Abstract
The influence of varied concentrations of sucrose and ammonical (NH +4 ) nitrogen on in vitro induction and expression of anthocyanin pigments from Rosa hybrida cv. ‘Pusa Ajay’ was investigated. Of two explants (petal and leaf discs) selected and cultured under two different conditions (light and dark), leaf discs were found to be most suitable for callus initiation. Profuse and early callus induction was observed when leaf discs of rose were cultured under total dark conditions on solid Murashige and Skoog (MS) medium supplemented with 4.0 mg l−1 2,4-dichlorophenoxyacetic acid (2,4-D). Early pigment initiation, enhancement and maximum anthocyanin production from calluses were recorded when leaf discs were cultured on Euphorbia millii (EM) medium supplemented with 7% sucrose compared with calluses cultured at 4% sucrose concentration under 16/8 h (light/dark) photoperiod regime. Reducing the concentration of NH +4 nitrogen in the solid MS medium led to slight improvement in anthocyanin production in rose leaf calluses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthocyanins are water-soluble vacuolar pigments in the diverse flavonoid group, which are largely responsible for blue, purple, red and orange colouration in higher plants (Zhang and Furusaki 1999). Today, interest in anthocyanin pigments has intensified because they can be used not only as food and beverage additives to obtain attractive natural red colouration (Jackman et al. 1987) but also as nutraceuticals to treat a number of human ailments (Wang and Jiao 2000; Gantet and Memelink 2002; Hou 2003; Lila 2004). The potential of utilizing anthocyanin pigments for different purposes motivated use of plant cell or callus cultures to produce several of these vital secondary metabolites irrespective of seasonal barriers.
In vitro anthocyanin production is well documented in several crops such as Vitis sp. (Chi and Cormier 1991), Oxalis linearis (Meyer and Staden 1995), Oxalis reclinata (Makunga et al. 1997), Platanus acerifolia (Alami and Clerivet 2000), Daucus carota (Sudha and Ravishankar 2003), Fragaria ananassa (Asano et al. 2002; Edahiro et al. 2005), Ipomoea batatas (Nishiyama and Yamakawa 2004), Raphanus sativus (Betsui et al. 2004), rose (Hennayake et al. 2006), Torenia fourunieri (Nagira et al. 2006) and Cleome rosea (Simões et al. 2009). However, research on in vitro induction of anthocyanin pigments from flower crops, especially roses, using solid callus cultures is non-existent to our knowledge. Earlier attempts in rose and some other crops were based on cell suspension cultures. Therefore, an attempt has been made to induce and express anthocyanin pigments in solid calluses derived from different explants of rose so that the extra effort of maintaining the cultures with continuous shaking can be dispensed with. Earlier studies indicated that in vitro induction of anthocyanin was strongly influenced by high sucrose concentration (Nagira and Ozeki 2004; Hennayake et al. 2006) and low NH +4 nitrogen concentration in the suspension culture media (Konczak-Islam et al. 2001; Piovan and Filippini 2007). The positive effect of high sucrose concentration on anthocyanin biosynthesis was related to high osmotic potential in the culture medium (Solfanelli et al. 2006) and change in the primary metabolism of the cells in case of reducing the NH +4 nitrogen concentration (Sato et al. 1996). The primary objective of our study is to induce anthocyanin pigments from rose on solid culture medium by manipulating the sucrose and NH +4 to nitrate (NO −3 ) nitrogen concentration.
Materials and methods
Plant material
The explants, i.e. petal (tight bud stage) and fully expanded young leaves (20–25 mm long), were collected during the winter/spring season from 3–4-year-old field-grown healthy and disease-free plants maintained at the Floriculture Research Farm of the Indian Agricultural Research Institute (IARI), New Delhi, India.
Explant preparation
Explants were washed with 0.1% Teepol® solution for 5 min, followed by thorough washing under running tap water for 8–10 min. Explants were then subjected to pre-treatment comprising 0.1% Carbendazim (BASF India Ltd., Mumbai) + 0.1% Mancozeb-45 (Rallis India Ltd., Mumbai) + 200 mg l−1 8-hydroxyquinoline citrate (HQC) for 3 h followed by surface sterilization with mercuric chloride (0.2%) for 4 min. The explants were then rinsed three times with sterilized double-distilled water. The whole petal and leaf discs of 5 × 5 mm size were used as explants for callus induction.
