Abstract
Various methods have been tried to prevent cell mortality during dehydration, but the reasons why microorganisms die when submitted to dehydration and rehydration are not well understood. The aim of this study was to further investigate the reasons for yeast mortality during dehydration. Osmotic dehydration and rehydration of Saccharomyces cerevisiae W303-1A were performed at different temperatures. Two different approaches were used: isothermic treatments (dehydration and rehydration at the same temperature), and cyclic treatments (dehydration at an experimental temperature and rehydration at 25°C), with significant differences in viability found between the different treatments. Dehydration at lower and higher temperatures gave higher viability results. These experiments allowed us to propose a hypothesis that relates mortality to a high water flow through an unstable membrane during phase transition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dehydration of Saccharomyces cerevisiae has been widely studied for many years, because of the use of this yeast in industry. Many researchers have tried to find ways to prevent cell mortality during dehydration, but mechanisms involved in this type of cell death are still not well understood. Experiments have shown that cell viability depends strongly on the osmotic pressure of the medium (Scott 1957; Esener et al. 1981), and that the kinetics of dehydration and rehydration are very important in prevention of cell death (Gervais et al. 1992; Gervais and Marechal 1994). Indeed, an optimum viability for a particular kinetic of progressive hyperosmotic modification on S. cerevisiae (Marechal and Gervais 1994) and lactic bacteria (Poirier et al. 1997) has been determined.
The increase in the osmotic pressure of the medium may have damaging effects on membranes, leading to structural modifications. Many authors have studied these changes, mostly on model membranes (Webb et al. 1993; Chapman 1994). At physiological temperature and hydration, the phospholipid bilayer of biological membranes is in a fluid lamellar liquid-crystalline phase. During dehydration, water molecules are removed, and phospholipid headgroups are forced close together, leading to an increase in van der Waals interactions between the fatty acyl chains, and the membrane is forced into a gel phase (Crowe et al. 1984a). During rehydration, the membrane must go through another phase transition, which returns it to the liquid-crystalline state. The phase transition temperature (T m) of phospholipids varies with the water activity of the surrounding medium and with membrane composition (fatty acid chain length, level of unsaturated fatty acids, and level of sterols). Biological membranes, which contain a heterogeneous mixture of lipids, could present domains where gel phase and liquid-crystalline phase lipid coexist, and could cause packing defects at boundaries between liquid-crystalline and gel phase domains (Cameron and Dluhy 1987). Membranes in such a state would not provide adequate barrier properties, as shown by Hammoudah et al. (1981), who observed significant changes in membrane permeability around the phase transition temperature. In these conditions, leakage of cytoplasmic content could occur, resulting in cell death (Crowe et al. 1989a, 1989b; Leslie et al. 1994, 1995). To prevent leakage, dehydration must be effected in the presence of sugars such as trehalose or saccharose (Eleutherio et al. 1993; Leslie et al. 1995; Crowe et al. 1996), or amino acids (Kets and De Bont 1994). As the water is removed, sugar molecules are thought to insert into the membrane and interact directly with the lipid headgroups by hydrogen bonding (Crowe et al. 1984b). Some studies have also shown that the temperature of the medium must be controlled to preserve cell viability (Walton and Pringle 1980; Hottiger et al. 1987; Suutari et al. 1990) and that rehydration temperature is also important, as shown for S. cerevisiae by Becker and Rapoport (1987). Viability seems to be preserved if rehydration is carried out at a temperature higher than the phase transition temperature, as proposed by Leslie et al. (1994) for S. cerevisiae, and Leslie et al. (1995) for Escherichia coli and Bacillus thuringiensis.
