 80 % (v/v) methanol in ultrasonic bath for 30 min at 4 °C.
Total phenolic (TP) contents were determined when methanolic extract were mixed with Folin–Ciocalteau reagent (Carlo Erba Reagenti, Rodano, Italy) and 0.7 M Na2CO3 (Singleton and Rossi 1965). Samples were incubated for 5 min at 50 °C and then cooled for 5 min at room temperature. Absorbance was measured spectrophotometrically at 765 nm. The concentration of TP was calculated using gallic acid as a standard. The results were expressed as milligrams of gallic acid equivalents (GAE) per gram of dry mass (mg GAE g−1 DM).
Total flavonoid (TF) contents were determined by using a method described by Makris et al. (2007). An aliquot of appropriately diluted (1:10–1:100, v/v) extract was mixed with 5 % NaNO2 and allowed to react for 5 min. Following this, 10 % AlCl3 was added and the mixture stood for further 5 min. Finally, to the reaction mixture 1 M NaOH and distilled water were added. Absorbance was measured spectrophotometrically at 510 nm. The concentration of TF was calculated from a calibration curve using catechin as a standard. The results were expressed as milligrams of catechin equivalents (CE) per gram of dry mass (mg CE g−1 DM).
Total flavanol (TFL) contents were determined in methanolic extracts with 4-(dimethylamino)-cinnamaldehyde (DMACA) reagent (Li et al. 1996). The DMACA reagent was added to the diluted (1:10–1:100, v/v) extracts. The samples were incubated for 10 min. at room temperature and absorbance was measured at 640 nm. The concentration of TFL was calculated using catechin as a standard. The results were expressed as milligrams of catechin equivalents (CE) per gram of dry mass (mg CE g−1 DM).
Total anthocyanin (TA) determination was performed as described by Giusti et al. (1999). Anthocyanins were extracted from freeze-dried lyophilized and powdered plant material with 1 % HCl/CH3OH (15/85, v/v), ultrasonicated for 60 min at 4 °C and than centrifugated at 13,000 rpm for 30 min. The absorbance of supernatant was measured at 530 nm. The concentration of TA was calculated using the molar extinction coefficient of cyanidin-3-glucoside (ε530 = 34,300 M−1 cm−1). The results were expressed as milligrams of cyanidin-3-glucoside equivalents (CyGE) per gram of dry mass (mg CyGE g−1 DM).
Non-enzymatic antioxidant properties (NEAOP) assay by β-carotene bleaching method
The NEAOP of methanolic extracts were estimated by using linoleic acid-β-carotene oxidation method adapted from Marron et al. (2002). A linoleic acid-β-carotene emulsion was prepared by mixing 10 mg of linoleic acid with 750 μL of 0.2 mg mL−1 chloroformic β-carotene solution and 100 mg of Tween 40 (polyoxyethylenesorbitan monopalmitate). Chloroform was evaporated under nitrogen flow for 10 min. The resulting mixture was adjusted to 25 mL with distilled water and shaken for 10 s. The reaction mixture was prepared as follows: 10 μL extract were adjusted with 15 μL 80 % (v/v) CH3OH and 225 μL of linoleic acid-β-carotene emulsion was added. The mixture was heated to 50 °C. The control consisted of 25 μL 80 % (v/v) CH3OH and 225 μL of linoleic acid-β-carotene emulsion. Absorbance was measured at 470 nm every 15 min for 45 min. Results were computed as the ratio of β-carotene protection of the extract to the control (80 % CH3OH). NEAOP was calculated using the following formula: NEAOP = ((B − A)/B) × 100, where A is variation of absorbance of samples between 0 and 45 min; B is variation of absorbance of control between 0 and 45 min.
Enzyme extraction and assays
The extraction procedure for determination of antioxidant enzyme assays was based on the method as previously described by Gadzovska et al. (2007). The enzyme extract was prepared by homogenizing 1 g of frozen sample in 2 mL 0.1 M KH2PO4/K2HPO4 buffer at pH 8.0, containing 2 mM ethylenediamine tetra-acetic acid (EDTA), 1.4 mM β-mercaptoethanol and 1 % (w/v) polyvinylpyrrolidone (PVP). The homogenate was centrifuged at 13,000 rpm for 20 min at 4 °C. The supernatant was collected for determination of protein content and enzyme assays. Protein contents in enzyme extracts were performed with a Bio-Rad Protein Assay Reagent (Bradford 1976) using bovine serum albumin as a standard.
POD assay was based on a method described by Gonzales et al. (1999). The reaction mixture contained 0.1 M NaH2PO4/Na2HPO4 buffer (pH 6.0), 20 mM guaiacol solution and 0.1 % (v/v) H2O2 and diluted enzyme extract (1:10). The absorbance was monitored in 40 s for a period of 1 min and 20 s at 420 nm. The rate of change in absorbance per minute was used to quantify POD activity using the molar extinction coefficient of the oxidized product tetraguaiacol ε420 = 6400 M−1 cm−1. POD specific activity was determined as the increase in absorbance and expressed in nkat mg−1 proteins.
CAT assay was based on a method described by Fu and Huang (2001). The reaction mixture contained 60 mM KH2PO4/K2HPO4, 30 % (v/v) H2O2 and diluted enzyme extract (1:10). The decomposition of H2O2 was measured as the decrease in absorbance at 240 nm. Absorbance was monitored in 60 s for a period of 10 min. The rate of change in absorbance per minute was used to quantify CAT activity using the molar extinction coefficient of H2O2 (ε240 = 43.6 M−1 cm−1). CAT activity was expressed in nkat mg−1 proteins.
PAL assay was determined according to Gadzovska et al. (2007, 2013). The reaction mixture contained 2 % (w/v) solution of l-phenylalanine in 50 mM Tris–HCl at pH 8.8 and enzyme extract. Enzyme assay mixtures were incubated at 40 °C for 60 min. PAL activity was determined by measuring the rate of formation of trans-cinnamic acid as increase in absorbance at 290 nm. Molar extinction coefficient of cinnamate was ε290 = 19,600 M−1 cm−1. The PAL activity was expressed in pkat mg−1 proteins.