Callus induction
Agar-based MS (Murashige and Skoog 1962) medium with 0.72% (w/v) agar-agar (HiMedia Lab., Mumbai, India) and 3% (w/v) sucrose was used as basal medium. pH was adjusted to 5.8 ± 0.1, and media were autoclaved for 20 min at 121.6°C and 15 psi. Callus was initiated from petal and leaf discs (placed adaxial side down) on solid MS medium supplemented with 1.0, 2.0, 3.0 and 4.0 mg l−1 2,4-D in test-tube (50 ml, borosilicate) containing 20 ml solid medium. The cultures were kept in the dark at 24 ± 1°C. Induced callus was sub-cultured on fresh agar-based MS medium at 25-day intervals.
Media composition for anthocyanin induction
Freshly initiated calluses were then transferred to test-tubes containing 20 ml solid EM medium (Yamamoto et al. 1981) supplemented with 0.2 mg l−1 2,4-D for multiplication and anthocyanin induction. Then, cultures were kept at 24 ± 1°C under 16/8 h (light/dark) photoperiod (108 μmol m−2 s−1) regime using cool-white fluorescent tubes (Phillips Electronics India Ltd., Kolkata, India) and sub-cultured at 15-day intervals. For anthocyanin induction in callus cultures, EM medium supplemented with 4%, 5%, 6% and 7% sucrose was used. A modified MS medium (Table 1) with 100% NH +4 nitrogen (control), MS medium with 50% strength of NH +4 nitrogen, and MS medium devoid of NH +4 nitrogen were used for anthocyanin induction. Cultures showing anthocyanin induction were harvested to measure total anthocyanin content.
Quantification of total monomeric anthocyanin
Quantification of total anthocyanin was done according to the method described by Wrolstad et al. (2005) on a UV-Vis double-beam spectrophotometer (Thermo Electrone Corp., USA) by using two buffer systems, i.e. potassium chloride buffer, pH 1.0 (0.025 M) and sodium acetate buffer (CDH Ltd., Mumbai, India), pH 4.5 (0.4 M). A known weight of sample (350–400 mg) was macerated with 80% methanol (S.D. Fine Chemicals, Mumbai, India) in a pre-chilled pestle and mortar. Then, sample was transferred to a vial and centrifuged at 10,000 rpm for 15 min on a refrigerated centrifuge (3K30; Sigma, Osterode, Germany). Final volume (25 ml) was prepared by adding 80% methanol to the supernatant. Then, final samples were prepared by taking 5 ml volume of sample and diluting separately in pH 1.0 and pH 4.5 buffers (5 ml each). Absorbance was measured at 520 and 700 nm using 1-cm-path-length cuvettes in a UV-Vis double-beam spectrophotometer. Anthocyanin content was calculated and expressed as cyanidin-3-glucoside mg kg−1 fresh weight (FW), using extinction coefficient (ε) of 29,600 l cm−1 mol−1 and molecular weight of 449.2 g mol−1. The final concentration of anthocyanin was calculated based on total volume of the extract and weight of sample using
where A = absorbance, MW = molecular weight (449.2), DF = dilution factor and ε = molar absorptivity (29,600)
Statistical analysis
Statistical analyses were carried out using one-way ANOVA, as suggested by Gomez and Gomez (1984). Each experiment comprised suitable replicates. All percentage data were subjected to arcsin √% transformation before analysis, and valid conclusions were drawn at the P ≤ 0.05 level of probability.
Results and discussion
Explant selection
Among the two explants used, minimum microbial contamination (15.60%) was recorded when leaf explants were used. This can be attributed to the short ontogenic age of the tissue, as observed by Vijaya et al. (1986) in rose. Highest culture initiation (78.77%) and earliest (8.84 days) callus induction were also recorded when leaf explants were used (Table 2). This may be due to the higher level of endogenous phyto-hormones in leaves. It is well known that in vitro culturability is dependent on the endogenous level of phyto-hormones and is enhanced by exogenously applied growth regulators (Mederos and Enriquez 1987).