Experiments carried out previously in our laboratory (Gervais et al. 1992; Marechal and Gervais 1994; Poirier et al. 1997, 1999, Gervais and Beney 2001) have allowed us to propose a hypothesis for understanding yeast cell viability after osmotic shock. Membrane permeabilization seems to be the most important element of cell death or survival during dehydration or rehydration. Moreover, Beney et al. (2001a) have shown that viability of the cells after osmotic shock is higher if experiments are carried out at low temperature, and that the high water flow rate cannot by itself explain the cell mortality following an osmotic shock (Beney et al. 2001b). It is well known that the phase transition of lipids is a function of the temperature and osmotic pressure of the medium. If we accept that cell mortality is related to membrane phase transition, then the combined effect of these two parameters is of great interest. The membrane phase transition temperature of S. cerevisiae W303-1A, as a function of the osmotic pressure, has already been studied by fluorescence polarization measurements (Laroche et al. 2001) at a wide range of temperatures (6–35°C) and at seven levels of osmotic pressure between 1.38 and 133.12 MPa. The aim of this study was to estimate yeast viability after combined thermal and osmotic treatments, and to verify, if possible, the relationship between the phase transition and the mortality observed.
Materials and methods
Yeast strain
S. cerevisiae W303-1A (MATa leu2-3,112 his3-11,15 trp1-1 can1-100 ade2-1 ura3-1) (Thomas and Rothstein 1989) was a gift from Laboratorium voor Moleculaire Celbiologie, Katholieke Universiteit Leuven.
Cultivation conditions
Cells were maintained on Petri dishes with a Malt Wickerman medium supplemented with 20 g l-1 agar (VWR International, Fontenay-sous-bois, France). S. cerevisiae W303-1A was grown aerobically as previously described (Gervais and Martínez de Marañon 1995) in 250-ml conical flasks containing 100 ml modified Malt Wickerman medium, composed of 10 g glucose (VWR International), 3 g pancreatic peptone (VWR International), 3 g yeast extract (Institut Pasteur, Paris, France), and 1.5 g NaH2PO4 (VWR International), in 1 l of a binary water/glycerol solution corresponding to an osmotic pressure of 1.38 MPa (51.09 g glycerol per 1 l water). This osmotic pressure corresponds to the optimal growth conditions for S. cerevisiae (Anand and Brown 1968). The pH was adjusted to 5.35 by addition of orthophosphoric acid (Sigma, Saint Quenti Fallavier, France) before sterilization by autoclaving at 121°C for 20 min. The flasks were shaken at 250 rpm on a rotary shaker (New Brunswick Scientific, Edison, N.J.) at 25°C for 48 h. One milliliter of a subculture was transferred into a conical flask containing the same medium and allowed to grow to early stationary phase (48 h, final population: 1×108 cells ml-1).
Preparation of binary water/glycerol solutions
The Norrish equation (Norrish 1966) was used to determine the mass of solute to be added to 1,000 g distilled water to obtain good osmotic pressure. The solute used in all these experiments was glycerol (Sigma) for which k is 1.16 (Chirife and Ferro-Fontan 1980), and X s =92.09 g mol-1. The osmotic pressure of all solutions was verified with a dew point osmometer (Decagon Devices, Pullman, Wash.).
Stress treatments
Cultures were centrifuged (5 min, 4,000 rpm) and washed twice in a binary water/glycerol solution (osmotic pressure: 1.38 MPa); the pellet was resuspended in 10 ml of the same medium. Cell suspensions and shock solutions were placed in a refrigerated bath (Huber, Bioblock Scientific, Illkirch, France) for 30 min at temperatures between 5°C and 25°C, or in a heating bath (Memert, Bioblock Scientific) for higher temperatures. The temperature of each solution was controlled during manipulations using a thermocouple. When the temperature was constant, an osmotic shock was obtained by the rapid introduction of 0.5 ml cell suspension into 10 ml binary water/glycerol solution (final osmotic pressure: 13.43 / 26.63 / 43.67 / 61.83 / 102.13 / 133.12 MPa). Cells were rehydrated by adding the appropriate quantity of distilled water to achieve an osmotic pressure of 1.38 MPa. Two types of routes were studied in the (T, Π) diagram: isotherms and cycles. Isotherm treatment corresponds to dehydration and rehydration at the same temperature, whereas cycles correspond to dehydration at an experimental temperature, changes in temperature up to 25°C, and then rehydration at 25°C. Sixty isotherms and 54 cycles were realized in the temperature range 5–35°C, and osmotic pressure range 13–133 MPa. Each experiment was repeated at least six times. Before measuring cell viability, samples were maintained at 25°C in order to perform cell viability measurements under the same conditions regardless of which experimental treatment had been applied to the cells.
Cell viability measurements
Cell viability after stress treatment was determined by vital staining (methylene blue stain), as previously described (Martínez de Marañon et al. 1999). The colony forming unit method underestimates the true viable cell count obtained by vital staining, especially under stress conditions (Jones 1987). The yeast suspension was suitably diluted and mixed in a 1:1 (v/v) ratio with a methylene blue solution [0.25 g l-1 methylene blue, 10 g l-1 glucose, 9 g l-1 sodium chloride, 0.42 g l-1 potassium chloride, 0.32 g l-1 calcium chloride, and 0.2 g l-1 sodium hydrogen carbonate, in a water/glycerol solution at 1.38 MPa (all components from VWR International)]. At least 300 cells were counted for each aliquot in a Malassez counting cell. The percentage of dead cells (blue-stained) was determined and related to initial viability.
Results
The strain used in this study, S. cerevisiae W303-1A, is quite resistant to osmotic stress. Regardless of the osmotic treatment applied, we did not observe more than a 2-log decrease in viability. Each experiment was repeated at least six times in order to calculate a precise average value. Confidence intervals of the mean at the 95% level were determined to be around ±5% of the viability, except for a few treatments where the maximal value was ±12% of the viability. The confidence interval values allowed us to discriminate significant differences between the results.
Two types of experiments were performed (cycles and isotherms), and viability was always determined at 25°C.
Isotherms
Isotherms correspond to dehydration and rehydration at the same temperature, both in shock. Results are presented in Figs. 1 and 2a. The first observation made from these results was that shock intensity influences viability, and for final osmotic pressures higher than 100 MPa, the maximum viability obtained was around 50 or 60% of the initial viability. Viability decreased as the osmotic shock intensity increased. The second observation was that viability obtained for sudden osmotic modification (shock) varies with the temperature at which the experiment was carried out.
Viability related to references is a function of temperature (final osmotic pressure: a 102.13 MPa, b 133.12 MPa). ● Viability after cycle treatment (rehydration at 25°C, after a hyperosmotic shock at temperature experiment), ○ viabilities after isotherm treatment (dehydration and rehydration at the same temperature). After rehydration, viability was measured at 25°C by vital staining (methylene blue stain). Each point corresponds to the average of at least six experiments, and confidence intervals of the mean at the 95% level were determined
For low osmotic pressure shifts, we did not observe significant viability differences with temperature. Nevertheless, for osmotic pressure levels higher than 100 MPa, viability varied with temperature. For 102.13 MPa (Fig. 1a), we observed viabilities around 60–70% when the experiment took place at a temperature lower than 10°C, or higher than 22°C. Between these two temperatures, cell viability decreased to 45%. For a more drastic stress (133.12 MPa; Fig. 1b), maximum viability obtained for temperatures lower than 10°C or higher than 22°C was around 55–65%, and decreased to just less than 40% for intermediate temperatures.
Mathematical treatment of viabilities obtained after shocks (MATLAB 6.1) allowed us to represent iso-mortality curves as a function of temperature and osmotic pressure (Fig. 2a). For low osmotic pressure shifts, we did not observe significant variations in viability with temperature. For high osmotic pressure, iso-mortality curves became strongly dependent upon temperature. For example, at an osmotic pressure of 75 MPa (Fig. 2a), viability was around 75%, whatever experimental temperature was used. However, for an osmotic pressure of 95 MPa, viability was around 60%, except for temperatures lower than 8°C or greater than 25°C, for which the viability was higher. For greater osmotic pressure shift (100 MPa), iso-mortality curves show an area for which viability is lower and dependent upon temperature. In fact, we can determine a specific range of temperatures, between 10°C and 22°C, for which viability is lower when osmotic shocks of more than 100 MPa were applied.
Cycles
We also measured the cell viability after cycles, i.e., dehydration at experimental temperature and rehydration at 25°C. Viability results obtained after cycles are presented in Figs. 1 and 2b. For both treatments (isotherm or cycle), we obtained the same profile when we plotted viability as a function of temperature. However, for high osmotic pressure shifts (>100 MPa) the mortality after cycle application is more important than that found with isotherms. In fact, the maximum viability obtained for a shock intensity of 102.13 MPa was around 45–50% (i.e., 10–20% less than for isotherms), with a decrease to 25–30% between 10°C and 22°C (Fig. 1a). For 133.12 MPa, (Fig. 1b) we also observed a decrease in viability, with the maximum about 40%, and minimum around 15%. Mathematical treatment of viabilities obtained after shocks has allowed us to represent iso-mortality curves as a function of temperature and osmotic pressure (Fig. 2b). As was the case for isotherms, for cycles we observed the highest mortality for osmotic shocks of more than 100 MPa, and similarly for temperatures in the range 10–22°C.
Discussion
The mortality of S. cerevisiae during osmotic shock as observed in this work could be related to the occurrence of a water flow through an unstable membrane in phase transition. Indeed, at T m (temperature of the main membrane phase transition, i.e., gel phase to liquid-crystalline phase), a portion of the phospholipid species is in a gel state, and the rest is in a liquid-crystalline state (due to the heterogeneous nature of lipids in the membrane). These domains could cause packing defects at boundaries between the liquid-crystalline and gel phases (Cameron and Dluhy 1987), so that the membrane would not provide adequate barrier properties as shown by Hammoudah et al. (1981), who observed changes in membrane permeability around T m. By modifying osmotic pressure, a water flow is created and, if a conformational change of phospholipids occurs simultaneously, with a low barrier property as a consequence, this water flow would result in membrane injury and leakage of the cytoplasm constituents into the surrounding medium leading to cell death. Conversely, if T m is crossed by modifying only temperature and not the hydration state of the cells, such water flow will not exist, because intracellular and extracellular concentrations are balanced; viability will then be preserved. Hence, mortality would only occur if there were conformational changes to the phospholipids during dehydration. Thus, mortality should occur only at particular temperatures, and for treatment temperatures that are outside the phase transition zone, cell viability should be preserved. Our results are in total agreement with this hypothesis. For isotherms, we observed a decrease in viability for temperatures between 10°C and 22°C, for a final osmotic pressure of 102.13 MPa. For this osmotic shock intensity, phase transition should then occur between 10°C and 22°C.
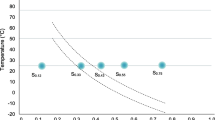
Some studies have shown that in a fully hydrated medium, phase transition occurred at around 10°C in yeast membranes (Crowe and Crowe 1992), and at more than 30°C in dried S. cerevisiae cells (Crowe et al. 1989b; Leslie et al. 1994). The T m of S. cerevisiae W303-1A has already been determined in the laboratory by fluorescence polarization using 1,6-diphenyl-1,3,5-hexatriene as a probe (Laroche et al. 2001), in the range 1.38–133.12 MPa and 5–30°C. Our results are in total agreement with these previous works and the hypotheses proposed. T m as a function of osmotic pressure already determined (Laroche et al. 2001) is reported in Fig. 3. For a hyperosmotic shock of more than 100 MPa, we observed viability to be significantly reduced between 10°C and 22°C, i.e., for treatments during which phase transition occurred.
Schematic representation of osmotic treatments and mortality observed. Curve corresponds to phase transition as previously determined by fluorescence polarization (Laroche et al. 2001), and the gray area shows treatments for which mortality was found to be high. Arrows Schematic representation of isotherm treatments performed during the study
Nevertheless, for osmotic pressure variations lower than 100 MPa, we did not observe significant mortality. In the range of temperature studied (5–30°C), and according to the S. cerevisiae membrane phase transition curve obtained by fluorescence polarization proposed on Fig. 3, the yeast passed through phase transition also for low shifts in osmotic pressure, even if we did not observe significant mortality. Thus, we can propose that, for low osmotic pressure shifts, the water flow resulting from the osmotic shock does not induce extensive damage to the membrane because of its low intensity, and that the cell can survive such a stress.
When dehydration and rehydration treatments took place at the same temperature (isotherms), viability was higher than for two different temperatures (cycles). In isotherms experiments, phase transition would have occurred twice: once during dehydration, and a second time during rehydration, whereas in cycle treatment, phase transition would have occurred only once. Nevertheless, when we performed cycles, we observed a lower viability than for isotherms, but for high osmotic pressure only. So, changing temperature when cells are in a high osmotic pressure medium may have dramatic effects on yeast cell viability during further rehydration, and to understand why will require further investigation.
This study has shown that viability of S. cerevisiae subjected to hyperosmotic shock is strongly dependent not only on the shock intensity, but also on the temperature at which it takes place. Results are in agreement with the proposed hypothesis, which relates mortality following rapid dehydration, or rehydration, to water flow through an unstable membrane in phase transition. Nevertheless, further experiments will be required to explain why viability after cycles treatment is lower than for isotherms treatment.
References
Anand JC, Brown AD (1968) Growth rate patterns of the so-called osmophilic and non-osmophilic yeasts in solutions of polyethylene glycol. J Gen Microbiol 52:205–212
Becker MJ, Rapoport AI, (1987) Conservation of yeasts by dehydration. Adv Biochem Eng Biotechnol 35:127–171
Beney L, Marechal PA, Gervais P (2001a) Coupling effects of osmotic pressure and temperature on the viability of Saccharomyces cerevisiae. Appl Microbiol Biotechnol 56:513–516
Beney L, Martinez de Marañon I, Marechal PA, Moundanga S, Gervais P (2001b) Osmotic destruction of Saccharomyces cerevisiae is not related to a high water flow rate across the membrane. Biochem Eng J 9:205–210
Cameron DG, Dluhy RA (1987) In: Gendreau RM (ed) Spectroscopy in the biomedical sciences. CRC Press, Boca Raton, Fla., pp 53–86
Chapman D (1994) The role of water in biomembranes structure. J Food Eng 22:367–380
Chirife J, Ferro-Fontan C (1980) A study of the water activity lowering behavior of polyethylene glycols in the intermediate moisture range. J Food Sci 45:1717–1719
Crowe JH, Crowe LM (1992) Membrane integrity in anhydrobiotic organisms: toward a mechanism for stabilizing dry cells. In: Somero GN, Osmond CB, Bolis CL (eds) Water and life. Springer, Berlin Heidelberg New York, pp 87–103
Crowe JH, Crowe LM, Chapman D (1984a) Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science 223:701–703
Crowe JH, Crowe LM, Chapman D (1984b) Infrared spectroscopic studies on interactions of water and carbohydrates with a biological membrane. Arch Biochem Biophys 232:400–407
Crowe JH, Crowe LM, Hoekstra FA (1989a) Phase transitions and permeability changes in dry membranes during rehydration. J Bioenerg Biomembr 21:77–91
Crowe JH, Hoekstra FA, Crowe LM (1989b) Membrane phase transitions are responsible for imbibitional damage in dry pollen. Proc Natl Acad Sci USA 86:520–523
Crowe LM, Reid DS, Crowe JH (1996) Is trehalose special for preserving dry biomaterials? Biophys J 71:2087–2093
Eleutherio ECA, Araujo PS, Panek AD (1993) Protective role of trehalose during heat stress in Saccharomyces cerevisiae. Cryobiology 30:591–596
Esener A, Bol G, Kossen N, Roels JA (1981) Effect of water activity on microbial growth. In: Moo Young M, Robinson CW, Vesina C (eds) Advances in biotechnology. Pergamon Press, Oxford, pp 339–344
Gervais P, Beney L (2001) Osmotic mass transfer in the yeast Saccharomyces cerevisiae. Cell Mol Biol 47:831–839
Gervais P, Marechal PA (1994) Yeast resistance to high levels of osmotic pressure: influence of kinetics. J Food Eng 22:399–407
Gervais P, Marechal PA, Molin P (1992) Effects of the kinetics of osmotic pressure variation on yeast viability. Biotechnol Bioeng 40:1435–1439
Gervais P, Martínez de Marañon I (1995) Effect of the kinetics of temperature variation on Saccharomyces cerevisiae viability and permeability. Biochim Biophys Acta 1235:52–56
Hammoudah MM, Nir S, Bentz J, Mayhew E, Stewart TP, Hui S, Kurian RJ (1981) Interactions of La2+ with phosphatidylserine vesicles: binding, phase transition, leakage, 31P-NMR and fusion. Biochim Biophys Acta 645:102–114
Hottiger T, Schmutz P, Wiemken A (1987) Heat-induced accumulation and futile cycling of trehalose in Saccharomyces cerevisiae. J Bacteriol 169:5518–5522
Jones RP (1987) Measures of yeast death and deactivation and their meaning. Process Biochem 22:118–128
Kets EPW, De Bont JAM (1994) Protective effect of betaine on survival of Lactobacillus plantarum subjected to drying. FEMS Microbiol Lett 116:251–256
Laroche C, Marechal PA, Beney L, Gervais P (2001) The effect of osmotic pressure on the membrane fluidity of Saccharomyces cerevisiae at different physiological temperatures. Appl Microbiol Biotechnol 56:249–254
Leslie SB, Teter SA, Crowe LM, Crowe JH (1994) Trehalose lowers membrane phase transition in dry yeast cells. Biochim Biophys Acta 1192:7–13
Leslie SB, Israeli E, Lighthart B, Crowe JH, Crowe LM (1995) Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl Environ Microbiol 61:3592–3597
Marechal PA, Gervais P (1994) Yeast viability related to water potential variation: influence of the transient phase. Appl Microbiol Biotechnol 42:617–622
Martínez de Marañon I, Chaudansson N, Joly N, Gervais P (1999) Slow heat rate increases yeast thermotolerance by maintaining the plasma membrane integrity. Biotechnol Bioeng 65:176–181
Norrish RS (1966) An equation for the activity coefficients and equilibrium relative humidities of the water in confectionery syrups. J Food Technol 1:25–39
Poirier I, Marechal PA, Gervais P (1997) Effects of the kinetics of water potential variation on bacteria viability. J Appl Microbiol 82:101–106
Poirier I, Marechal PA, Richard S, Gervais P (1999) Saccharomyces cerevisiae viability is strongly dependent on rehydration kinetics and the temperature of dried cells. J Appl Microbiol 86:87–92
Scott WJ (1957) Water relations of food spoilage microorganisms. Adv Food Res 7:83–127
Suutari M, Liukkonen K, Laakso S (1990) Temperature adaptation in yeasts: the role of fatty acids. J Gen Microbiol 136:1469–1474
Thomas BJ, Rothstein R (1989) Elevated recombination rates in transcriptionally active DNA. Cell 56:619–630
Walton EF, Pringle JR (1980) Effect of growth temperature upon heat sensitivity in Saccharomyces cerevisiae. Arch Microbiol 124:285–287
Webb MS, Hui SW, Steponkus PL (1993) Dehydration-induced lamellar-to-hexagonal-II phase transitions in DOPE/DOPC mixtures. Biochim Biophys Acta 1145:93–104
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laroche, C., Gervais, P. Achievement of rapid osmotic dehydration at specific temperatures could maintain high Saccharomyces cerevisiae viability. Appl Microbiol Biotechnol 60, 743–747 (2003). https://doi.org/10.1007/s00253-002-1167-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-002-1167-5