CHI enzyme assay was based on the method described by Gadzovska et al. (2007, 2013). CHI was assayed in 60 mM KH2PO4/K2HPO4 buffer at pH 8.0, containing 50 mM KCN to inhibit POD activity. Reaction was initiated by mixing enzyme extract and 2′4,4′,6-tetrahydroxychalcone. Enzyme assay mixture was incubated at 30 °C for 45 min. The kinetics of the reaction was monitored by measuring the decrease in absorbance at 400 nm. Molar extinction coefficient of 2′4,4′,6-tetrahydroxychalcone was ε400 = 33,113 M−1 cm−1. The CHI activity was expressed in pkat mg−1 proteins.
High performance liquid chromatography and electrospray ionization mass spectrometry (HPLC/ESI–MS) analysis of naphtodianthrones
HYP and PHYP extractions were performed as described by Gadzovska et al. (2005). A Shimadzu LC-6A liquid chromatograph equipped with a fluorescence detector Shimadzu RF-535 (λexc = 236 nm and λem = 592 nm) was used for end-point detection. HPLC analyses were carried out at 25 °C on a Hypersil reversed-phase C18 column (150 × 4.6 mm, 5 μm, Interchim, France). Mobile phase A was triethylammonium acetate buffer (0.01 M) at pH 7.0 and phase B was mixture of methanol and acetonitrile (5:4, v/v). The analyses followed linear gradient program with a flow rate of 1.5 mL min−1 with 20 μL injected volume. Linear gradient combinations were started with 60 % B (0–3 min), 92 % B (4–9 min), and 100 % B (10 min). Total run time was 10 min. Standard solutions of HYP (0–100 μg ml−1) were prepared from pure commercially available standard (Sigma, France). The PHYP was isolated from plant extracts and purified onto semi preparative Nucleosil C18 column (250 × 10 mm, 5 μm, Interchim, France). Standard solutions of PHYP were prepared in concentration range of 0–100 μg ml−1. Chromatograms were performed at 590 nm. All reagents were HPLC grade from Merck (Germany).
As previously described by Gadzovska et al. (2005), mass spectra of naphtodianthrones were acquired using a LCQ Deca mass spectrometer, equipped with an atmospheric pressure chemical ionization source (Thermo-Finnigan). The instrument was operated in the negative ion mode, scanning from m/z 150–600. Operating conditions were: sheath gas, 65 psi; auxiliary gas (nitrogen), 10 psi; ESI needle voltage, 4.5 kV; capillary temperature, 250 °C; capillary voltage, −12 V. For multiple MS (MS2) spectra of selected precursor ions, the activation energy was 53 % for HYP and 50 % for PHYP. Compounds were introduced to the fused silica-lined ESI needle by syringe pump at 5 µL min−1 flow rates. Data acquisition and processing were performed with Xcalibur software (version 1.2).
FTIR (Fourier Transform Infrared) spectroscopy
FTIR spectra were recorded between 4000 and 400 cm−1 at 4 cm−1 resolution on a Nicolet spectrophotometer using KBr discs containing 1 % of the dry samples and corrected for the KBr background. The window between 800 and 2000 cm−1 which show information of polysaccharides, lignin and protein was selected to compare cell wall modifications between control and elicited samples. For each sample, 16 spectra were averaged following baseline-correction and normalization at 1050 cm−1.
Statistical analyses
The experiments were independently repeated twice under the same conditions and all analyses were performed in triplicate. Error bars of graphs show the standard error of mean value (±SE). The statistical analyses were performed with the SPSS statistical software program (SPSS version 11.0.1 PC, USA, IL). Means were expressed with their standard error and compared by one-way ANOVA (GML procedure). All statistical tests were considered significant at p < 0.05.
Results
Biomass production of H. perforatum cell suspensions
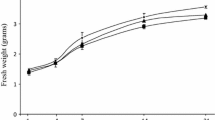
The influence of three fungal mycelia extracts (Fusarium, Phoma and Botrytis) on cell viability and fresh biomass production of H. perforatum cell suspensions was evaluated (Fig. 1a–c). Outgoing results showed that long term treatment (after day 14) with fungal elicitors caused browning and aggregation in elicited cells. Though all tested mycelia extracts caused the greenish cell culture into brown ones, the intensity of brown coloration slightly varied depending on the fungal species (Fig. 1a). All elicitors used had similar effects on H. perforatum cell viability tested by vital staining (Fig. 1b). Cell viability in all elicited cultures was decreased compared with the untreated controls. The effect of treatment with Botrytis on cell viability (84.17 ± 5.14 %) was minor compared with that of Fusarium (75.55 ± 4.02 %) and Phoma (77.83 ± 8.25 %). Fresh weight of elicited cells was always below or equal to control values, all along the culture duration (Fig. 1c). Biomass production of elicited cells was not significantly different compared to control till day 7 of post-elicitation. From day 14 to day 21, it was clear that fresh biomass production in elicited cells was notably lower for about 20–33 % compared to control cells. In addition, cells elicited with Fusarium and Phoma exhibited almost identical kinetics in cell growth inhibition.
Phenylpropanoid and naphtodianthrone productions of H. perforatum cell suspensions
The production of phenylpropanoids (TP, TF, TFL, TA) and naphtodianthrones (HYP and PHYP) in H. perforatum cell cultures were examined according to the type of elicitor and post-elicitation period. The effect of fungal mycelia extracts on TP accumulation in cell suspensions is indicated in Fig. 2a. Elicited cells produced significantly increased TP contents from day 4 to day 21 compared to control cells. The production of TP increased more rapidly upon Fusarium elicitation at day 4 (3.5-fold) and remained stable for a longer period of elicitation. Cells elicited with Phoma and Botrytis showed a markedly higher accumulation of TP at day 7 and 14 (from 2.5 to threefold, respectively) compared to corresponding controls. The TP contents in all treated cells stay elevated up to 2.5-fold at the end of post-elicitation (day 21). The production of TF in elicited cells was significantly increased from day 4 to day 21 compared to control (Fig. 2b). Fusarium extract remarkably enhanced TF production at day 7 (ninefold) compared to control cells. With regards to Phoma and Botrytis elicitation, notably increased TF amounts (about fivefold) were observed at day 7 and 14, respectively, compared to corresponding controls. Elicited cell suspensions exhibited similar response in enhancement of TF content (about 2.5-fold) at day 21. The TFL content in elicited cells was not largely changed during the first 7 days of treatment and began to increase from day 14 of post-elicitation (Fig. 2c). Elicited cell suspensions showed significantly higher TFL contents at day 14 and 21 (from 6.5 to eightfold, respectively) compared to control. All applied elicitors gave a similar trend in TFL production over the same culture period. The amount of TA in elicited cells was linearly increased from the beginning until the end of the culture period (Fig. 2d). In all the corresponding treated samples, an increase of TA contents was observed from day 1 of post-elicitation (up to 3.5-fold), reaching a maximum at day 7 (about fivefold) and remained stable up to day 21. Outgoing results showed that fungal mycelia caused elicitation of phenylpropanoid production (TP, TF and TA) during the entire cultivation period, while TFL amounts were enhanced at the end of post-elicitation. Among the tested fungal elicitors, Fusarium possesses greater stimulating action on phenylpropanoid production, while Phoma and Botrytis showed a weaker elicitor activity.
Production of phenylpropanoids and naphtodianthrones in Hypericum perforatum cell suspensions. a Total phenolics (TP). b Total flavonoids (TF). c Total flavanols (TFL). d Total anthocyanins (TA). e Hypericin (HYP). f Pseudohypericin (PHYP). g Phenylalanine ammonia lyase activity (PAL). h Chalcone isomerase activity (CHI). GAE gallic acid equivalents, CE catechin equivalents, CyGE cyanidin-3-glucoside equivalents, DM dry mass. Asterisk denoted values indicate significant differences between control and elicited cells (p < 0.05)
H. perforatum cell suspensions were found to respond strongly and rapidly towards the applied fungal elicitors through the enhanced synthesis of both naphtodianthrones, HYP (Fig. 2e) and PHYP (Fig. 2f). The HYP and PHYP production in elicited cells was significantly increased (from three to fourfold) compared to corresponding controls all along the post-elicitation period. Present results indicate that fungal elicitors showed a similar stimulating activity on naphtodianthrone production in cell cultures. In addition, the production of both naphtodianthrones (HYP and PHYP) was positively correlated with all estimated phenylpropanoids (Table 1).
The activities of two key enzymes of the phenylpropanoid/flavonoid pathways, PAL and CHI were monitored to estimate general channelling in the different metabolic pathways in H. perforatum cells upon fungal elicitation. The activities of PAL (Fig. 2g) and CHI (Fig. 2h) were strongly induced in all elicited cells but at distinct levels depending on type of elicitor and culture period. In cell suspensions, PAL activity was enhanced upon treatment with fungal elicitors, particularly in those elicited with Fusarium mycelia extracts. The PAL activity in Fusarium elicited cells peaked at day 1, the value of which was about 24-fold that of the control. Afterwards, PAL activity was gradually decreased but remained significantly higher till the end of the experiment (about threefold) compared to control. Mycelia extracts of Phoma and Botrytis markedly induced PAL activity in cells at day 14 (from 15- to 17.5-fold, respectively) compared to corresponding controls. With regards to CHI enzyme, enhanced activity for Fusarium elicited cells was observed during the entire post-elicitation period. Phoma and Botrytis stimulated the CHI activity in cell suspensions from day 4 to day 21 of culture. It is worth noting that all tested fungal elicitors markedly stimulated CHI activity (about 26-fold) at the end of the experiment in comparison to control samples. In addition, PAL and CHI activities in elicited cells showed remarkably positive correlations with phenylpropanoid and naphtodianthrone productions (Table 1). Simultaneous induction of PAL and CHI activities in H. perforatum elicited cells as observed in our study indicated that fungal mycelia extracts could be proposed as efficient elicitors of phenylpropanoid and naphtodianthrone biosynthesis.
Antioxidant activity of H. perforatum cell suspensions
The experiments have been undertaken to analyze the influence of fungal mycelia extracts on NEAOP and enzymatic activity of POD and CAT in cell suspensions. The NEAOP in elicited cells was not largely changed during the first 7 days and began to increase after day 14 of post-elicitation period (Fig. 3a). Elicited cells showed from 2.5- to fourfold increase in NEAOP at day 14 of post-elicitation compared to control. It is worth noting that Fusarium was shown as superior elicitor of NEAOP in treated cells than other fungal elicitors (Phoma and Botrytis). The activities of antioxidant enzymes POD and CAT in H. perforatum cell suspensions are shown in Fig. 3b, c, respectively. A strong and rapid increase in POD activity of elicited cells until day 7 of post-elicitation was noticed. At the beginning of the experiment, similar pattern in elevation of POD activity was observed in cells elicited with Fusarium and Phoma (about twofold), while Botrytis treated cells showed 1.6-fold higher enzyme activity compared to control cells. Among the tested fungi, Phoma exhibited higher stimulatory effect on POD activity compared to other elicitor used, particularly at day 4 and 7. The CAT activity in elicited cells was not significantly changed during the first 7 days of post-elicitation and began to increase thereafter (up to twofold) compared to control cells.
Statistical analysis demonstrated that NEAOP was in significant positive correlation with phenylpropanoid and naphtodianthrone productions. On the other hand, NEAOP was negatively correlated with the activities of antioxidant enzymes POD and CAT, while both antioxidant enzymes were in significant positive correlation (Table 1). Taken together, these results showed that fungal elicitors mediated certain type of defense responses in H. perforatum cultures evidenced by substantial modification of the cell redox system.
Cell wall modifications of H. perforatum cell suspensions
In order to extend the characterisation of elicitor-induced cell wall modifications, we analysed the cell suspensions by FTIR spectroscopy. Overall, the fingerprint profile of FTIR spectra from the alkali-treated walls of untreated H. perforatum cell suspensions and the corresponding elicited samples showed little variations (Fig. 4a). The spectra showed the pre-eminence of bands in the 1200–1000 wavelength regions which are characteristic for polysaccharides (as the bands centered at 1150 and 1100 cm−1 for C–O–C vibration and C–C stretching in cellulose or pectins). Other peaks can be assigned to C–H deformation in polysaccharides (1425 cm−1) and non-conjugated C=O groups (1750 cm−1), (Pandey and Pitman 2003; Alonso-Simon et al. 2004). The most important band at 1650 cm−1 can be assigned to conjugated aldehyde or ketone and/or the carbonyl groups of amide II (proteins). However, the accompanying amide N–H stretch at 1540 cm−1 suggested that the broad absorbance at 1650−1 cm was mostly indicative of proteins (Stewart et al. 1994). Additionally, the lack of clear-cut peak or shoulder at 1600 and 1510 cm−1 suggested that lignin was a minor component of the alkali-treated cell walls. Nevertheless, difference spectra between elicited and control samples (Fig. 4b) showed distinct profiles for each treatment. They revealed that the most pronounced variations were related to the protein bands which were slightly more intensive in the alkali-treated walls of elicited cells. In the case of cell walls isolated from Fusarium elicited samples, higher signals at 1650 and 1540 cm−1 were clearly distinguished. In addition, difference spectra (Fig. 4b) highlighted a significant peak at 1450 cm−1 and a broad one at 1220–1270 cm−1 which have been respectively reported as indicative of lignin aromatic ring and guaiacyl ring (Faix 1991). Therefore, even if lignin appeared as a minor component of fungal elicited cell-walls, the contribution of this polymer might require further investigations to confirm data from FTIR analysis.
Discussion
Biomass production of H. perforatum cell suspensions
It has been reported that the effect of elicitors on cell growth is dependent on the type of elicitor, concentrations used and the cell growth stage when the elicitor is applied (Namdeo 2007). Present results showed that exogenously applied fungal mycelia extracts negatively affected cell viability and fresh biomass production of H. perforatum cell cultures. Such a result has already been reported when H. perforatum cell suspensions have been elicited with JA, SA and fungal mycelia from A. flavus (Gadzovska et al. 2007, 2013; Gadzovska-Simic et al. 2012). On the other side, JA significantly stimulated biomass production of H. perforatum cells (Walker et al. 2002), while treatments with SA, P. cinnamoni and Agrobacterium failed to show any stimulatory effect on cell growth (Walker et al. 2002; Tusevski et al. 2014). These findings indicated that the response of H. perforatum cells to a particular elicitor may vary between different cell lines and culture conditions. The suppression of cell growth and viability upon elicitation has been suggested to be caused by the diversion of metabolic flux, i.e. the activation of secondary metabolism over primary metabolism (Sivakumar and Paek 2005). Even that growth of elicited cells is often negatively correlated with secondary metabolite production, results presented here clearly demonstrated that fungal mycelia extracts could be used as effective elicitors for obtaining valuable bioactive compounds from H. perforatum cells.
Phenylpropanoid and naphtodianthrone productions of H. perforatum cell suspensions
Fungal mycelia extracts from Fusarium, Phoma and Botrytis induced a strong accumulation of phenylpropanoids and naphtodianthrones in H. perforatum elicited cells. Fusarium was shown as superior elicitor in stimulation of TP and TF production, while all tested fungal elicitors gave a similar response with respect to TFL and TA accumulation. Phenylpropanoid production in H. perforatum cell suspensions has also been stimulated using JA, SA, A. flavus and Agrobacterium as elicitors (Gadzovska et al. 2007, 2013; Gadzovska-Simic et al. 2012; Tusevski et al. 2014). The elevation of TP and flavonoid compounds in H. polyanthemum acclimatized plants upon elicitation with fungus Nomuraea rileyi suggested that these bioactive compounds are inducible part of plant defense response (Meirelles et al. 2013). Therefore, the increase in phenylpropanoid production could be due to the activation of defense pathway of plant cells by extracellular products released from the fungal mycelia. The specific and diverse effects of fungal elicitors, as observed in this study, are most likely to be implicated with unique modes of recognition upon interactions with fungi and the complexity of elicitor signal transduction, resulting in plant defense response (Berrocal-Lobo and Molina 2008).
Though phenylpropanoid production has been investigated in H. perforatum elicited cells, there is still an increased demand for further research on naphtodianthrones as major pharmacologically active compounds. Present results suggested that fungal elicitors stimulated HYP and PHYP production during the entire post-elicitation period. The production of HYPs in H. perforatum cultures has also been stimulated upon treatments with fungal elicitors such as P. cinnamoni, C. gloeosporioides and A. flavus (Walker et al. 2002; Sirvent and Gibson 2002; Gadzovska-Simic et al. 2012). The levels of HYPs presented in H. perforatum plant tissues as a response to C. gloeosporioides invasion have been directly toxic to the fungus and caused inhibition of mycelia growth in vitro (Sirvent and Gibson 2002). Also, fungal spores from P. capsici and Diploceras hypericinum were found to be able to elicit an increase in HYP biosynthesis in greenhouse-grown H. perforatum and H. pruinatum (Cirak et al. 2005). Therefore, it could be assumed that fungal elicitors mimic stress conditions and the enhancement in HYP and PHYP levels appear to have a potential role in defense strategy of Hypericum cells.
The present study showed that fungal elicitors stimulated PAL and CHI activities, but Fusarium mycelia extract was the most potent for the enzyme activation. This rapid PAL activation could be associated with the presence of various Fusarium cell wall active compounds such as proteins, glycoproteins, lipids and free oligosaccharides that trigger early plant defense reactions (de Ascensao and Dubery 2003). These findings are supported by the increment of PAL activity in different Fusarium elicited cell cultures (Yuan et al. 2002; Nita-Lazar et al. 2004). Even that PAL is the key regulatory enzyme leading to the formation of a wide range of phenylpropanoid metabolites (Dixon et al. 2002), the activity of CHI is essential for the biosynthesis of flavonoids (Ververidis et al. 2007). The increase in CHI activity associated with flavonoid accumulation in cucumber plants upon elicitation with fungal pathogen Sphaeroteca fuliginea highlighted the implication of these phenolic compounds in plant defense system (Fofana et al. 2002). Moreover, the coordinated induction of PAL and CHI has already been reported in our previous studies for H. perforatum cells, calli and shoots elicited with JA and SA (Gadzovska et al. 2007, 2013). It was demonstrated about twofold increase PAL and CHI activities in shoots and calli, while enzyme activities in cells were remarkably higher (from 50 to 60-fold) upon treatments with SA. For these data, the most efficiency could only result either, in a better exposition of cells to exogenously applied elicitors into the liquid medium, or in possible difficulties in penetration of elicitor components into shoots cultivated on solid medium. Therefore, the facilitated penetration of fungal elicitors in cells in suspension could efficiently trigger PAL and CHI activities. If such a consequence existed in fungal elicited cells, it could be possible that the changes in phenolic levels would consist in a response to the enzymatic modifications.
The PAL as the entrypoint enzyme into phenylpropanoid metabolism regulates the production of the monomers necessary for lignin biosynthesis and other phenolic compounds in plant cells. Induced lignification is one of the several plant responses to microbial attack or elicitation (Lattanzio et al. 2006). Defense lignin is known to create a physical barrier thereby limiting pathogen invasion in the plant tissue and this polymer being highly resistant to enzymatic degradation by microorganisms. Since it is generally difficult to quantify lignin in weakly-lignified cells, we decided to investigate any potential modification in lignin accumulation by using FTIR analysis. The analysis of cell wall modifications by FTIR showed that necrotrophic pathogen Botrytis or biotrophic pathogens Fusarium and Phoma gave similar responses in cell suspensions. Present results demonstrated that fungal mycelia extracts did not notably affect lignin accumulation in elicited cell walls. In contrast, Fusarium treated flax cells showed that lignin accumulation in cell walls correlated with an increase in PAL activity (Hano et al. 2006). Similarly, Zhao et al. (2005) reported that fungal elicitors stimulated the phenylpropanoid pathway leading to accumulation of lignin which is required for cell wall reinforcement against pathogen invasion. The discrepancies with our results could possibly be explained by an activation of alternative naphtodianthrone pathway rather than lignin biosynthesis through phenylpropanoid pathway which is associated with induced PAL activity in H. perforatum fungal elicited cells.
Antioxidant activity of H. perforatum cell suspensions
In fungal-host interaction, plant cells produced a certain amount of ROS via an oxidative burst that may affect not only the invading pathogen, but also the host cells themselves, unless the host cells have a high antioxidant capacity (Nowogórska and Patykowski 2015). The cooperative functioning of enzymatic and non-enzymatic antioxidants plays an important role in ROS scavenging and maintaining the physiological redox status of plant cells (Gill and Tuteja 2010). When the antioxidant activity of H. perforatum cell suspensions was evaluated in response to fungal elicitation, the results consistently indicated an early up-regulation of POD activity, while NEAOP and CAT activity were enhanced at the end of post-elicitation. Comparison of antioxidant enzyme activities showed that the lower response of CAT activity to fungal treatments in the early phase of post-elicitation maybe compensated by the increased POD activity. These results suggested that CAT activity may be essential for the protection of fungal elicited cells during the long-term treatment. These enzymes acting concurrently to remove H2O2 in elicited cells and POD rather than CAT participated in the early protection of cells against fungal-mediated ROS production. These results are in good agreement with previous studies reporting an increase in plant antioxidant enzymes under fungal attack (Polkowska-Kowalczyk et al. 2007; Nowogórska and Patykowski 2015).
A significant correlation between enhanced NEAOP and phenylpropanoid/flavonoid accumulation in fungal elicited cells was found, indicating the potential role of phenolic compounds as antioxidants. The naphtodianthrone (HYP and PHYP) productions and NEAOP also correlated significantly, but to a lower extent compared with phenylpropanoid production. Even if antioxidant activities from H. perforatum extracts are usually derived from polyphenolic compounds (Silva et al. 2005), these effects do not always correlate with the presence of naphtodianthrones. It was shown that HYP which has six hydroxyl groups demonstrate lower antioxidant activity, probably due to its hydrophobicity (Conforti et al. 2002). The contribution of naphtodianthrones to NEAOP was expected because β-carotene bleaching assay used in this study is suitable for estimation of hydrophobic antioxidants (Kulisic et al. 2004). Therefore, the accumulation of phenolic compounds within the elicited cells indicates an attempt to maintain the cellular redox homeostasis in order to minimize the consequences of biotic stress. This study clearly demonstrated that H. perforatum defense system was triggered by the phenylpropanoid/naphtodianthrone-mediated elevation of NEAOP in fungal elicited cells.
Effects of fungal elicitors on the secondary metabolism channelling
Phenylpropanoid biosynthetic pathways are among the most frequently observed metabolic activities that are induced upon infection of plants with pathogens or treatment of plant tissue or cultured cells with different elicitors. The strong and rapidly stimulating effect of fungal elicitors on plant secondary metabolite accumulation attracts considerable attentions and research efforts (de Ascensao and Dubery 2003; Li et al. 2014). In the present study, an increase in phenylpropanoid and naphtodianthrone productions with simultaneous elevation of PAL and CHI activities indicated that fungal elicitation is an effective strategy for enhancement of various groups of phenolic compounds. Concerning naphtodianthrone biosynthesis, it proceeds via a polyketide pathway in which a polyketide synthases (HpKS1 and HpKS2) intervenes (Karppinen and Hohtola 2008). However, the final stages of HYP biosynthesis have been suggested to be conducted by a phenolic coupling protein HYP-1 (Bais et al. 2003). In our previous study, we found that SA did not influence the Hyp-1 mRNA accumulation in H. perforatum cells (Gadzovska et al. 2013). As basic structural unit of HYP is emodin anthrone, the search for possible bysinthetic routes for antraquinone synthesis revealed the existence of an alternative pathway, through chorismate/o-succinylbenzoic acid pathway (Pillai and Nair 2014). Therefore, we can consider that stimulated PAL activity upon fungal elicitation might trigger the defense responses of H. perforatum cell suspensions through activation of synthetic pathway of naphtodianthrones.
Many investigations have been conducted to establish and to enhance HYP and PHYP production using different H. perforatum in vitro culture systems (Kirakosyan et al. 2000; Walker et al. 2002; Sirvent and Gibson 2002; Tusevski et al. 2014). Results from our previous studies (Gadzovska et al. 2005, 2007, 2013) showed that naphtodianthrone production (HYP and PHYP) of regenerated plantlets reached 150–500 μg g−1 DW, which was higher than that of in vitro shoots (200–400 μg g−1 DW), calli (135–200 μg g−1 DW) and cell suspension cultures (20–50 μg g−1 DW).The HYP and PHYP accumulation in plantlets and shoots was related to leaf morphogenesis and apparition of dark oil glands, while calli and cells cultivated on hormonal supplemented medium were able to acquire certain degree of differentiation and capability for naphtodianthrone production (Gadzovska et al. 2005, 2013). Present results showed that all three types of fungal elicitors had more universal effects on stimulated production of naphtodianthrones (HYP and PHYP) in H. perforatum cell suspensions, as previously reported for elicitation with JA and SA (Gadzovska et al. 2007, 2013). On the other side, Walker et al. (2002) demonstrated that JA enhanced HYP production in H. perforatum cell cultures, while SA and fungal elicitor P. cinnamoni failed to show any stimulatory effects. These results indicate that plant cell response to a particular elicitor might vary between different cell lines and culture conditions. It has been well documented that the elicitor-induced secondary metabolite production is mediated by endogenous signalling pathways involving signal molecules such as nitric oxid, JA, SA and H2O2. In this view, fungal elicitors induced HYP production in H. perforatum cells through a JA dependent signalling pathway (Xu et al. 2005) or NADPH oxidase-mediated H2O2 signalling pathway (Xu et al. 2011). It is still unclear whether elicitors acted on the same or distinct signalling pathways and further research is needed to better understand the effects of various elicitors on secondary metabolites partitioning in H. perforatum cell suspensions. The system described here represents a promising approach for studying the biosynthetic pathways of plant secondary metabolites within a short cultivation time. Thus, we proposed that secondary metabolite production in H. perforatum cell suspensions can be partially modified by fungal elicitation and well controlled cultures could be used as a source for rapid and increased production of naphtodianthrones as major pharmacologically active compounds.
Conclusions
In summary, the present work showed that fungal mycelia extracts from F. oxysporum ssp lini, Phoma exigua and Botrytis cinerea are efficient elicitors for enhancement of secondary metabolite production in H. perforatum cell suspensions. A strong accumulation of phenylpropanoids and flavonoids was related to markedly higher activities of PAL and CHI in fungal elicited cells. Moreover, H. perforatum elicited cells synthesized and stored significant quantities of naphtodianthrones (HYP and PHYP) indicating that these compounds represent a main part of defense strategy triggered by fungal elicitors. Antioxidant activity in fungal elicited cells remains significantly elevated throughout the post-elicitation period, suggesting a modification in the accumulation of phenolic compounds that exhibit high antioxidant properties. The investigation of inter-relationship between phenylpropanoid and naphtodianthrone productions with antioxidant activity will be a promising field to understand and elucidate possible mechanisms for utilization of Hypericum cells as sources of bioactive compounds in food and pharmaceutical industry. Regarding the commercialization of plant cell culture processes, different strategies to increase biosynthesis of secondary metabolites and to alter the product spectrum have to be investigated. We believe that only continuation and increase of efforts in this field will lead to controllable and successful biotechnological production of valuable phenylpropanoids and naphtodianthrones, and as yet unknown plant bioactive compounds.
Abbreviations
- CAT:
-
Catalase
- CHI:
-
Chalcone isomerase
- FTIR:
-
Fourier transform infrared spectroscopy
- HYP:
-
Hypericin
- JA:
-
Jasmonic acid
- NEAOP:
-
Non-enzymatic antioxidant properties
- PAL:
-
Phenylalanine ammonia lyase
- PHYP:
-
Pseudohypericin
- POD:
-
Peroxidase
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
- TA:
-
Total anthocyanins
- TFL:
-
Total flavanols
- TF:
-
Total flavonoids
- TP:
-
Total phenolics
References
Alonso-Simon A, Garcia-Angulo A, Acebes JL (2004) FTIR spectroscopy monitoring of cell wall modifications during the habituation of bean (Phaseolus vulgaris L.) callus cultures to dichlobenil. Plant Sci 167:1273–1281
Bais HP, Vepachedu R, Lawrence CB, Stermitz FR, Vivanco JM (2003) Molecular and biochemical characterization of an enzyme responsible for the formation of hypericin in St. John’s wort (Hypericum perforatum L.). J Biol Chem 34:32413–32422
Berrocal-Lobo M, Molina A (2008) Arabidopsis defense response against Fusarium oxysporum. Trends Plant Sci 13:145–150
Boerema GH, de Gruyter J, Noordeloos ME, Hamers MEC (2004) Phoma identification manual. Differentiation of specific and intra-specific taxa in culture. CABI Publishing, Wallingford
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cirak C, Aksoy HM, Ayan AK, Saglam B, Kevseroglu K (2005) Enhanced hypericin production in Hypericum perforatum and Hypericum pruinatum in response to inoculation with two fungal pathogens. Plant Prot Sci 4:109–114
Conforti F, Statti GA, Tundis R, Menichini F, Houghton P (2002) Antioxidant activity of methanolic extract of Hypericum triquetrifolium Turra aerial part. Fitoterapia 6:479–483
de Ascensao AR, Dubery IA (2003) Soluble and wall-bound phenolics and phenolic polymers in Musa acuminata roots exposed to elicitors from Fusarium oxysporum f.sp. cubense. Phytochemistry 63:679–686
Dixon RA, Achnine L, Kota P, Liu CJ, Reddy MKS, Wang L (2002) The phenylpropanoid pathway and plant defence-a genomics perspective. Mol Plant Pathol 3:371–390
Faix O (1991) Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 45:21–27
Fofana B, McNally DJ, Labbé C, Boulanger R, Benhamou N, Séguin A, Bélanger RR (2002) Milsana-induced resistance in powdery mildew-infected cucumber plants correlates with the induction of chalcone synthase and chalcone isomerase. PMPP 61:121–132
Fu J, Huang B (2001) Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ Exp Bot 45:105–114
Gadzovska Simic S, Tusevski O, Maury S, Delaunay A, Joseph C, Hagège D (2014) Effects of polysaccharide elicitors on secondary metabolite production and antioxidant response in Hypericum perforatum L. shoot cultures. Sci World J. doi:10.1155/2014/609649
Gadzovska S, Maury S, Ounnar S, Righezza M, Kascakova S, Refregiers M, Spasenoski M, Joseph C, Hagège D (2005) Identification and quantification of hypericin and pseudohypericin in different Hypericum perforatum L. in vitro cultures. Plant Physiol Biochem 43:591–601
Gadzovska S, Maury S, Delaunay A, Spasenoski M, Joseph C, Hagege D (2007) Jasmonic acid elicitation of Hypericum perforatum L. cell suspensions and effects on the production of phenylpropanoids and naphtodianthrones. Plant Cell Tiss Org 89:1–13
Gadzovska S, Maury S, Delaunay A, Spasenoski M, Hagège D, Courtois D, Joseph C (2013) The influence of salicylic acid elicitation of shoots, callus, and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell Tiss Org 113:25–39
Gadzovska-Simic S, Tusevski O, Antevski S, Atanasova-Pancevska N, Petreska J, Stefova M, Kungulovski D, Spasenoski M (2012) Secondary metabolite production in Hypericum perforatum L. cell suspensions upon elicitation with fungal mycelia from Aspergillus flavus. Arch Biol Sci 64:113–121
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures soybean root cells. Exp Cell Res 50:148–151
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Giusti MM, Rodriguez-Saona LE, Wrolstad RE (1999) Spectral characteristics, molar absorptivity and color of pelargonidin derivates. J Agric Food Chem 47:4631–4637
Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43:205–227
Gonzales LF, Rojas CM, Perez JF (1999) Diferulate and lignin formation is related to biochemical differences of wall-bound peroxidases. Phytochemistry 50:711–717
Hano C, Addi M, Bensaddek L, Cronier D, Baltora-Rosset S, Doussot J, Maury S, Mesnard F, Chabbert B, Hawkins S, Lainé E, Lamblin F (2006) Differential accumulation of monolignol-derived compounds in elicited flax (Linum usitatissimum) cell suspension cultures. Planta 223:975–989
Karppinen K, Hohtola A (2008) Molecular cloning and tissue-specific expression of two cDNAs encoding polyketide synthases from Hypericum perforatum. J Plant Physiol 165:1079–1086
Karuppusamy S (2009) A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plants Res 3:1222–1239
Kirakosyan A, Hayashi H, Inoue K, Charchoglyan A, Vardapetyan H (2000) Stimulation of the production of hypericins by mannan in Hypericum perforatum shoot cultures. Phytochemistry 53:345–348
Kirakosyan A, Sirvent TM, Gibson DM, Kaufman PB (2004) The production of hypericins and hyperforin by in vitro cultures of St. John’s wort (Hypericum perforatum). Biotechnol Appl Biochem 39:71–81
Košuth J, Koperdáková J, Tolonen A, Hohtola A, Cellárová E (2003) The content of hypericins and phloroglucinols in Hypericum perforatum L. seedlings at early stage of development. Plant Sci 165:515–521
Kulisic T, Radonic A, Katalinic V, Milos M (2004) Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem 85:633–640
Laroche C, Gervais P (2003) Achievement of rapid osmotic dehydration at specific temperatures could maintain high Saccharomyces cerevisiae viability. Appl Microbiol Biotechnol 60:743–747
Lattanzio V, Lattanzio VM, Cardinali A (2006) Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem Adv Res 661:23–67
Leonard KJ, Bushnell WR (2004) Fusarium Head Blight of Wheat and Barley. APS Press, USA
Li YG, Tanner G, Larkin P (1996) The DMACA-HCl protocol and the threshold proanthocyanidin content for bloat safety in forage legumes. J Sci Food Agr 70:89–101
Li P, Luo H, Meng J et al (2014) Effects of oligosaccharides from endophytic Fusarium oxysporum Dzf17 on activities of defense-related enzymes in Dioscorea zingiberensis suspension cell and seedling cultures. Electron J Biotechn 17:156–161
Makris DP, Boskou G, Andrikopoulos NK (2007) Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J Food Comp Anal 20:125–132
Marron N, Delay D, Petit JM, Dreyer E, Kahlem G, Delmotte FM, Brignolas F (2002) Physiological traits of two Populus × euramericana clones, Luisa Avanzo and Dorskamp, during a water stress and re-watering cycle. Tree Physiol 22:49–858
Meirelles G, Pinhatti AV, Sosa-Gomez D, Rosa LMG, Rech SB, von Poser GL (2013) Influence of fungal elicitation with Nomuraea rileyi (Farlow) Samson in the metabolism of acclimatized plants of Hypericum polyanthemum Klotzsech ex Reichardt (Guttiferae). Plant Cell Tiss Org 112:379–385
Morel G, Wetmore RH (1951) Tissue culture of monocotyledons. Am J Bot 38:138–140
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murch SJ, Saxena PK (2006) St. John’s wort (Hypericum perforatum L.): challenges and strategies for production of chemically consistent plants. Can J Plant Sci 86:765–771
Murthy HN, Lee EJ, Paek KY (2014) Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tiss Org 118:1–16
Nahrstedt A, Butterweck V (2010) Lessons learned from herbal medicinal products: the example of St. John’s wort. J Nat Prod 73:1015–1021
Namdeo AG (2007) Plant cell elicitation for production of secondary metabolites: a review. Pharmacogn Rev 1:69–79
Nita-Lazar M, Heyraud A, Gey C, Braccini I, Lienart Y (2004) Novel oligosaccharides isolated from Fusarium oxysporum L. rapidly induce PAL activity in Rubus cells. Acta Biochim Pol 51:625–634
Nowogórska A, Patykowski J (2015) Selected reactive oxygen species and antioxidant enzymes in common bean after Pseudomonas syringae pv. phaseolicola and Botrytis cinerea infection. Acta Physiol Plant 37:1–10
Pandey KK, Pitman AJ (2003) FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Inter Biodeter Biodegr 52:151–160
Pillai PP, Nair AR (2014) Hypericin biosynthesis in Hypericum hookerianum Wight and Arn: Investigation on biochemical pathways using metabolite inhibitors and suppression subtractive hybridization. Comptes Rendus Biol 337:571–580
Polkowska-Kowalczyk L, Wielgat B, Maciejewska U (2007) Changes in the antioxidant status in leaves of Solanum species in response to elicitor from Phytophthora infestans. J Plant Physiol 164:1268–1277
Silva BA, Ferreres F, Malva JO, Dias ACP (2005) Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts. Food Chem 90:157–167
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Sirvent T, Gibson D (2002) Induction of hypericins and hyperforin in Hypericum perforatum L. in response to biotic and chemical elicitors. Physiol Mol Plant Pathol 60:311–320
Sivakumar G, Paek KY (2005) Methyl jasmonate induce enhanced production of soluble biophenols in Panax ginseng adventitious roots from commercial scale bioreactors. Chem Nat Compd 41:669–673
Solomon D, Adams J, Graves N (2013) Economic evaluation of St. John’s wort (Hypericum perforatum) for the treatment of mild to moderate depression. J Affect Disord 148:228–234
Stewart D, Lyon GD, Tucker EJB (1994) A Fourier-transform infrared spectro6scopic and microscopic study of the infection of potato tubers by Erwinia carotovora ssp. carotovora in aerobic and anaerobic conditions. J Sci Food Agric 66:145–154
Tusevski O, Petreska Stanoeva J, Stefova M, Gadzovska Simic S (2014) Agrobacterium enhances xanthone production in Hypericum perforatum cell suspensions. Plant Growth Regul. doi:10.1007/s10725-014-9989-6
Ververidis F, Trantas E, Douglas C, Vollmer G, Kretzschmar G, Panopoulos N (2007) Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: chemical diversity, impacts on plant biology and human health. Biotechnol J 2:1214–1234
Walker ST, Pal Bais H, Vivanco MJ (2002) Jasmonic acid-induced hypericin production in cell suspension cultures of Hypericum perforatum L (St. John’s wort). Phytochemistry 60:289–293
Xu MJ, Dong JF, Zhu MY (2005) Nitric oxide mediates the fungal elicitor-induced hypericin production of Hypericum perforatum cell suspension cultures through a jasmonic-acid-dependent signal pathway. Plant Physiol 139:991–998
Xu M, Sheng J, Wang H, Dong J (2011) Involvement of NADPH oxidase-mediated H2O2 signaling in PB90-induced hypericin accumulation in Hypericum perforatum cells. Plant Cell Tiss Org 105:47–53
Yuan YJ, Li JC, Hu ZD, Wu JC, Zeng AP (2002) Fungal elicitor-induced cell apoptosis in suspension cultures of Taxus chinensis var. mairei for taxol production. Proc Biochem 38:193–198
Yue W, Ming QL, Lin B, Rahman K, Zheng CJ, Han T, Qin LP (2014) Medicinal plant cell suspension cultures: pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit Rev Biotechnol 0:1–18
Zhao J, Davis L, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333
Acknowledgments
This work and a Ph.D. grant (Sonja Gadzovska) was supported by the Ministère des Affaires Etrangères (Programme COCOP: Réseau d’Enseignement régional Postgraduate en Biologie, Grant No. DSUR-NGE-4B1-505). We thank Dr S. Ounnnar, S. Pochon, G. Moreau for their technical help. We acknowledge Dr F. Brignolas for his help in statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gadzovska Simic, S., Tusevski, O., Maury, S. et al. Fungal elicitor-mediated enhancement in phenylpropanoid and naphtodianthrone contents of Hypericum perforatum L. cell cultures. Plant Cell Tiss Organ Cult 122, 213–226 (2015). https://doi.org/10.1007/s11240-015-0762-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0762-y