Callus induction
Growth and morphogenesis of plant tissue under in vitro conditions are largely governed by culture media composition. Earliest callus induction (9.67 days) was noticed in leaf explants when MS medium was fortified with 4.0 mg l−1 2,4-D, whereas petal explants took longer (11.56 days) to induce callus. The frequency of callus formation from these explants also exhibited a similar trend. Callus induction was noticed initially on the punctured places, followed by other (Fig. 1a, b, c) parts. The highest frequency of callusing (91.11%) was recorded when leaf explants were cultured on MS medium fortified with 4.0 mg l−1 2,4-D, followed by 3.0 mg l−1 (82.22%), which was statistically non-significant (P ≤ 0.05). The frequency of callusing in petal explants was significantly lower (77.24%) at 4.0 mg l−1 2,4-D. A similar trend was observed in generation of callus biomass in Oxalis reclinata (Makunga et al. 1997) and in Hibiscus sabdariffa (Mazukami et al. 1988). The differential ability of the two explants to induce callus at the same 2,4-D concentration could have been due to different levels of endogenous auxins present in these explants. Earlier, Yi-xun et al. (2009) and Wang and Bao (2007) also observed that optimum level of 2,4-D induced high callusing in Anthurium andraeanum Hort. and Viola wittrockiana, respectively, which was further enhanced by addition of benzyl aminopurine. Only calluses obtained from leaf explants were used for anthocyanin induction, considering the early response and higher biomass accumulation.
Effects of different sucrose concentrations on anthocyanin induction
In this study the influence of varied levels of sucrose on anthocyanin induction and expression indicated that earliest expression (5.0 days) in maximum number of cultures (82.5%) was observed when EM medium was supplemented with 7% sucrose. It is well documented that sugars might play an important role in induction of anthocyanin biosynthesis in leaves or in flower petals, and also in red autumn leaves (Ishikura 1973). Earlier studies indicated that sugars such as sucrose and glucose are required as substrates for anthocyanin biosynthesis. It was also postulated that other sugars, such as sorbitol and mannitol, which cannot act as substrates for plant metabolism, play an important role as osmotic stress signals in triggering induction or promotion of anthocyanin biosynthesis in plant cells (Nagira and Ozeki 2004). Increasing sucrose concentration was found to significantly enhance anthocyanin yield in callus or suspension cultures of Euphorbia millii (Yamamoto et al. 1981), Daucus carota (Rajendran et al. 1992), Aralia cordata Thunb (Sakamoto et al. 1993), Fragaria ananassa (Mori and Sakurai 1994, 1995), Perilla frutescens (Zhong and Yoshida 1995), Vitis vinifera (Decendit and Merillon 1996), Vacciniurn pahalae (Smith et al. 1997), Vitis vinifera (Pasqua et al. 2005) and Torenia fourunieri (Nagira et al. 2006), as observed in our study.
Calluses obtained from leaves took significantly less time (9.75 days) for enhancement of anthocyanin pigments when cultured on EM medium supplemented with 7% sucrose. A similar trend was observed (Fig. 2a, b, c) when estimating the quantity of anthocyanin (50.49 mg kg−1 FW). Intense red colouration and high quantity of anthocyanin were estimated in cultures having 6% or 7% sucrose. The red pigmentation became more intense as the concentration of sucrose in the culture medium was increased to 7% (Fig. 3d). It is apparent that sugar does not act as a promoter of anthocyanin biosynthesis but rather as an inducer, as reported in Arabidopsis seedlings (Mita et al. 1997), Terminalia catappa (Dube et al. 1993) and Hedera helix leaves (Murray et al. 1994). Taking this phenomenon as a clue, we too applied high concentrations of sucrose in the culture medium as the anthocyanin inducer, which invariably led to higher anthocyanin accumulation in the calluses. The augmentative effects of sugars on anthocyanin accumulation have been reported mainly for sucrose, glucose or mannitol in cultured cells of various species (Do and Cormier 1991a, b; Rajendran et al. 1992; Suzuki 1995; Tholakalabavi et al. 1997).
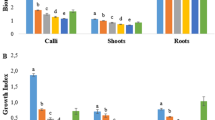
Effects of sucrose concentration on anthocyanin production from rose leaf calluses cultured on solid EM medium supplemented with 0.2 mg l−1 2,4-D: a frequency of response (%); b days taken for initiation and enhancement of anthocyanin; c total anthocyanin content (mg kg−1 FW). Vertical bars represent standard error. Asterisk indicates recommended dose of sucrose in EM basal medium
The decrease of cell growth in media containing high sucrose concentration, as observed in our study, could be due to inhibition of nutrient uptake owing to increase in the osmotic potential or high viscosity of the medium. The increase in anthocyanin induction and expression was specific to sucrose-induced osmoticum rather than salt-induced (high-salinity) osmoticum, as suggested by Nagira and Ozeki (2004). Besides, the earlier available hypothesis indicates that accumulation of phenylalanine in cells as a result of growth suppression at higher osmoticum is utilized in anthocyanin production, and the concomitant reduction in cell density increases the amount of permeating light, which further increases anthocyanin content in pigmented cells (Sato et al. 1996). The capacitance of the cultured cells indicated a difference in membrane structure between cells cultivated with different sucrose concentrations, supporting the hypothesis that cell membrane permeability was increased at higher sucrose concentration (Zhang and Furusaki 1999).
In plants, sugars represent not only energy sources and structural components but also act as physiological signals regulating expression of a variety of genes (Jang et al. 1997). Hennayake et al. (2006) postulated that glycosylation of cyanidin might be promoted if cells absorbed sugar from sucrose-rich medium, thereby improving anthocyanin production. Solfanelli et al. (2000) explained that sugar acts as a signalling molecule, whose signal transduction pathway may lead to activation or inactivation of gene expression, as observed in Arabidopsis thaliana. Vitrac et al. (2000) reported that sucrose signal transduction leading to anthocyanin accumulation involves phosphorylation of hexoses by hexokinase. The effect of high concentration of sucrose on both cell growth and anthocyanin production was also reported in other in vitro studies (Sakamoto et al. 1993; Narayan and Venkataraman 2002).
It was observed that, as the concentration of sucrose was reduced, anthocyanin induction drastically reduced in the cultures. The minimum anthocyanin accumulation (1.48 mg kg−1 FW) was estimated at 4% followed by 5% (7.20 mg kg−1 FW) sucrose (Figs. 2c, 3a, b). Higher concentration of sucrose (6% and 7%) in the culture medium might have induced high osmotic stress, which resulted in slow growth of callus and rhizogenesis. It is interesting to observe that in vitro induced roots and root hairs also exhibited anthocyanin biosynthesis when sucrose supplementation was either 6% or 7% (Fig. 3e, f). Our literature search revealed that there is no such anthocyanin biosynthesis in roots and root hairs in other species that were utilized for anthocyanin induction and expression. Such de novo biosynthesis and expression of anthocyanin in roots and root hairs also open up a new avenue for in vitro pigment production (Fig. 3e, f).
Increase in sucrose concentration in basal media above normally used concentrations (2–3%) has been reported to enhance production of various phenolic compounds including anthocyanin (Mizukami et al. 1991) in Hibiscus sabdariffa. Those researchers clearly demonstrated that, among the different carbon sources, only sucrose and glucose were equally effective in inducing anthocyanin production, whereas fructose and maltose improved anthocyanin formation only marginally. Other carbon sources such as xylose, galactose, mannose and lactose did not support anthocyanin production. It was also hypothesized that the altered C:N ratio may also influence the phenolic metabolism, resulting in increased anthocyanin production. Such a favourable response in anthocyanin production is limited to a few carbon sources such as sucrose and glucose but not with other sugars, which prompts further investigations.
It is well documented that osmotic stress imposed by sugars provides the impetus necessary to induce anthocyanin biosynthesis. However, the mechanism operating in the cells that activates anthocyanin biosynthesis is not yet clear. In this study, we have demonstrated that the media requirement for callus induction (MS medium supplemented with different concentrations of 2,4-D) and anthocyanin production (EM medium with different sucrose and NH +4 to NO −3 nitrogen concentration) were different. Such media preference could be due to a shift of cells from growth state to metabolite production state, as suggested by Simões et al. (2009).
Effects of NH +4 to NO −3 nitrogen
The nitrogen source is another important factor affecting anthocyanin production by plant cell cultures. In general, depletion of some nutrients led to enhancement of secondary metabolites, but with growth limitations (Narayan et al. 2005). This study revealed that reducing the NH +4 nitrogen and increasing the NO −3 nitrogen concentration in the MS medium (Table 1) resulted in improvement of anthocyanin biosynthesis. Maximum frequency of response (36.12%) at an early period (10.20 days) was recorded when cultures were maintained on MS medium devoid of NH +4 nitrogen. Quantification of total anthocyanin in proliferated calluses also exhibited a similar trend. It was hypothesized that the ratio of NH +4 to NO −3 nitrogen improved anthocyanin production, which was mainly due to change in primary cellular metabolism (Yamakawa et al. 1983; Sakamoto et al. 1994; Sato et al. 1996; Konczak-Islam et al. 2001; Nagira and Ozeki 2004).
Anthocyanin induction was not observed in cultures that were grown on MS medium with full-strength NH +4 nitrogen (control). When the medium was devoid of NH +4 nitrogen, the anthocyanin enhancement was early (Fig. 4b). Highest anthocyanin production (6.58 mg kg−1 FW) was recorded in cultures maintained on MS medium devoid of NH +4 nitrogen (Fig. 4c). However, in medium having full-strength NH +4 nitrogen, anthocyanin production was significantly low (0.75 mg kg−1 FW). Thus, the ratio of NH +4 to NO −3 nitrogen markedly affected production of anthocyanin pigments. These findings are in concurrence with earlier findings of Yamamoto et al. (1981) and Rao and Ravishankar (2002). Similar response to reduced NH +4 nitrogen was also reported by Simões et al. (2009), who postulated that the increase in anthocyanin content in callus culture of Cleome rosea was probably due to nutritional stress induced by reduction of salt concentration to quarter strength.
Effects of NH +4 nitrogen level on anthocyanin production from rose leaf calluses cultured on solid MS medium supplemented with 0.2 mg l−1 2,4-D: a frequency of response (%); b days taken for initiation and enhancement of anthocyanin; c total anthocyanin content (mg kg−1 FW). Vertical bars represent standard error. Asterisk indicates recommended dose of NH +4 nitrogen in MS basal medium
Production of anthocyanin pigments was considerably higher when cultures were grown on EM medium compared with MS medium. Our results support the hypothesis that modification of the nutrient composition in the plant cell culture medium can play an important role in induction of anthocyanin pigments under in vitro conditions, as proposed by Collin (2001).
The protocol standardized for successful in vitro induction of anthocyanin pigments by manipulating the carbon source (sucrose) and altering the nutrient status (reduced NH +4 , increased NO −3 nitrogen) of the culture medium opens up an exciting avenue to produce these nutraceutical pigments at will without seasonal boundaries. Such pigment production can be more reliable, simpler and more predictable. In vitro cultures can yield pigments of defined purity and standard in a short span of time. At the same time, anthocyanin pigments can be produced in large volume without interference from other compounds that occur in field-grown plants.
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- ANOVA:
-
Analysis of variance
- EM:
-
Euphorbia millii medium
- FW:
-
Fresh weight
- MS:
-
Murashige and Skoog medium
- NH +4 :
-
Ammonical ion
References
Alami I, Clerivet A (2000) Cyanidin 3-glucoside accumulation in plane tree (Platanus acerifolia) cell suspension cultures. Biotech Lett 22:87–89
Asano S, Ohtsubo S, Nakajima M, Kusunoki M, Kaneko K, Katayama H, Nawa Y (2002) Production of anthocyanins by habituated cultured cells of Nyoho strawberry (Fragaria ananassa Duch.). Food Sci Tech Res 8:64–69
Betsui F, Nishikawa NT, Shimomura K (2004) Anthocyanin production in adventitious root cultures of Raphanus sativus L. cv. Peking Koushin. Plant Biotechnol 21(5):387–391
Chi BD, Cormier F (1991) Effects of low nitrate and high sugar concentrations on anthocyanin content and composition of grape (Vitis vinifera L.) cell suspension. Plant Cell Rep 9:500–504
Collin HA (2001) Secondary product formation in plant tissues cultures. Plant Growth Reg 34:119–134
Decendit A, Merillon JM (1996) Condensed tannin and anthocyanin production in Vitis vinifera cell suspension cultures. Plant Cell Rep 15:762–765
Do CB, Cormier F (1991a) Accumulation of peonidin 3-glucoside enhanced by a high osmotic stress in grape (Vitis vinifera L.) cell suspension. Plant Cell Tiss Org Cult 24:49–54
Do CB, Cormier F (1991b) Effects of low nitrate and high sugar concentrations on anthocyanin content and composition of grape (Vitis vinifera L.) cell suspension. Plant Cell Rep 9:500–504
Dube A, Charti S, Laloraya MM (1993) Inhibition of anthocyanin synthesis and phenylalanine ammonia-lyase activity of Co21 in leaf disks of Terminalia catappa. Physiol Plant 88:237–242
Edahiro J, Nakamura M, Seki M, Furusaki S (2005) Enhanced accumulation of anthocyanin in cultured strawberry cells by repetitive feeding of L-phenylalanine into the medium. J Biosci Bioeng 99:43–47
Gantet P, Memelink J (2002) Transcription factors: tools to engineer the production of pharmacologically active plant metabolites. Trends Pharmacol Sci 23:563–569
Gomez KA, Gomez AA (1984) Statistical Procedures for Agricultural Research, 2nd edn. Wiley, New York, USA, pp 8–29
Hennayake CK, Takagi S, Nishimura K, Kanechi M, Uno Y, Inagaki N (2006) Differential expression of anthocyanin biosynthesis genes in suspension culture cells of Rosa hybrida cv. Charleston. Plant Biotechnol 23:379–385
Hou DX (2003) Potential mechanisms of cancer chemoprevention by anthocyanins. Curr Mol Med 3(2):149–159
Ishikura N (1973) The changes in anthocyanin and chlorophyll content during the autumnal reddening of leaves. Kumamoto J Sci 11:43–50
Jackman RL, Yada RY, Tung MA, Speers RA (1987) Anthocyanins as food colorants–review. J Food Biochem 11:201–247
Jang JC, Leon P, Sheen J (1997) Hexokinase as a sugar sensor in higher plants. Plant Cell 9:5–19
Konczak-Islam I, Nakatani M, Yoshinaga M, Yamakawa O (2001) Effect of ammonium ion and temperature on anthocyanin composition in sweet potato cell suspension culture. Plant Biotechnol 18:109–117
Lila MA (2004) Anthocyanins and human health: an in vitro investigative approach. J Biomed Biotech 5:306–313
Makunga NP, Staden JV, Cress WA (1997) The effect of light and 2, 4-D on anthocyanin production in Oxalis reclinata callus. Plant Growth Reg 23:153–158
Mazukami H, Tomita K, Ohashi H, Hiraoka N (1988) Anthocyanin production in callus cultures of roselle (Hibiscus sabdariffa L.). Plant Cell Rep 7:553–556
Mederos S, Enriquez MJ (1987) In vitro propagation of golden times roses, factors affecting shoot tips and axillary bud growth and morphogenesis. Acta Hort 212:619–624
Meyer HJ, Staden JV (1995) The in vitro production of anthocyanin from callus cultures of Oxalis linearis. Plant Cell Tiss Org Cult 40:55–58
Mita S, Murano N, Akaike M, Nakamura K (1997) Mutants of Arabidopsis thaliana with pleiotropic effects on the expression of the gene for alpha-amylase and on the accumulation of anthocyanin that are inducible by sugars. Plant J 11:841–851
Mizukami H, Nakamura M, Tomita K, Higuchi K (1991) Effect of macronutrients on anthocyanin production in roselle (Hibiscus sabdariffa L.) callus cultures. Plant Tiss Cult Lett 8:14–20
Mori T, Sakurai M (1994) Production of anthocyanin from strawberry cell suspension cultures; effects of sugar and nitrogen. J Food Sci 59:588–593
Mori T, Sakurai M (1995) Effects of riboflavin and increased sucrose on anthocyanin production in suspended strawberry cell cultures. Plant Sci 110:147–153
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murray JR, Smith AG, Smith AG, Hackett WP (1994) Differential dihydroflavonol reductase transcription and anthocyanin pigmentation in the juvenile and mature phases of ivy (Hedera helix L.). Planta 194:102–109
Nagira Y, Ozeki Y (2004) A system in which anthocyanin synthesis is induced in regenerated torenia shoots. J Plant Res 117:377–383
Nagira Y, Ikegami K, Koshiba T, Ozeki Y (2006) Effect of ABA upon anthocyanin synthesis in regenerated torenia shoots. J Plant Res 119:137–144
Narayan MS, Venkataraman LV (2002) Effect of sugar and nitrogen on the production of anthocyanin in cultured carrot (Daucus carota) cells. J Food Sci 67:84–86
Narayan MS, Thimmaraju R, Bhagyalakshmi B (2005) Interplay of growth regulators during solid-state and liquid-state batch cultivation of anthocyanin producing cell line of Daucus carota. Process Biochem 40:351–358
Nishiyama Y, Yamakawa T (2004) Effect of medium composition on the production of anthocyanins by hairy root cultures of Ipomoea batatas. Plant Biotechnol 21:411–414
Pasqua G, Monacelli B, Mulinacci N, Rinaldi S, Giaccherini C, Innocenti M, Vinceri FF (2005) The effect of growth regulators and sucrose on anthocyanin production in Camptothea acuminata cell cultures. Plant Physiol Biochem 43:293–298
Piovan A, Filippini R (2007) Anthocyanins in Catharanthus roseus in vivo and in vitro: a review. Phytochem Rev 6:235–242
Rajendran L, Ravishankar GA, Venkataraman LV, Prathiba KR (1992) Anthocyanin production in callus cultures of Daucus carota is influenced by nutrient stress and osmoticum. Biotech Lett 14:707–712
Rao SR, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153
Sakamoto K, Iida K, Sawamura K, Hajiro K, Asada Y, Yoshikawa T, Furuya T (1993) Effects of nutrients on anthocyanin production in cultured cells of Aralia cordata. Phytochemistry 33:357–360
Sakamoto K, Iida K, Sawamura K, Hajiro K, Asada Y, Yoshikawa Y, Furuya T (1994) Anthocyanin production in cultured cells of Aralia cordata Thunb. Plant Cell Tiss Org Cult 36:21–26
Sato K, Nakayama M, Shigeta J (1996) Culturing conditions affecting the production of anthocyanin in suspended cell cultures of strawberry. Plant Sci 113:91–98
Simões C, Bizarri CHB, da Silva Cordeiro L, de Castro TC, Coutada LCM, da Silva AJR, Albarello N, Mansur E (2009) Anthocyanin production in callus cultures of Cleome rosea: modulation by culture conditions and characterization of pigments by means of HPLC-DAD/ESIMS. Plant Physiol Biochem 47:895–903
Smith MAL, Madhavi DL, Fang Y, Tomczak MM (1997) Continuous cell culture and product recovery from wild Vaccinium pahalae germplasm. J Plant Physiol 150:462–466
Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P (2000) Sucrose specific induction of the anthocyanin biosynthetic pathway in arabidopsis. Plant Physiol 140:637–646
Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P (2006) Sucrose specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140:637–646
Sudha G, Ravishankar GA (2003) Influence of putrescine on anthocyanin production in callus cultures of Daucus carota mediated through calcium ATPase. