Abstract
Somatic embryogenesis receptor kinase (SERK) gene is known to be a marker of somatic embryogenesis in several plant species. The present study reported the isolation and characterization of a SERK gene ortholog, designated as McSERK, in Momordica charantia, an important medicinal plant. The complete coding region of McSERK was found to encode a 627 amino acid protein which contained an N-terminal signal peptide, a leucine zipper, five leucine rich repeats, a serine-proline-proline domain, a transmembrane domain, a kinase domain and the C-terminal region, depicting the typical characteristic features of SERK-family proteins. Phylogenetic analysis suggested that McSERK was highly similar to the SERK proteins of Cucumis sativus, Glycine max and Medicago truncatula. Homology modeling was attempted to construct the three dimensional structure of McSERK protein which showed that it corresponded to a monomeric protein. McSERK expression was high in embryogenic callus but its expression was relatively low in different plant organs. The high expression of McSERK transcript in the embryogenic callus confirmed its association with somatic embryogenesis in M. charantia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Somatic embryogenesis, an important plant propagation method, has become an indispensable tool for crop improvement. It is a complex developmental program in which competent somatic cells undergo restructuring through a series of morphological, biochemical and molecular changes to form embryogenic cells. Cellular totipotency, a unique feature of higher plants, forms the basis of somatic embryogenesis that enables somatic cells to form an embryo which is eventually converted to a whole plant. Somatic embryogenesis facilitates large scale production of transgenic plants for commercial application.
The molecular alterations of somatic embryogenesis involve a differential gene expression pattern (Chugh and Khurana 2002), which is triggered by a series of signal cascades. Therefore, identification of specific genes involved in the molecular regulation of somatic embryogenesis has attracted considerable attention from plant biotechnologists. It has become evident that the molecular events leading to the formation of somatic embryos are regulated by a group of receptor-like kinases (RLKs) containing leucine-rich repeats (LRRs) in their extracellular domain. RLKs comprise a large gene family responsible for various signal transduction processes. The unique feature of RLK is characterized by the presence of an N-terminal signal peptide (SP), a single transmembrane (TM) and cytoplasmic kinase domain containing serine/threonine and an extracellular domain (Tichtinsky et al. 2003). Leucine-rich-repeat RLKs (LRR–RLKs) form the largest group of RLKs. Among the various genes involved in the molecular regulation of somatic embryogenesis, substantive effort has been made to isolate a novel gene known as somatic embryogenesis receptor kinase (SERK) that belongs to the LRR–RLK superfamily. The gene encodes a RLK containing an N-terminal SP, an extracellular leucine zipper (LZ), a single TM domain, five leucine rich repeats (LRRs) in its extracellular domain, a distinct serine-proline-proline (SPP) domain, a serine/threonine kinase domain and the C-terminal region. The first SERK gene (DcSERK) was identified by Schmidt et al. (1997) in embryogenic cultures of carrot. Since then a large number of DcSERK homologs have been characterized in many different monocot and dicot plants. Expression of SERK gene during somatic embryogenesis and its association with embryogenic competence have been demonstrated in a wide variety of plants including Arabidopsis thaliana (Hecht et al. 2001), Medicago truncatula (Nolan et al. 2003), Zea mays (Baudino et al. 2001), Helianthus annuus (Thomas et al. 2004), Ocotea catharinensis (Santa-Catarina et al. 2004), Poa pratensis (Albertini et al. 2005), Citrus unshiu (Shimada et al. 2005), Oryza sativa (Hu et al. 2005), Triticum aestivum (Singla et al. 2008), Vitis vinifera (Maillot et al. 2009), Musa acuminata (Huang et al. 2009), Cocos nucifera (Pérez-Núñez et al. 2009), Ananas comosus (Ma et al. 2012), Cyrtochilum loxense (Cueva et al. 2012), and Garcinia mangostana (Rohani et al. 2012). Extensive characterization of SERK has, however, been carried out in A. thaliana and M. truncatula. Five and six SERK genes have been identified in A. thaliana (AtSERK 1–5) and M. truncatula (MtSERK 1–6) respectively, indicating that in Arabidopsis and Medicago, SERK exists as a gene family. Apart from playing a major role in embryogenesis, SERK has also been known to be involved in biotic and abiotic stress responses (Santos and Aragão 2009; Elmaghrabi et al. 2013). SERK plays many other important functions during different developmental processes such as organ differentiation in flower, tapetum development, microspore maturation and male sporogenesis in A. thaliana (Lewis et al. 2010; Colcombet et al. 2005; Albrecht et al. 2005). SERK expression has also been observed in procambium and immature vascular cells (Kwaaitaal and de Vries 2007).
Momordica charantia, a plant from Cucurbitaceae family, is mainly found in the tropics. The plant possesses many medicinal properties including antidiabetic, antitumor, anticancer, anti-inflammatory, antiviral and cholesterol lowering effects (Ahmed et al. 2001). The fruits of M. charantia have been used in traditional medicine for its hypoglycemic property (Miura et al. 2001). Several studies have demonstrated in vitro propagation of M. charantia via organogenesis using shoot tip, nodal explants etc. (Agarwal and Kamal 2004; Sultana and Bari Miah 2003). An earlier study by our group has been able to develop an efficient protocol for somatic embryogenesis in M. charantia using leaf explants (Paul et al. 2009); however, there is no report of regeneration of M. charantia by somatic embryogenesis. Since the regeneration potential of M. charantia is low, therefore, it is important to understand the molecular mechanism underlying the formation of embryogenic callus. The present investigation was therefore focused on identifying and characterizing the gene principally involved in the molecular regulation of somatic embryogenesis, and determining its expression during somatic embryogenesis in M. charantia. Although SERK has been extensively studied in many plant species, experimental evidence pertaining to the three dimensional structure of this protein has not as yet surfaced. In our study, we have successfully isolated and characterized an ortholog of SERK in M. charantia and further investigated its expression during the developmental stages of somatic embryogenesis as well as in different plant organs. In addition we have tried to predict the three dimensional structure of Momordica SERK (McSERK) using bioinformatic tools. The results of the present report would broader the horizon of biotechnological advancements in the field of crop science.
