Abstract
The co-cultivation of tissue explants with beneficial microbes induces numerous developmental and metabolic alterations in the resulting plantlets conferring enhanced tolerance to abiotic and biotic stresses. In the present study we have co-cultivated plant growth promoting rhizobacteria (PGPR) exhibiting multiple plant growth promoting activities with the in vitro raised saffron cormlets for evaluating various morphogenetic responses like proliferation, germination and weight increment of cormlets. The results obtained indicate the significant effect of Pseudomonas sp., Bacillus subtilis and Pantoea sp. in weight increment of cormlets. Proliferation of cormlets was also significantly improved with Pantoea sp. + Bacillus subtilus + Pseudomonas sp. on MS liquid medium. Similarly, the co-cultivation with Acinetobacter haemolyticus, Accintobacter lwoffii and Pantoea sp. resulted in 100 % germination of cormlets. The root system of cormlets was found denser and thicker than the control cormlets. However, rhizobacterial cormlets exhibited lower values of root length than non-treated cormlets. This study represents earliest report across the globe with suitable and reproducible protocol for corm development through PGPRs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Kashmir valley has an inimitable significance in saffron production in entire India covering exclusively 4,496 hectares. In the recent years, there has been 83 % decline in cultivation resulting in consequent 21.5 % decrease in production and 72 % in productivity (Husaini et al. 2010). The decline has been attributed to many factors, such as conventional cultivation practices, pathogenic infections to corms and reduction in arable land suitable for saffron cultivation (Kamili et al. 2007). Sterility in saffron which limits the application of conventional breeding approaches for its further improvement is major constraint for its low productivity. In vitro mass production of pathogen free corms offers a tremendous scope. However, the current protocol available for in vitro corm production is inadequate (Devi et al. 2011; Parray et al. 2012) for the production of multiple flowering corms. To validate and commercialize the technique for enhanced saffron production, there is an immediate need to improve the existing protocol. Hence, progress of modern technology for quick propagation methods and high yielding varieties is considered to be important from commercial point of view. In vitro co-culture of tissue explants with beneficial microbes induces developmental and metabolic changes in the resulting plantlets which confer them enhanced tolerance against varied abiotic and biotic stresses, this phenomenon is known as “biotization” (Nowak 1998). PGPR’s have been studied for the past century as possible inoculants for increasing plant productivity (Kloepper et al. 1991) and several mechanisms have been postulated to explain how PGPR stimulate plant growth under in vivo conditions. Souissi et al. 1997 inoculated leafy spurge callus tissue with two rhizobacterial isolates, namely Pseudomonas fluorescens isolate LS 102 and Flavobacterium balustinum isolate LS 105 to investigate the mode and/or mechanism of action of potential biocontrol agents on their host plants. The prime objective of this study was to determine the efficacy of already screened PGPR strains with PGP activities on growth of cormlets of Crocus sativus L. under in vitro conditions. These PGR strains are already documented for incased plant growth on various crops including the C. sativus L. under greenhouse and field conditions (Sharaf-Eldin et al. 2008). Keeping in view the present knowledge, co-cultivation of rhizobacterial strains with in vitro raised cells/tissues etc. seems to be one of the appropriate approaches for mass propagation of the desired plant. In this context, the present study was under taken to evaluate the role of rhizobacterial strains isolated from saffron soil in in vitro saffron culture for enhancing proliferation, weight and germination of in vitro cormlets.
Materials and methods
In vitro culture and isolation/identification of rhizobacterial strains.
In vitro culture
The collection, sterilization, establishment of in vitro culture of saffron corms was carried out as reported previously (Parray et al. 2012).
Plant material
Corms of C. sativus L. Kashmirianus were collected from Pampore area of Kashmir, J&K, India and were thoroughly washed with detergent Extran (0.5 %) and Tween-20 (surfactant) with tap water followed by rinsing with double distilled water. Subsequently these were surface disinfected with 70 % ethanol for 1 min followed by 0.5 % HgCl2 (w/v) for 6 min and washed five times with sterilized double distilled water. All the chemicals, unless otherwise specified, were obtained from Hi-Media Mumbai Pvt. Ltd.
Culture media
MS basal medium supplemented with different concentrations of sucrose (Hi-media), Difcobacto agar (Qualigens, India) and different concentrations of plant growth regulators (PGRs; Hi-media) were prepared. The pH was adjusted to 5.6–5.8 with 1 N HCl or 1 N NaOH and finally dispensed into 100 and 250 ml Erlenmeyer flasks (borosilicate glass) plugged with non-absorbent cotton. The media were sterilized in an autoclave for 15 min at 121 °C and cultures were incubated at 25 ± 2 °C under 16 h photoperiod, illuminated with cool white fluorescent tubes, at irradiance of 100 μmol m−2s−1. The experiments were performed in completely randomized block design, repeated three times; each treatment had 05 replicates.
Establishment of in vitro culture
The sterilized corms were cut with a sharp scalpel into slices and these were cultured on MS (1/2) medium with different concentrations of auxins and cytokinins. Since in our previous report (Parray et al. 2012) highest number of comlets (70) were obtained via callusing on MS (1/2) medium supplemented with TDZ (20 μM) + IAA (10 μM) after 8 weeks, and this media combination was used in consecutive experiments for the use of PGPR for the cormlet proliferation. The in vitro regenerated cormlets sub-cultured on MS + BAP (20 µM) + NAA (15 µM) resulted in 90 % germination and accordingly this media combination was used for further enhancement in germination of cormlets. Similarly, the in vitro raised cormlets sub cultured on plant nutrient medium (MS) attained a maximum corm weight of 2.5 g using TDZ (15 μM) + IAA (12.5 μM) and BAP (20 µM) + NAA (15 µM) and these two media combinations were separately used for further increment in weight of cormlets via cocultivation of PGPRs.
Isolation and Identification of PGPRs
The isolation, identification and in vitro screening of rhizobacterial strains for PGP traits was carried out as per our previous published report (Parray et al. 2013).
Sampling process and bacterial isolation/identification
The rhizosphere saffron soil samples were collected in the month of September to October 2010 during the flowering of saffron corms from the saffron field in Pampore area, J&K, India for isolation of rhizobacterial strains with beneficial traits like siderophore production, phosphate solubilization and IAA production. The corms were uprooted and the soil adhering to the roots which represent rhizosphere soil were shaken from the roots and collected in sterilized plastic bags. The soil samples were then transported to the microbiology laboratory of the Centre of Research for Development (CORD), University of Kashmir, for immediate processing. To isolate bacteria, 1 g of soil sample was transferred to 9 ml distilled water and was serially diluted. Diluted suspensions were spread plated on LB agar medium and were incubated at 28 °C for 24 h. Representative colonies were randomly selected from the countable plates and re-streaked onto new plates of the different media to obtain pure colonies. A total of 23 isolates obtained in this manner were maintained on agar slants. Because many isolates were morphologically indistinguishable in culture, preliminary characterization procedures including cytochrome oxidase (Kovacs 1956), oxidative fermentation (Hugh and Leifson 1953), catalase and motility tests were conducted. Based on these preliminary microscopic/macroscopic characterizations, six (06) isolates were chosen from the original 23 isolates and were subjected to biochemical tests using strain specific biochemical kits (Hi-media).The three types of kits used as per the strain were KB002 for Gram negative rods, KB013 for gram Positive bacillus and KB001 for Enterobacteriaceae. The strains were identified as per the chart sheet of the kits to the nearest value. The strains were further authenticated using Vitex-2 sophisticated equipment comprising of 64 tests at Dr Qadri’s laboratory, Karanagar (J&K) India. Pure cultures of isolates stored at −80 °C in nutrient broth supplemented with 200 mg/g glycerol were used for the screening of growth promoting activities.
Screening for PGP activities
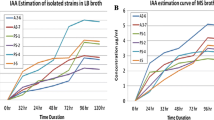
Quantitative estimation of IAA production was detected by the method as described by Brick et al. (1991). The amount of soluble phosphate was measured by the colorimetric method as described by King (1932) and percentage of siderophore production by PGPRs was detected as described by Schwyn and Neilands (1987).
Co-cultivation
The six rhizobacterial strains—Pseudomonas sp. JCORD01, Klebsiella sp. JCORD04, Bacillus subtilis JCORD06, Acinetobacter haemolyticus-JCORD08, Acinetobacter lwofii-JCORD09 and Pantoea sp. JCORD23 were selected on the basis of their plant growth promoting properties (Parray et al. 2013). The trails were carried out either using single or multiple inoculums of strains. The 30–70 days old in vitro raised cormlets were co-cultivated with rhizobacterial strains on nutrient MS medium (Murashige and Skoog 1962) for desired morphogenetic responses. The MS medium used was supplemented with 3 % sucrose solidified with 8 % agar augmented with both auxins and cytokinins however, their concentrations and combinations varies with treatments. The medium was adjusted to pH 5.6 prior to autoclaving at 15 psi for 25 min. After inoculation, all the cultures were kept for incubation under cool fluorescent tubes at day night regime of 16 h photoperiod with light intensity of 1,500–3,000 lux at a constant temperature of 25 ± 3 °C. Relative humidity between 60 and 70 % was maintained. However, the broth cultures were incubated in an orbital shaker (Remi) at 60 rpm and were exposed to continuous fluorescent light at 25 ± 5 °C for a month. The 1-month duration was sufficient for plantlets to absorb all nutrients that were available in the media. The 30 ml MS liquid medium was replenish with same fresh medium at 2 week intervals.
For inoculum preparation, each isolate was grown separately in nutrient broth (Kado and Heskett 1970). The density of colony forming units (c.f.u.) in cell suspensions of each isolate was estimated from absorbance measurements at 600 nm. A density of approximately 1 × 107–1 × 108 cfu/ml was used in all experiments. The inoculum suspensions were kept in a refrigerator at 4 °C prior to use. About 0.1 ml of 1 × 107–1 × 108 cfu/ml of rhizobacterial strains was used for inoculation purposes and rhizobacterial cells were inoculated at a distance of about 2 cm from the explants (Mahmood et al. 2010).
The whole experiment for co-cultivation was divided into three sequential experiments. Exp. I—Each explant was inoculated with individual rhizobacterial strains. Exp. II—Each explant was co-cultured with different combinations of rhizobacterial strains. Exp. III—Each explant was cultured without any rhizobacterial strains (Control). The combinations of strains was first tested on nutrient MS media to observe the antagonistic effects of strains and later on were recommended for the co-cultivation. The cultures were than incubated in a culture room in a same way as for the cultures kept under normal growth conditions. After 30 to 45 days of culture, if possible, the bacterized cells were transferred to the fresh MS medium i.e. without bacterial strains. Growth responses in terms of germination, cormlet size and number were recorded after 10–12 weeks of culture period.
Growth evaluation of in vitro raised corms under field conditions
The in vitro raised cormlets were categorized into three groups, with a minimum weight of 3.0, 4.0, and 5.0 g. These cormlets were taken out from culture vials and after shade drying (7 days) in a well ventilated room with room temperature (25 °C), were transplanted into small pots containing clay loamy soil. Misting of the cormlets was done as per the need until the germination of cormlets.
Statistical analysis
The whole experiment was performed in a randomized complete block with 05 replications and one–two plant parts per replication. Data were subjected to analysis of variance using SSPP software version 17.0 (SAS Institute Inc., Cary, NC, USA). The growth response of explants under normal conditions and also under the influence of PGPR treatments were considered significant according to the magnitude of the F value (P ≤ 0.005).
Results
In this study, the isolated pure rhizobacterial strains with PGP traits were co-cultivated with in vitro raised cormlets of C. sativus and morphogenetic responses of in vitro cormlets like their multiplication, germination and weight increment was studied.
Multiplication of cormlets cultured with rhizobacterial strains
The in vitro raised cormlets were co-cultured with different combinations of PGPRs for enhancing the multiplication of cormlets in ½ MS liquid media augmented with TDZ (20 µM)/IAA (10 µM). About 30 ± 2.54 cormlets were obtained on ½ MSliq. + TDZ (20 µM) + IAA (10 µM) without PGPRs (Fig. 1a). The MS liquid medium proved to be effective in terms of proliferation of cormlets co-cultivated with PGPRs. Among all the treatments, the combination of Pantoea sp., Bacillus subtilus and Pseudomonas sp. was found to be the best treatment resulting in cormlet proliferation up to 50 after 12 weeks of culture period (Table 1; Fig. 1b). However, from the data obtained pertaining to the cormlet proliferation, it was observed that Pantoea sp. was found to be the useful strain either individually or in combination with B. subtilis or Klebsiella sp.
In vitro cormlet development of Crocus sativus L. under the influence of rhizobacterial strains under in vitro conditions: a multiple cormlets on ½ MS (liquid) + TDZ (20 μM) + IAA (10 μM) {Control} b multiple cormlets on ½ MS (liquid) + TDZ (20 µM) + IAA (10 µM) + Pantoea sp. + B. subtilus + Pseudomonas sp. c complete germination on MS + BAP (20 µM) + NAA (15 µM) {Control} d complete germination on MS + BAP (20 µM) + NAA (15 µM) + Acinetobacter lwofii + Acinetobacter haemoliticus + Pantoea sp. e increase in weight of cormlets on MS + BAP (20 µM) + NAA(15 µM) {Control} f increase in weight of cormlets on MS + BAP (20 µM) + NAA(15 µM) + Bacillus subtilis + Pantoea sp. + Acinetobacter haemoliticus g, h irregular cormlet formation on MS + BAP (20 µM) + NAA (15 µM) + Bacillus subtilis + Acinetobacter lwofii + Acinetobacter haemoliticus i, j increase in weight of cormlets on MS + TDZ (15 µM) + IAA (10 µM) + Pseudomonas sp. + Bacillus subtilis +Pantoea sp.
Germination of cormlets co-cultured with rhizobacterial strains
Experiments were also carried out to study germination of in vitro raised cormlets co-cultivated with six PGPRs (Pantoea sp., B. subtilus, Pseudomonas sp., Klebsella sp., A. lwoffii and A. haemoliticus) on MS nutrient medium + BAP (20 µM) + NAA (15 µM). The germination response of cormlets was recorded about 90 % without the PGPRs (Fig. 1c). The Pseudomonas sp. and Klebsella sp. facilitated germination of cormlets with rhizogenesis with average germination initiation after 35 and 28 days respectively. Similarly, here in these trials the combination of PGPRs was found effective for germination of cormlets. The maximum germination response (100 %) of cormlets with germination initiation after 26 days was observed on MS medium co-cultivated with A. lwoffii, A. haemoliticus and Pantoea sp. (Figure 1d) followed by 95 % germination response of cormlets co-cultiavted with A. lwoffii and A. haemoliticus inoculants after 12 weeks of culture period. The PGPRs from native saffron rhizosphere were found effective in plantlet formation both with root and shoots as compared to control (Table 2).The root system of cormlets was found denser and thicker than the control cormlets. The root number as well as shoot number increased over time in both bacterised and non-treated cormlets, although values for former were always significantly higher In the case of root length, rhizobacterial cormlets showed lower values than non-treated cormlets, although differences were non-significant. The root system exhibited interesting observation as illustrated in Table 2, the highest number of roots (2.74 ± 0.77) as well as maximum root weight (0.380 ± 0.06 g) were observed on the MS + BAP (20 µM) + NAA (15 µM) + A. haemolyticus + Accintobacter lwoffii +Pantoea sp., however, root length was reduced to 1.41 ± 0.45 cm. While the control samples exhibited maximum root length (3.5 ± 0.54 cm), and minimum root weight (0.095 ± 0.028 g). Thus it can be conjectured from the results that there exists inverse response between the weight and number of roots with respect to length of root system.
Increase in weight of cormlets co-cultivated with plant growth isolates
In this experiment, trials were carried out for enhancing the weight of cormlets on MS medium augmented with two phytohormonal combinations i.e. MS + BAP (20 µM) + NAA (15 µM) and MS + TDZ (15 µM) + IAA (10 µM). The average initial weight of cormlets used in these experiments was 0.45 g. The average weight of cormlets obtained on above respective media combinations without the PGPRs was only 2.5 g (Fig. 1e). However, the weight of cormlets varied significant (P < 0.005) after co-cultivation with PGPRs.
In first trial, MS + BAP (20 µM) + NAA (15 µM) combination was used for co-cultivation. The PGPRs were inoculated either singly or in combination. The combination of PGPRs proved to be effective for enhancing the weight of cormlets. Here, the significant increase in weight (4.42 g) of cormlets was obtained on MS medium co-cultivated with B. subtilis, A. lwofii and A. haemoliticus (Fig. 1f) followed by 4.22 g on nutrient medium co-cultivated with B. subtilis, Pantoea sp. and A. haemoliticus after 12 weeks of culture period. It was also observed that only 2.93 g increase in weight of cormlets was observed on MS medium co-cultivated with A. lwoffi (Table 3). However, in some cases irregular shaped cormlets were formed (Fig. 1g, h). It is pertinent to mention here that some of the bacterized cormlets had to be transferred onto same fresh nutrient medium without any strain and growth of cormlets was recovered.
In another trial, cormlets were co-cultivated with PGPRs on MS medium + TDZ (15 µM) + IAA (10 µM). Among the PGPRs, Pseudomonas sp., A. lwofii, B. subtilis and Pantoea sp. were found effective in enhancing the weight of cormlets. The significant weight (P < 0.005) of cormlets 5.01 g was observed on nutrient medium co-cultivated with Pseudomonas sp., B. subtilis and Pantoea sp. with average 40 % response (Figs. 1i, j) followed by 4.18 g on same medium co-cultivated with Pseudomonas sp., B. subtilis and A. lwofii after 12 weeks of culture period.
Again, in this experiment, combination of strains was found to be effective for weight increment. The significant difference with respect to control (P < 0.005) in terms of cormlet weight was observed due to the bacterial inoculation (Table 3). It was also observed that besides the PGPRs, the varied hormonal media combination proved to be also an important factor that affects the weight of cormlets as in our study TDZ/IAA combination was found more responsive for the same. In the present study, a reproducible protocol for multiple cormlet development using normal growth promoters as well as the PGPRs was developed.
The in vitro raised cormlets with varied size ranges 3–5 g as illustrated in Table 4 were transferred to green house for evaluating the growth response. The results observed demonstrate 79 % of vegetative growth response with 5.0 g corm weight.
Discussion
Our study reveals that the PGPRs exhibit significant impact on growth and development of cormlets with respect to control under in vitro condition. The effects of PGPRs are considered to be highly specific with respect to plant and bacterial genotypic combination (Rennie and Larson 1979). The results of the present study support the hypothesis that all the six PGPRs were able to promote the growth of cormlets under in vitro conditions which is attributed to host specific variations (Smith and Goodman 1999). The isolates were selected based on their ability to produce high levels of auxin (IAA) and/or other functional assays for plant growth promotion (Parray et al. 2013).
An extensive literature is available documenting the beneficial aspects of screening and employing PGPR’s from crop plants however, there is scarce research done on corm development under in vivo conditions in saffron via PGPR (Ambardar and Vakhlu 2013; Parray et al. 2013). Sharaf-Eldin et al. (2008) reported that inoculation of saffron (C. sativus L.) corms with B. subtilis FZB24 under ex-vitro conditions significantly increased leaf length, flowers per corm, weight of the first year flower stigma, total stigma biomass and significantly declined the time period requisite for corm sprouting and the number of shoots which could be basis for development of protocol for use of native saffron PGI in tissue culture for growth and development of cormlets.
The present hypothesis is further supported by Om et al. (2009) who reported that inoculation of oil palm tissues with selected diazotrophic rhizobacteria during in vitro micropropagation enabled early associative interactions between the bacteria and the host plants that allowed better adaptation of the host plants to environmental conditions and a higher survival rate for the host plants. All the PGPRs i.e. Pseudomonas sp., Klebsella sp., B. subtilis, A. lwofii, A. haemoliticus and Pantoea sp. were able to enhance the growth of cormlets either alone or in combination in our study. The microbial symbiosis with plants can synthesize hormones similar to those produced by the plant as growth regulator such as auxins, gibberellins and cytokinins (Melo 1998). Jimtha et al. 2014 identified of Ralstonia sp. from somatic embryogenic cultures of banana (Musa accuminata AAA cv. Grand Naine) and confirmed its plant growth promoting properties including indole acetic acid and siderophore production.
Among them, auxins (IAA) are one of the most well-known phytohormones because of their important role in the initial processes of lateral and adventitious root formation (Gaspar et al. 1996) and shoot elongation (Yang et al. 1993). The other possible mechanisms may be nutrient solubility or fixation and siderophore production, activation of phosphate solubilization (Lalande et al. 1989). Further the efficiency of microbes in plant growth promotion and the absence of competition from other soil-borne microbes under controlled conditions is well documented (Carletti et al. 1998; Weller 2007).
In the present study, PGPRs significantly enhanced the growth and development of cormlets. Pantoea sp. was found to exhibit profound effect on enhancing the size of in vitro cormlets and its role is being reported (Huo et al. 2012) under hydroponic culture in Panicum maximum. Under in vitro condition PGPRs significantly produce biochemical and histological modifications, rhizogenesis, growth promotion and reduction of hyperhydricity of in vitro cultured plants (Frommel et al. 1991; Mahmood et al. 2012).The co-cultivation of multiple PGPRs with saffron cormlets significantly improves growth and development of cormlets which may be the case of synergism (Mahmood et al. 2010). The co-cultivation of multiple PGPRs in other plants is well documented, two strains of P. maltophilia in vitro soybean cotyledon explants (Yang et al. 1991), Acetobacter diazotrophicus (R12) and Azospirillum brasilense (Sp7) with in vitro oil Palm plants (Om et al. 2009).
Thomas et al. 2010 evaluated the efficiency of native isolates of P. fluorescens, A. brasilense, and Trichoderma harzianum on rooting and acclimatization of in vitro-grown shoots and plantlets of tea. They have evaluated acclimatization of rooted plantlets in soil amended with these bio-inoculants, either independently or in diverse combinations, enhanced plantlet survival. Root rot or wilting of tissue culture-derived plants was not evaluated in bio-inoculant-treated plants, as they exhibited comparatively elevated activities of defense enzymes, together with peroxidase and phenylalanine ammonia lyase.
Employing regular plant growth regulators like {cytokinins (BAP and TDZ) and auxins (NAA and IAA)} supplemented in nutrient medium proved beneficial in our study. The impact of bacterial auxin production on plant root growth likely depends not only on endogenous root auxin levels, but also on the existence of other bacterial characteristics that may minimize auxin impact (Kende 1993).
In present study, both phosphate solubilizing, B. subtilis and Pseudomonas sp were found efficient in enhancing the weight and germination of cormlets which may be due to their myoinositol solubilizing capacity in the nutrient medium and the results obtained during the present study are in agreement with the findings of Mohan and Radhakrishnan (2012) who reported similar observation after co cultivating the PSB (phosphate solubilizing bacteria) isolates KED-4 B. subtilis and TCO-6 (P. fluorescence) with in vitro raised teak plantlets. Similarly for the in vitro rooting of banana plantlets (Mahmood et al. 2010) and hardening of tissue cultured tea plantlets (Pandey et al. 2000) both Bacillus and Pseudomonas species were effective.
Co-cultivation of saffron cormlets with PGPRs led to the growth of cormlets without the actual root system. Certainly IAA-producing bacteria evidently alter root elongation and root architecture, but still it is not clear whether impacts of these bacteria on shoot growth are because of direct long-distance IAA signaling or indirect effects of altered root system performance on water and nutrient capture (Richardson 2001). The main reasons for enhancing the growth of cormlets may be due to the release phytohormones or other volatile compounds that act directly or indirectly to activate plant immunity or regulate plant growth and morphogenesis (Soundarapandian and Dhandayuthapani 2010). Also the identification of signals from free-living bacteria and fungi that interact with plants in a beneficial way reveal that the classic plant signals such as auxins and cytokinins can be produced by microorganisms to efficiently colonize the root and modulate root system architecture and while the other classes of signals, including N-acyl-L-homoserine lactones used by bacteria for cell to cell communication, can be perceived by plants to modulate gene expression, metabolism and growth (Ortiz-Castro et al. 2009). Plants produce a wide range of organic compounds including sugars, organic acids and vitamins, which can be used as nutrients or signals by microbial populations (Ortiz-Castro et al. 2009).The complete regeneration protocol for cormlet development using both plant growth regulators as well as PGPRs were used under in vitro conditions.
The eventual success of micro propagation on a commercial scale depends on the ability to transfer plants out of culture on a large scale, at low cost and with high survival rates. After field transfer the in vitro grown plantlets were unable to compete with soil microbes and to cope up with the environmental conditions. In order to increase growth and reduce mortality in plantlets at the acclimatization stage, efforts are focused on the control of both physical and chemical environment and bio-hardening of micro propagated plantlets (Chandra et al. 2010).
Abbreviations
- BAP:
-
Benzyl aminopurine
- NAA:
-
Naphthalene acetic acid
- IAA:
-
Indole acetic acid
- PGP:
-
Plant growth promoting
- PGPR:
-
Plant growth promoting rhizobacteria
- PSB:
-
Phosphate solubilizing bacteria
References
Ambardar S, Vakhlu J (2013) Plant growth promoting bacteria from bacteria from Crocus sativus rhizosphere. World J Microbiol Biotechnol 29:2271–2279
Brick JM, Bostock RM, Silversone SE (1991) Rapid in situ assay for indole acetic acid production by bacteria immobilized on nitrocellulose membrane. Appl Environ Microbiol 57:535–538
Carletti SM, Llorente BE, Rodriguez EA, Tandecarz JS (1998) Jojoba inoculation with Azospirillum brasilense stimulates in vitro root formation. Plant Tissue Cult Biotechnol 4:165–174
Chandra S, Bandopadhyay R, Kumar V, Chandra R (2010) Acclimatization of tissue cultured plantlets from laboratory to land. Biotechnol Lett 32(9):1199–1205
Devi K, Sharma K, Singh M, Ahuja PS (2011) In vitro cormlet production and growth evaluation under greenhouse conditions in saffron (Crocus sativus L.) - A commercially important crop. Eng Life Sci 11:189–194
Frommel M, Nowak J, Lazarovits G (1991) Growth enhancement and developmental modifications of in vitro grown potato (Solanum tuberosum ssp. tuberosum) as affected by a nonfluorescent Pseudomonas sp. Plant Physiol 96:928–936
Gaspar T, Kevers C, Penel C, Greppin H, Reid DM, Thorpe TA (1996) Plant hormones and plant growth regulators in plant tissue culture. Vitro Plant Cell Div Biol 32:272–289
Hugh R, Leifson E (1953) The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram negative rods. J Bacteriol 66:24–26
Huo W, Chun-hua Z, Ya C, Meng P, Hui Y, Lai-qing L, Qing-sheng C (2012) Paclobutrazol and plant-growth promoting bacterial endophyte Pantoea sp. enhance copper tolerance of guinea grass (Panicum maximum) in hydroponic culture. Acta Physiol Plant 34:139–150
Husaini AM, Kamili AN, Wani MH, Teixeira da Silva JA, Bhat GN (2010) Sustainable Saffron (Crocus sativus L. Kashmirianus) Production: technological and policy interventions for Kashmir. FPSB 4:116–127
Jimtha JC, Smitha PV, Anisha C, Deepthi T, Meekha G, Radhakrishnan EK, Gayatri GP, Remakanthan A (2014) Isolation of endophytic bacteria from embryogenic suspension culture of banana and assessment of their plant growth promoting properties. Plant Cell Tiss Organ Cult 118:57–66
Kado CI, Heskett MG (1970) Selective media for isolation of Agrobacterium, Corynebacterium, Erwinia, Pseudomonas, and Xanthomonas. Phytopath 60:969–976
Kamili AS, Nehvi FA, Trag AR (2007) Saffron-A legendry crop of Kashmir Himalaya. J Himal Ecol Sustain Dev 2:1–12
Kende H (1993) Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol 44:283–307
King A (1932) An improved method for the colorimetric determination of phosphate. Biochem J 26(2):292–297
Kloepper JW, Rodriguez-Kabana R, McInroy JA, Collins DJ (1991) Analysis of population and physiological characterization of microorganism in rhizosphere of plants with antagonistic properties to phytopathogenic nematodes. Plant Soil 136:95–102
Kovacs N (1956) Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature 178:703
Lalande R, Bissonnette N, Coutlee D, Antoun H (1989) Identification of rhizobacteria from maize and determination of their plant growth promoting potential. Plant Soil 115:7–11
Mahmood M, Rahman ZA, Saud HM, Shamsuddin ZH, Subramaniam S (2010) Influence of rhizobacterial and agrobacterial inoculation on selected physiological and biochemical changes of banana cultivar, berangan (AAA) plantlets. J Agric Sci 2(1):115–137
Melo IS (1998) Rizobacterias promotoras de crescimento de plantas: descriçao e potencial de uso na 98 ERTURK ET AL. Biol Res 43, 2010, 91–98 agricultura. In: Melo IS, Azevedo JL (eds). Ecologia Microbiana. EMBRAPA Meio Ambiente, Jaguariuna, p. 86–116
Mohan V, Radhakrishnan A (2012) Screening of phosphate solubilizing bacterial rhizobacteria (PGPR) and endophytes with medicinal plants-new avenues for phytochemicals. J Phytol 2(7):91–100
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–497
Nowak J (1998) Benefits of in vitro “Biotization” of plant tissue cultures with microbial inoculants. Vitro Cell Dev Biol Plant 34(2):122–130
Om AC, Amir HA, Chan LK, Zamzuri I (2009) Microbial inoculation improves growth of oil palm plants (Elaeis guineensis Jacq). Trop Life Sci Res 20(2):71–77
Ortiz-Castro R, Contreras-Cornejo HA, Macias-Rodríguez L, Lopez-Bucio J (2009) The role of microbial signals in plant growth and development. Plant Signal Behav 4(8):701–712
Pandey A, Palni LMS, Bagm N (2000) Biological hardening of tissue culture raised tea plants through rhizosphere bacteria. Biotech Lett 22:1087–1091
Parray JA, Kamili AN, Hamid R, Husaini AM (2012) In vitro cormlet production of saffron (Crocus sativus L. Kashmirianus) and their flowering response under greenhouse. GM Crops Food biotechnol agric Food 3(4):289–295
Parray JA, Kamili AN, Reshi ZA, Hamid R, Qadri RA (2013) Screening of beneficial properties of rhizobacteria isolated from Saffron (Crocus sativus L.) rhizosphere. Afr J Microbiol Res 7(23):2905–2910
Rennie RJ, Larson RI (1979) Nitrogen fixation associated with disomic chromosome substitution lines of spring wheat. Can J Bot 57:2771–2775
Richardson AE (2001) Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Aust J. Plant Physiol 28:897–906
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Sharaf-Eldin M, Shereen E, Jose-Antonio F, Helmut J, Ronald C, Jose G, Pamela W (2008) Bacillus subtilis FZB24 affects flower quantity and quality of Saffron (Crocus sativus). Planta Med 74:732–734
Smith KP, Goodman RM (1999) Host variation for interactions with beneficial plant-associated microbes. Ann Rev Phytopathol 37:473–491
Souissi T, Kremer RJ, White JA (1997) Interaction of rhizobacteria with leafy spurge (Euphorbia esula L.) callus tissue cells. Plant Cell Tissue Organ Cul 47(3):279–287
Soundarapandian S, Dhandayuthapani K (2010) Interaction of plant growth promoting isolates for the growth improvement of Tectona grandis Linn. Res J Microbiol 7:101–113
Thomas J, Ajay D, Kumar RR, Mandal AKA (2010) Influence of beneficial microorganisms during in vivo acclimatization of in vitro-derived tea (Camellia sinensis) plants. Plant Cell Tissue Organ Cul 101(3):365–370
Weller DM (2007) The nature and application of biocontrol microbes III: Pseudomonas spp. Pseudomonas biocontrol agents of soilborne pathogens. Am Pathol Soc 97:2–0250
Yang T, Law DM, Davies PJ (1993) Magnitude and kinetics of stem elongation induced by exogenous indole 3-acetic acid in intact light grown pea seedlings. Plant Physiol 102:717–724
Yang YS, Wada K, Goto M (1991) In vitro formation of nodular calli in soybean (Glycine max L.) induced by co-cultivated Pseudomonas maltophilia. Jpn J Breed 41:595–604
Acknowledgments
This study was supported by DBT, GoI, New Delhi funded project vide letter no: BT/PR5525/PVD/16/980/2012 Dtd.20/11/2012, the assistance of which is highly acknowledged. The authors are thankful to department of Microbiology SKIMS Soura Srinagar and Dr. Qadri’s Lab, Karanagar, J&K for helping in identifying rhizobacterial strains.
Conflict of interest
The authors hereby declare no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parray, J.A., Kamili, A.N., Reshi, Z.A. et al. Interaction of rhizobacterial strains for growth improvement of Crocus sativus L. under tissue culture conditions. Plant Cell Tiss Organ Cult 121, 325–334 (2015). https://doi.org/10.1007/s11240-014-0703-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0703-1