Materials and methods
Establishment of embryogenic culture
Momordica charantia seeds were procured from local farmers and propagated in the green house. Seeds used for somatic embryogenesis induction were from the same stock. The seeds were first decoated and then imbibed in sterile distilled water overnight. Following imbibition, the seeds were surface sterilized in 10 % sodium hypochlorite (commercial bleach) solution for 20 min with vigorous shaking and washed four to five times (each 5 min) with sterile distilled water to remove excess bleach. The seeds were then inoculated aseptically in germination medium containing 3 % (w/v) sucrose [Sisco Research Laboratories (SRL), Mumbai, India] and 0.9 % (w/v) agar (SRL, Mumbai, India) and kept for 10 days to germinate into seedlings.
Embryogenic callus cultures were induced from leaf discs cut from 10 day old seedlings following the standard method of Paul et al. (2009). The explants were aseptically inoculated in MS (Murashige and Skoog 1962) medium containing 0.5 mgL−1 NAA (α-Naphthaleneacetic acid) and 5 mgL−1 BAP (6-Benzylaminopurine) as plant growth regulators and incubated over a period of 21 days. The pH of the medium was adjusted between 5.6 and 5.8 (using 1 N KOH) prior to autoclaving. The cultures were maintained in a plant growth chamber (GC-300 TLH, Lab Companion, Korea) at a temperature of 22–25 °C and a relative humidity of 55–60 % under Philips fluorescent day-light tubes emitting 32 × 108 mol s−1 m−2 for 16/8-h duration in light/dark photoperiods. Subculturing was done after 21 days in the same medium using a similar combination and concentrations of plant growth regulators.
Histology
For histological analysis, globular structures from embryogenic callus of M. charantia were fixed in FAA fixative (a solution of formalin: glacial acetic acid: ethanol, 1:1:18 v/v/v) for 24 h. Fixed tissues were dehydrated gradually in an increasing ethanol series (50, 60, 70, 80, 90, 100 %) for 30 min each in case of 50, 60, 70, 80 and 90 % ethanol and for 1 h in 100 % ethanol. Dehydrated tissues were then embedded in paraffin wax. Sections of 10 μM thickness were cut using a rotary microtome (Spencer Lens, NewYork, USA) and taken on glass slides. After removing wax and rehydrating the tissues, the sections were double stained with Haematoxylin and Safranin. Stained sections were mounted on slides using Canada balsam and observed under compound light microscope (Motic, Germany).
Primer designing
The primers for M. charantia SERK (McSERK) were designed from the published sequences in NCBI (National Centre for Biotechnology Information) databank. Reported sequences of SERK amino acids were aligned using ClustalW multiple sequence alignment program and conserved domains were identified. Specific primers for McSERK were designed from the conserved regions using primer3 software (Version 4.0). During the initial screening of McSERK, a partial sequence was obtained first which showed high similarity with the reported cDNA sequences of Cucumis SERK1 (Accession No. XP_004138361.1) and Medicago SERK1 (Accession No. AAN64293.1), so these two sequences were specially used for designing the primers of the 5′ and 3′ regions of the coding domain of McSERK. Actin was used for normalization in both densitometric analysis and Real-Time PCR. The primers for Momordica actin were also designed from the published actin sequences of Cucurbitaceae family based on the process mentioned above. The sequences of all the primers are listed in Table 1.
Cloning and sequencing of Momordica SERK (McSERK) gene
Genomic DNA was extracted from 21 day old embryogenic callus following the method of Edward et al. (1991) with minor modifications. Callus tissues were finely crushed in the DNA extraction buffer [200 mM Tris–HCl (Tris-Amresco, Ohio, USA, HCl-SRL, Mumbai, India), pH 7.5; 200 mM NaCl (SRL, Mumbai, India); 25 mM EDTA (SRL, Mumbai, India), pH 8.0; 0.5 % SDS (SRL, Mumbai, India)] followed by centrifugation. The supernatant was collected and mixed with equal volume of phenol–chloroform (1:1) (SRL, Mumbai, India) and centrifuged. This was repeated twice and after that the supernatant was washed with chloroform. Finally the clear upper aqueous phase was collected carefully; 3 M ammonium acetate (Merck, Mumbai, India) and an equal volume of isopropanol (SRL, Mumbai, India) were added to it and mixed slowly. The mixture was centrifuged; pellet was washed with 70 % ethanol (SRL, Mumbai, India) and air dried. The DNA was dissolved in sterile triple distilled water. The quality and concentration of the extracted DNA was checked by agarose gel electrophoresis and spectrophotometry (HITACHI UV–VIS [U-2800, Tokyo, Japan] spectrophotometer) respectively.
Primer pair SF1 and SR1 (Table 1), designed specifically for McSERK gene, were used to amplify Momordica SERK fragment. Polymerase chain reaction was carried out with these primers. Amplification reactions were set at a final volume of 25 μl containing 30 ng genomic DNA, 1× Taq DNA polymerase buffer, 0.2 mM dNTPs, 1.5 mM MgCl2, 1 U Taq polymerase and 0.4 μM each of forward and reverse primers. The thermal amplification parameters for the PCR reaction were as follows: an initial denaturation at 94 °C for 5 min followed by 35 cycles of amplification (94 °C for 1 min, 55 °C for 1 min, 72 °C for 1 min 30 s) and a final extension step at 72 °C for 30 min. Amplifications were carried out in a Thermal Cycler (Applied Biosystem, CA, USA). The PCR products were subjected to 1.5 % (w/v) agarose gel electrophoresis and visualized in a Gel documentation system (BioRad Molecular Imager, GelDoc XR, Milan, Italy).
The fragment of McSERK, isolated from genomic DNA, was purified using PCR Clean-up Kit from Chromous Biotech (Bengaluru, India) essentially abiding by the manufacturer’s instructions with a few modifications. It was then cloned in pTZ57R/T vector using Ins T/A clone PCR Product Cloning Kit (Fermentas, USA) in the competent Escherichia coli strain XL1-Blue. The recombinant plasmid DNA was used to transform XL1-Blue competent cells. Selection of transformed clones was performed by blue/white selection. Positive clones were further scrutinized by colony PCR using positive clones as template with similar reaction conditions. Colonies producing the desired fragment in colony PCR were cultured overnight for plasmid isolation. Plasmid DNA was isolated from positive clones using Plasmid DNA isolation Kit (Qiagen, Germany). Plasmid DNA containing the desired fragment was sequenced from both directions with M13 Universal forward and Universal reverse primers and submitted to GenBank (KC347724.1). The sequence of McSERK gene was analyzed by BLAST (blastn) algorithm.
Isolation of Momordica SERK (McSERK) cDNA
Total RNA was extracted from 21 day-old embryogenic callus of M. charantia, using RNeasy Plant Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions with a few modifications. All the reagents and plastic materials were treated with 0.1 % diethylpyrocarbonate (Sigma, St. Louis, MO, USA) prior to use in the RNA extraction procedure. The RNA preparation was subjected to DNase treatment to eliminate any contaminating DNA. Spectrophotometric analysis was carried out to determine the concentration of the extracted RNA. The RNA preparation was free of any contaminating genomic DNA. This was confirmed using 1 % agarose gel electrophoresis.
Reverse transcription PCR (RT-PCR) was carried out with the extracted RNA sample using QIAGEN One Step RT-PCR Kit (Qiagen, Germany) according to the manufacturer’s instructions with a few modifications. RT-PCR was performed initially with the primer pair SF1 and SR1 (Table 1), designed specifically for McSERK. Concentration of template RNA for RT-PCR reaction was 2 μg/50 μl reaction volume. The thermal cycler conditions were as follows: reverse transcription at 50 °C for 30 min followed by 40 cycles of amplification (95 °C for 15 min, 94 °C for 1 min, 55 °C for 1 min, 72 °C for 1 min 30 s) and a final extension at 72 °C for 10 min. The RT-PCR fragments were run on 1.5 % agarose gel and visualized in Gel documentation system. The cDNA fragment of McSERK was purified using PCR Clean-up Kit and sequenced. For isolating the complete coding region of McSERK, further RT-PCR reactions were carried out with the primer pairs SF2/SR2 and SF3/SR3 (Table 1). SF2/SR2 and SF3/SR3 set of primers were used to isolate the 5′ and 3′ end of the coding sequence of McSERK respectively. The reaction conditions for RT-PCR for these two sets of primers were the same as above. The RT-PCR fragments isolated using these two sets of primers were purified and sequenced. Finally the complete coding sequence of McSERK was deduced from the obtained sequences. The complete coding sequence of McSERK cDNA was submitted to GenBank (JX863894.3). The sequence was subjected to BLAST (blastn) algorithm for searching homology with known SERK sequences.
Isolation of Momordica actin
Momordica β-actin was isolated by reverse transcription PCR using the primer pair AF/AR. Amplification parameters were: reverse transcription at 50 °C for 30 min followed by 40 cycles of amplification (95 °C for 15 min, 94 °C for 1 min, 55 °C for 1 min, 72 °C for 1 min 30 s) and a final extension at 72 °C for 10 min. The isolated actin fragment was purified and sequenced. In case of McActin, a 102 bp fragment was obtained.
Prediction of putative signal peptide and protein
The complete coding region of McSERK was subjected to “ORF Finder” program (http://www.ncbi.nlm.nih.gov/gorf/gorf.html) for determining the open reading frame (ORF). The presence of a SP was confirmed using “SignalP 4.1 Server” (http://www.cbs.dtu.dk/services/SignalP/) program (Petersen et al. 2011). The TM region was predicted by “TMpred” (http://www.ch.embnet.org/software/TMPRED_form.html). Prediction of the putative protein of Momordica SERK was done with the help of PSORT (Prediction of Protein Sorting Signals and Localization Sites in Amino Acid Sequences) (http://www.psort.hgc.jp/form.html) and Scan Prosite (http://www.prosite.expasy.org/scanprosite/). A schematic representation of McSERK protein was drawn using MyDomains image creator tool (http://www.prosite.expasy.org/mydomains/).
Phylogenetic analysis
To analyze the relationship between McSERK protein and known SERK protein sequences of other species, an alignment was done with the predicted amino acid sequence of McSERK and other reported SERK sequences in the ClustalW multiple sequence alignment program. A phylogenetic tree was constructed using PHYLIP program (version 3.69) enabling 100 bootstraps in SEQBOOT program provided by the PHYLIP package. The multiple sequence alignment file (phylip format) was first run in SEQBOOT generating 100 bootstrap replicates. The outfile of this program was run in Protpars for parsimony based phylogenetic analysis restricting the number of jumbles to 10. The output tree of this program was run using Consense and the unrooted phylogenetic tree was finally constructed using the neighbor-joining (NJ) method as provided by the program njplot (http://pbil.univ-lyon1.fr/software/njplot/html).
Molecular modeling of McSERK
The deduced amino acid sequence of McSERK was submitted to the PSIPRED Protein Sequence Analysis Workbench (http://www.cs.ucl.ac.uk/psipred/). PSIPRED performs comparative automated homology modeling that uses BioSerf (version2.0). The best template search was performed by pGenTHREADER (Profile Based Fold Recognition) and GenTHREADER (Rapid Fold Recognition). BRASSINOSTEROID INSENSITIVE 1-associated receptor kinase 1 (Protein Data Bank ID 3UIM, chain A) was taken as a template to generate the three dimensional structure of McSERK. Finally the homology model was generated by selecting the best scoring hits using PSI-BLAST, pGenTHREADER and HHPred against the current template. The structure was reconstructed using the DeepView/Swiss-Pdb Viewer (version 4.10). A hydropathy plot was generated in ExPASy-ProtScale (http://web.expasy.org/protscale/) using Kyte and Doolittle (1982) scale with default parameters and window size 9.
McSERK expression analysis by densitometry
The relative mRNA expression pattern of McSERK at different stages of embryo development (14, 21, 28, 35 and 42 day) was determined densitometrically from the band intensity of the agarose gel. Total RNA was extracted from different stages of embryogenesis mentioned earlier and the RNA samples were subjected to reverse transcription PCR using the primer pair RTSF/RTSR. The thermal cycler conditions were as follows: reverse transcription at 50 °C for 30 min followed by 40 cycles of amplification (95 °C for 15 min, 94 °C for 1 min, 60 °C for 1 min, 72 °C for 1 min 30 s) and a final extension at 72 °C for 10 min. The RT-PCR fragments were run on 1.5 % agarose gel and visualized in the Gel documentation system. Densitometric analysis was performed with ImageJ software. Expression profile was normalized against Momordica β-actin gene. Data was represented as mean ± SEM. Statistical significance was performed by one way ANOVA using Kyplot software. Group means were compared by Student’s t test. Differences of the data at P ≤ 0.05 were considered significant.
McSERK expression analysis by real-time PCR
The relative expression of McSERK transcript at different stages of somatic embryogenesis was measured using Real-Time PCR in a Step One Plus Real-Time PCR thermocycler (Applied Biosystems, CA, USA). Expression of McSERK was also analyzed in different plant organs of M. charantia. Primers for Real-Time PCR of McSERK were designed using primer express software (Version 3.0) and are listed in Table 1. The primer pair RTSF/RTSR was used for Real-Time PCR. First strand cDNA was synthesized with 2 μg total RNA using high-capacity RNA-to-cDNA kit (Applied Biosystems, CA, USA) according to the manufacturer’s instruction. Each reaction mixture consisted of 2× Power Cyber Green PCR master mix (Applied Biosystems, CA, USA), diluted cDNA, and 10 pmol each of the forward and reverse primers. The thermal cycler condition was as follows: an initial hold at 95 °C for 10 min, 1 cycle followed by 40 cycles of denaturation at 95 °C for 30 s, annealing and elongation at 60 °C for 1 min. The gene encoding Momordica β-actin was used as endogenous control. The relative expression of McSERK transcript was normalized with Momordica β-actin gene expression in each sample using actin gene specific primers (Table 1). A melt curve analysis was also included in order to ensure the amplification of the desired fragment.
The relative gene expression pattern was presented using the 2−ΔΔCT method (Livak and Schmittgen 2001). The data of relative gene expression was analyzed by Step One software (Version 2.1) (Applied Biosystems, CA, USA). All the experiments were performed in triplicate and calibrated to the expression in a 14 day old callus. A negative control was maintained in each reaction. The results documented here are means of triplicates, and the bars indicate mean ± SEM. Statistical significance of the data was analyzed by analysis of variance (ANOVA) using KyPlot software.
Results
Induction of somatic embryogenesis
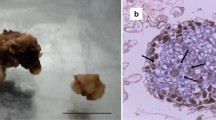
Leaf explants were used for inducing embryogenic callus culture in M. charantia. Explants started to swell within 7–10 days of culture and callus formation was initiated within 14 days from the edges of the explant. Somatic embryogenesis was successfully induced in MS media supplemented with 0.5 mgL−1 NAA and 5 mgL−1 BAP. Initiation of somatic embryo formation was observed at 21 day. The calli were light green in colour and the texture was hard and compact. Somatic embryos developed during the initial phase of somatic embryogenesis were globular in structure (Fig. 1a, b). Complete maturation of somatic embryos took 42 days. The data is in conformity with our previous reports as described by Paul et al. (2009).
Morphological features of somatic embryogenesis in M. charantia (a) Initiation of callus formation after 14 days of culture (b) Globular embryogenic callus formation after 21 days of culture (c) Histological section of 21 day old embryogenic callus of M. charantia with globular structure (Bar 100 μM)
Histology
Histological sections of 21 day old embryogenic callus revealed the emergence of globular somatic embryo from the periphery of the embryogenic callus with prominent vasculatures. The development of globular somatic embryo was found to be attached to the callus mass at its base (Fig. 1c). Embryogenic cells were isodiametric, nucleated and contained dense cytoplasm.
Sequence analysis of Momordica SERK gene
Polymerase chain reaction (PCR) was carried out with gene specific primers to isolate SERK gene from M. charantia. A 912 bp fragment of McSERK gene was obtained after sequencing which was submitted to GenBank (Accession No. KC347724.1). The 912 bp sequence of McSERK gene contained an intron of 309 bp length, deduced by aligning the DNA and cDNA sequence of McSERK. The McSERK sequence showed high homology (80–90 %) with almost all the reported SERK sequences in other plant species when subjected to BLAST (blastn) analysis.
Reverse transcription PCR was performed to isolate a cDNA fragment of Momordica SERK (McSERK) from embryogenic callus culture using the SF1/SR1 primer pair. The fragment was purified and sequenced. During this primary screening, initially a 608 bp cDNA fragment of McSERK was obtained. The sequence showed maximum similarity with Cucumis sativus (Accession No. XP_004138361.1) and M. truncatula (Accession No. AAN64293.1) SERK1 genes, so the primers were designed especially from these two sequences for obtaining the complete coding region of McSERK. Primer pairs SF2/SR2 and SF3/SR3 were used for isolating the 5′ and 3′ end of the coding region respectively. Finally a total of 1,884 bp coding sequence of McSERK was deduced from the sequences of the fragments isolated using SF1/SR1, SF2/SR2 and SF3/SR3 primer pairs. The sequence was submitted to GenBank (JX863894.3). The 1,884 bp McSERK sequence, when subjected to BLAST (blastn) tool, showed high similarity (70–90 %) with all reported SERK sequences in other plant species but McSERK was found to be similar to that of SERK1 genes in other species. Both McSERK DNA and cDNA shared highest homology with SERK1 sequences of C. sativus and M. truncatula. The high sequence similarity of McSERK with other SERK sequences proved that it was a member of the SERK gene family.
Sequence analysis of McSERK predicted protein
The cDNA sequence of McSERK confirmed the presence of an 1,884 bp ORF. The 1,884 bp cDNA sequence of McSERK was found to encode a 627 amino acid protein upon in silico translation. The McSERK protein, when subjected to BLAST (pblast) analysis, showed high identity (80–90 %) with SERK sequences of other plant species available in the NCBI data bank. Maximum similarity of McSERK was observed with C. sativus SERK1 (XP_004138361.1), M. truncatula MtSERK1 (AAN64293.1), Glycine max GmSERK (ACJ64717.1), Carica papaya CpSERK (ABS32228.1), Citrus sinensis CsSERK (ACP20180.1) and C. unshiu CuSERK1 (BAD32780.1). Multiple sequence alignment of McSERK protein with reported SERK sequences displayed the presence of all characteristic domains of SERK proteins (Fig. 2). The sequence contained an N-terminal SP which was immediately followed by a LZ, five LRRs, a single TM domain, a kinase domain and the C-terminal region. Very weak similarity was observed between the SPP motif of McSERK with other SERK members indicating the signature characteristic of SERK family proteins. The possible cleavage site of the SP was found between amino acid position 28 and 29 as predicted by SignalP (Fig. 3a). TMpred analysis showed a strongly preferred model of membrane spanning TM helix between amino acid 234–276 (Fig. 3b). McSERK was found to be a member of the serine/threonine kinase protein family having eleven different kinase domains as observed in the ScanProsite prediction. The probable protein kinase-ATP binding site was predicted between amino acid positions 309–331. The schematic representation of McSERK protein with all characteristic domains of SERK is annotated in Fig. 3c.
Multiple sequence alignment of predicted amino acid sequence showing sequence similarity of McSERK with reported SERK sequences. Characteristic domains of McSERK protein are indicated by respective arrows (SP signal peptide, LZ leucine zipper, LRR1 to LRR5 leucine rich repeats 1–5, SPP serine-proline-proline, TM transmembrane, Kinase, C-terminal end). Accession numbers included in this analysis are as follows: AAB61708.1 (DcSERK), Daucus carota; AAK68074.1 (AtSERK3), Arabidopsis thaliana; AAN64293.1 (MtSERK), Medicago truncatula; AAV58833.2 (CnSERK), Cocos nucifera; ABS32228.1 (CpSERK), Carica papaya; ACJ64717.1 (GmSERK), Glycine max; ACP20180.1 (CsSERK), Citrus sinensis; ACY91853.1 (AaSERK), Araucaria angustifolia; AEB40066.1 (CpSERK), Cyclamen persicum; AEP14551.1 (TaSERK), Triticum aestivum; BAD32780 (CuSERK), Citrus unshiu; NP_001105132.1 (ZmSERK), Zea mays; NP_001233866.1 (SlSERK), Solanum lycopersicum; NP_174683.1 (AtSERK2), Arabidopsis thaliana; NP_177328.1 (AtSERK1), Arabidopsis thaliana; NP_178999.2 (AtSERK4), Arabidopsis thaliana; NP_179000.3 (AtSERK5), Arabidopsis thaliana; XP_004138361.1 (CsSERK), Cucumis sativus
Phylogenetic tree construction
A phylogenetic tree was constructed using PHYLIP 3.69 package and evaluated by 100 bootstrap values. The phylogenetic tree clearly indicated that McSERK shares high sequence similarity with different plant species (Fig. 4). Maximum similarity of McSERK was observed with the predicted C. sativus SERK1, GmSERK and MtSERK1 sequence indicating that these four proteins may have originated from common ancestors. Therefore, McSERK is supposed to be an ortholog of these SERK proteins. Further, CpSERK (Accession No. ABS32228.1), CsSERK (Accession No. ACP20180.1) and CuSERK1 (Accession No. BAD32780.1) are three close neighbors of McSERK.
Phylogenetic tree of McSERK with other reported SERK sequences depicting the interrelationship of different SERKs. Percentage bootstrap values are represented along the branch length. Position of McSERK is indicated by an arrow. The GenBank accession numbers of these sequences are in accordance with the accession numbers in Fig 2
Molecular modeling of McSERK
The calculated molecular mass of the predicted protein of McSERK was found to be 68.870 kD with a hypothetical pI value of 5.49. Template based homology modeling using the template 3UIM (BAK1) from Protein Data Bank suggested the probable three dimensional structure of McSERK protein (Fig. 5a). McSERK showed 93.3 % sequence identity with the template and a p value of 8e−23. The 3D model predicted by PSIPRED represented McSERK as a monomeric protein. The LRR region was found to be made up of β-sheets and the SPP domain was predicted as coil. The hydropathy plot of McSERK, generated by ProtScale, showed a high hydrophobicity score in the TM region whereas the score was relatively low in the C-terminal end of the McSERK protein (Fig. 5b).
Expression of SERK gene by densitometry and real-time PCR
Reverse transcription PCR was carried out to study the expression level of SERK gene during embryogenic development in M. charantia and the expression level was determined from the relative band intensity of agarose gel (Fig. 6a). It was clear from the RT-PCR data that SERK is expressed in all the stages of embryogenesis in M. charantia; however initially, SERK expression was low in 14 day old callus. A sudden increase (1.3-fold with respect to the 14 day old callus) in expression of SERK was observed during the initiation phase of embryogenesis. But maximum band intensity was observed in 28 day old embryogenic callus indicating the highest expression (1.4-fold as compared to the 14 day old callus) of SERK transcript during this developmental period (Fig. 6b). SERK expression level was found to decrease with an increasing period of callus culture and lowest expression was observed in fully matured embryogenic callus (42 day).
a Gel photograph of McSERK expression during somatic embryogenesis at different developmental stages. b Graphical representation of band intensity represents the change in expression of McSERK at different stages of somatic embryogenesis in M. charantia. Data represents the mean ± SEM. Asterisks indicate significant differences at p < 0.05 (∗), p < 0.01 (∗∗), or p < 0.001 (∗∗∗) compared to 14 day old callus. c Real Time PCR expression profile of McSERK transcript at different stages of somatic embryogenesis and in different plant organs of M. charantia. Results are represented as the mean ± SEM
In order to precisely analyze the accumulation of SERK transcript, Real Time PCR was performed with samples of different developmental stages of somatic embryogenesis (14, 21, 28, 35 and 42 day) and in different plant organs. The real time PCR profile indicated the accumulation of McSERK transcript all through the developmental process of somatic embryogenesis (Fig. 6c); however, expression of SERK was relatively low in 14 day old callus. During the initiation of somatic embryogenesis expression of SERK was found to increase suddenly which was 2.1-fold higher than the 14 day old callus. A significant increase (3.4-fold) in SERK expression was observed in 28 day old embryogenic callus compared to the 14 day old callus. Accumulation of McSERK transcript was maximum in 28 day old callus of M. charantia which was suddenly found to decline in 35 day old callus. McSERK accumulation was decreased with increasing days of callus culture and was found to be lowest at 42 day. McSERK expression was also detected in different plant organs of M. charantia. Accumulation of McSERK transcript in leaves was considerably low (0.2-fold) compared to embryogenic callus but it was found to be the highest among all the plant organs tested. However, McSERK expression was not detected in flower and fruit of M. charantia.
Discussion
Somatic embryogenesis has become an integral part of transgenic crop production which is very often applied for crop improvement in various commercial applications. But the molecular mechanism underlying the regulatory process of somatic embryogenesis is still illusory (Santa-Catarina et al. 2004). Momordica charantia is a valuable crop of tropical countries. It possesses high nutritive value and medicinal importance. Hence it is very often used by researchers for different biotechnological purposes. It has been observed that the plant shows recalcitrance towards somatic embryogenesis, therefore, studying the process at molecular level would provide better knowledge of somatic embryogenesis in this plant. The present investigation reported establishment of somatic embryogenesis in M. charantia. Morphological and histological observations revealed the presence of globular somatic embryo in the embryogenic callus derived from leaf explants of M. charantia. According to Rohani et al. (2012), globular structures are most prominent stage of leaf explant derived somatic embryogenesis during its early phase. The embryogenic cells, reported in the present study, were closely packed with dense cytoplasm which was also observed by Chung et al. (2007) during somatic embryogenesis of Dendrobium. The globular somatic embryos clearly showed the presence of vascular tissues. Dos Santos et al. (2006) and Elviana et al. (2011) also suggested the occurrence of vascular systems in globular somatic embryos while working with G. max and mangosteen.
The prerequisite of molecular regulation of somatic embryogenesis is the expression of SERK gene, responsible for playing an effective role in the signaling process of embryo formation. Molecular analysis of somatic embryogenesis in different plant species documented the involvement of SERK gene in this process. Salaj et al. (2008) and Somleva et al. (2000) observed that SERK is expressed during the early stages of embryogenesis. In our study we have successfully isolated and characterized a SERK, designated as McSERK, from embryogenic callus of M. charantia. Since the first report of SERK in carrot (Schmidt et al. 1997) many researchers have successfully isolated SERK from many different plant species. Among the dicots, Arabidopsis and Medicago are known to contain five and six different SERK genes respectively (Hecht et al. 2001; Nolan et al. 2003) whereas in case of monocots the highest number of SERK genes have been isolated in Z. mays and T. aestivum (Baudino et al. 2001; Singla et al. 2008). The complete sequence of a SERK (McSERK) gene from M. charantia was documented in the present study. The high sequence similarity between McSERK and other SERK sequences including AtSERK1, suggested that it is a SERK homolog. McSERK shared a high degree of similarity with Cucumis SERK1 and MtSERK1 which indicated that McSERK could be an ortholog of Cucumis and Medicago SERK1. Among the various isoforms of SERKs, SERK1 has been known to play a substantial role in embryogenic competence in a variety of plant species. MtSERK1 and AtSERK1 both are known to be involved in somatic embryogenesis. On the basis of the sequence similarity of McSERK with these two sequences, it could be proposed that McSERK imparts positive influence on somatic embryogenesis process in M. charantia.
The sequence analysis of McSERK protein confirmed that it is a member of the LRR–RLK superfamily. The protein showed the presence of all characteristic features of SERK family proteins. The most distinct character of the SERK proteins which makes them special from other RLKs is the existence of an SPP motif that varies among species (Albrecht et al. 2008). This unique feature was also observed in McSERK which validated the fact that the protein isolated in the present study belongs to the SERK family. It has been suggested by earlier researchers that this SPP motif acts as a hinge to provide flexibility to the extracelluar region of SERK proteins (Hecht et al. 2001). The N-terminal SP, the LZ, the LRRs and SPP of McSERK were supposed to be located in the extracellular region whereas the kinase and C-terminal domains were found to be in the intracellular portion. The TM domain of McSERK separated the intracellular and extracellular portions of the protein. Schmidt et al. (1997) proposed that the LRR domains play an important role in protein–protein interaction.
The phylogenetic analysis documented the relationship of McSERK with the SERK sequences of other plant species. McSERK was grouped in the same cluster where Cucumis SERK1, GmSERK and MtSERK1 were situated. This result indicated that SERKs of these four different plant species might have originated from a common ancestor during speciation. The close relationship of McSERK with reported SERK sequences proved that this gene is highly conserved throughout the plant kingdom. Cueva et al. (2012) also suggested a similar kind of explanation after analyzing the phylogenetic relationship of different monocot and dicot SERKs. In their study they have observed that all monocot SERK and all dicot SERKs reside in two different clusters. They have also reported that the SERK gene family of O. sativa appears in the same branch and explained this as an effect of gene duplication after speciation.
The molecular model of McSERK provided an insight of the hypothetical three dimensional structure of the SERK protein. The model was found to be highly similar with that of BRASSINOSTEROID INSENSITIVE 1-associated receptor kinase 1.
Somatic embryogenesis is triggered by a series of signal cascades with the active participation of a myriad of genes. Plant growth regulators play an important role to stimulate these genes. SERK is known to be upregulated by auxin in both root-forming and embryogenic cultures (Nolan et al. 2003). In a recent study by Nolan et al. (2009), it has been observed that not only auxin but cytokinin also promotes somatic embryogenesis. They have also demonstrated the positive effect of NAA and BAP in somatic embryogenesis induction as well as MtSERK expression. In our study we have used NAA and BAP to induce somatic embryogenesis in M. charantia. According to Zhang et al. (2011) there is a clear correlation between SERK expression and somatic embryogenesis. They have showed that alteration in plant growth regulator combination causes change in expression patterns of different SERKs. An up-regulation of McSERK was observed during the induction phase of somatic embryogenesis. Accumulation of McSERK transcript increased when cells became competent to form embryogenic mass after 21 days of callus culture and reached its highest level at 28 day. With the passage of time SERK expression gradually declined and after complete maturation of somatic embryos SERK expression reduced significantly. The expression pattern of McSERK, i.e., an initial up-regulation followed by an intermittent decrease with subsequent embryogenic developmental phases, could be used to correlate the association of SERK with embryogenic competence of somatic cells in M. charantia. During the initial period of somatic embryo formation, a sudden elevation in SERK transcript level portrays the positive role of SERK in triggering the somatic embryogenesis process in plants. The results of the present study are in accordance with the earlier reports of Hecht et al. (2001), Cueva et al. (2012) and Ma et al. (2012). It has been observed that AtSERK1 expression increases up to the formation of the heart stage after which expression ceases (Hecht et al. 2001). The similarity in the expression pattern of McSERK with that of AtSERK1 points to the fact that McSERK could be a functional homolog of AtSERK1. Expression of McSERK was also detected in different plant organs of M charantia but to a basal level. A very low level of McSERK transcript was detected in leaf, stem and root of M. charantia; however, McSERK was not detected in flower and fruit. In case of AtSERK1 too, a lower level of expression in other plant organs was observed than in embryogenic callus (Hecht et al. 2001). McSERK expression was also found to be significantly low in the plant organs tested with respect to embryogenic callus. This indicates that McSERK could be directly involved in the establishment of somatic embryogenesis in M. charantia. Many different isoforms of SERK gene have been isolated in different plant species which have varying functions. Few other genes namely Leafy cotyledon 1 (LEC1), LEC2 and Baby Boom (BBM) are also known to be ectopically expressed during somatic embryo formation (Zhang et al. 2011). But maximum reports emphasize the direct involvement of SERK in somatic embryogenesis. In a recent attempt, Song et al. (2008) was able to identify a SERK homolog in banana that takes part in disease resistance response in addition to playing an important role in somatic embryogenesis. Huang et al. (2010) have also made a similar observation. During the process of somatic embryogenesis somatic or stem cells are programmed to form pluripotent cells which are further converted to totipotent cells capable of forming somatic embryos through a complex signal transduction. It has been proposed earlier that only a small population of cells in an embryogenic culture retain the ability of embryogenic competence and form somatic embryos (Hecht et al. 2001). Therefore the pattern of expression of McSERK transcript in embryogenic cell culture described in the present study indicates that all the stem cells of M. charantia explants are first converted to pluripotent cells; however, some of them become totipotent to be converted into somatic embryos. The expression of McSERK was found to be high in all these cells because these pluripotent cells eventually undergo developmental changes through different signaling pathways and some of them form embryos. This corresponds to the fact that SERK transduces a signal that effectuates changes in the pluripotent cells and on further transformations, some of these cells become totipotent to form somatic embryo.
The results evidenced in the present article would provide better insight into the regulatory aspects of SERK induction for making cells competent during in vitro somatic embryogenesis in M. charantia. This then would help to formulate a new approach for regenerating M. charantia that would eventually open up a new vista in plant biotechnology.
References
Agarwal M, Kamal R (2004) In vitro clonal propagation of Momordica charantia L. Indian J Biotechnol 3(3):426–430
Ahmed I, Lakhani MS, Gillett M, John A, Raza H (2001) Hypotriglyceridemic and hypocholesterolemic effects of anti-diabetic Momordica charantia (karela) fruit extract in streptozotocin induced diabetic rats. Diabetes Res Clin Pract 51:155–161
Albertini E, Marconi G, Reale L, Barcaccia G, Porceddu A, Ferranti F, Falcinelli M (2005) SERK and APOSTART: candidate genes for apomixis in Poa pratensis. Plant Physiol 138:2185–2199
Albrecht C, Russinova E, Hecht V, Baaijens E, Vries SD (2005) The Arabidopsis thaliana somatic embryogenesis receptor-like kinases1 and 2 control male sporogenesis. Plant Cell 17:3337–3349
Albrecht C, Russinova E, Kemmerling B, Kwaaitaal M, de Vries S (2008) Arabidopsis somatic embryogenesis receptor kinase proteins serve brassinosteroid-dependent and -independent signaling pathways. Plant Physiol 148:611–619
Baudino S, Hansen S, Brettshneider R, Hecht VFG, Dresselhaus T, Lors H, Dumas C, Rogowsky PM (2001) Molecular characterization of two novel maize LRR receptor-like kinase, which belong to the SERK gene family. Planta 213:1–10
Chugh A, Khurana P (2002) Gene expression during somatic embryogenesis: recent advances. Curr Sci 83(6):715–730
Chung HH, Chen JT, Chang WC (2007) Plant regeneration through direct somatic embryogenesis from leaf explants of Dendrobium. Biol Plant 51:346–350
Colcombet J, Boisson-Dernier A, Ros-Palau R, Vera CE, Schroeder JI (2005) Arabidopsis somatic embryogenesis receptor kinase 1 and 2 are essential for tapetum development and microspore maturation. Plant Cell 17:3350–3361
Cueva A, Concia L, Cella R (2012) Molecular characterization of a Cyrtochilum loxense somatic embryogenesis receptor-like kinase (SERK) gene expressed during somatic embryogenesis. Plant Cell Rep 31:1129–1139
Dos Santos KGB, Mariath JEA, Moco MCC, Bodanese-Zanettini MH (2006) Somatic embryogenesis from immature cotyledons of soybean (Glycine max (L.) Merr.): ontogeny of somatic embryos. Braz Arch Biol Technol 49(1):49–55
Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19(6):1349
Elmaghrabi AM, Ochatt S, Rogers HJ, Francis D (2013) Enhanced tolerance to salinity following cellular acclimation to increasing NaCl levels in Medicago truncatula. Plant Cell Tiss Organ Cult 114:61–70
Elviana M, Rohani ER, Ismanizan I, Normah MN (2011) Morphological and histological changes during the somatic embryogenesis of mangosteen. Biol Plant 55:731–736
Hecht V, Vielle-Calzada JP, Hartog MV, Schmidt ED, Boutilier K, Grossniklaus U, de Vries SC (2001) The Arabidopsis somatic embryogenesis receptor kinase1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol 127:803–816
Hu H, Xiong L, Yang Y (2005) Rice SERK1 gene positively regulates somatic embryogenesis of cultured cell and host defense response against fungal infection. Planta 222:107–117
Huang X, Lu XY, Zhao JT, Chen JK, Dai XM, Xiao W, Chen YP, Chen YF, Huang XL (2009) MaSERK1 gene expression associated with somatic embryogenic competence and disease resistance response in banana (Musa spp.). Plant Mol Biol Rep 28:309–316
Huang X, Lu XY, Zhao JT, Chen JK, Dai XM, Xiao W, Chen YP, Chen YF, Huang XL (2010) MaSERK1 gene expression associated with somatic embryogenic competence and disease resistance response in banana (Musa spp.). Plant Mol Biol Rep 28:309–316
Kwaaitaal MACJ, de Vries S (2007) The SERK1 gene is expressed in procambium and immature vascular cells. J Exp Bot 58(11):2887–2896
Kyte J, Doolittle R (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132
Lewis MW, Leslie ME, Fulcher EH, Darnielle L, Healy P, Youn JY, Liljegren SJ (2010) The SERK1 receptor-like kinase regulates organ separation in Arabidopsis flower. Plant J 62(5):817–828
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Ma J, He Y, Wu C, Liu H, Hu Z, Sun G (2012) Cloning and molecular characterization of a SERK gene transcriptionally induced during somatic embryogenesis in Ananas comosus cv. Shenwan. Plant Mol Biol Rep 30:195–203
Maillot P, Lebel S, Schellenbaum P, Jacques A, Walter B (2009) Differential regulation of SERK, LEC-like and pathogenesis related genes during indirect secondary somatic embryogenesis in grapevine. Plant Physiol Biochem 47:743–752
Miura T, Itoh C, Iwamoto N, Kato M, Kawai M, Park SR, Suzuki I (2001) Hypoglycemic activity of the fruit of the Momordica charantia in type 2 diabetic mice. J Nutr Sci Vitaminol 47:340–344
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nolan KE, Irwanto RR, Rose RJ (2003) Auxin up-regulates MtSERK1 expression in both Medicago truncatula root-forming and embryogenic cultures. Plant Physiol 33:218–230
Nolan KE, Kurdyukov S, Rose RJ (2009) Expression of the somatic embryogenesis receptor-like kinase1 (SERK1) gene is associated with developmental change in the life cycle of the model legume Medicago truncatula. J Exp Bot 60(6):1759–1771
Paul A, Mitter K, Raychaudhuri SS (2009) Effect of polyamines on in vitro somatic embryogenesis in Momordica charantia L. Plant Cell Tiss Organ Cult 97:303–311
Pérez-Núñez MT, Souza R, Sáenz L, Chan JL, Zúñiga-Aguilar JJ, Oropeza C (2009) Detection of a SERK-like gene in coconut and analysis of its expression during the formation of embryogenic callus and somatic embryos. Plant Cell Rep 28:11–19
Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786
Rohani ER, Ismanizan I, Noor NM (2012) Somatic embryogenesis of mangosteen. Plant Cell Tiss Organ Cult 110:251–259
Salaj J, von Recklinghausen IR, Hecht V, de Vries SC, Schel JHN, van Lammeren AAM (2008) AtSERK1 expression precedes and coincides with early somatic embryogenesis in Arabidopsis thaliana. Plant Physiol Biochem 46:709–714
Santa-Catarina C, Hanai LR, Dornelas MC, Viana AM, Floh EIS (2004) SERK gene homology expression, polyamines and amino acids associated with somatic embryogenic competence of Ocotea catharinensis Mez. (Lauraceae). Plant Cell Tiss Organ Cult 79:53–61
Santos MO, Aragão FJ (2009) Role of SERK genes in plant environmental response. Plant Signal Behav 4(12):1111–1113
Schmidt ED, Guzzo F, Toonen MA, de Vries SC (1997) A leucine rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 124:2049–2062
Shimada T, HirabayashI T, Endo T, Fujii H, Kita M, Omura M (2005) Isolation and characterization of the somatic embryogenesis receptor-like kinase gene homologue (CitSERK1) from Citrus unshiu Marc. Sci Hortic 103:233–238
Singla B, Khurana JP, Khurana P (2008) Characterization of three somatic embryogenesis receptor kinase genes from wheat, Triticum aestivum. Plant Cell Rep 27:833–843
Somleva MN, Schmidt EDL, de Vries SC (2000) Embryogenic cells in Dactylis glomerata L. (Poaceae) explants identified by cell tracking and by SERK expression. Plant Cell Rep 19:718–726
Song D, Li G, Song F, Zheng Z (2008) Molecular characterization and expression analysis of OsBISERK1, a gene encoding a leucine-rich repeat receptor-like kinase, during disease resistance responses in rice. Mol Biol Rep 35:275–283
Sultana RS, Bari Miah MA (2003) In vitro propagation of Karalla (Momordica charantia Linn.) from nodal segment and shoot tip. J Biol Sci 3:1134–1139
Thomas C, Meyer D, Himber C, Steinmetz A (2004) Spatial expression of a sunflower SERK gene during induction of somatic embryogenesis and shoot organogenesis. Plant Physiol Biochem 42:35–42
Tichtinsky G, Vanoosthuyse V, Cock JM, Gaude T (2003) Making inroads plant receptor kinase signalling pathways. Trends Plant Sci 8:231–237
Zhang S, Liu X, Lin Y, Xie G, Fu F, Liu H, Wang J, Gao S, Lan H, Rong T (2011) Characterization of a ZmSERK gene and its relationship to somatic embryogenesis in a maize culture. Plant Cell Tiss Organ Cult 105:29–37
Acknowledgments
The authors sincerely acknowledge the financial assistance provided by Council of Scientific and Industrial Research [CSIR No. 38/(1261)/EMR II, dt. 17.05.10].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talapatra, S., Ghoshal, N. & Raychaudhuri, S.S. Molecular characterization, modeling and expression analysis of a somatic embryogenesis receptor kinase (SERK) gene in Momordica charantia L. during somatic embryogenesis. Plant Cell Tiss Organ Cult 116, 271–283 (2014). https://doi.org/10.1007/s11240-013-0401-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-013-0401-4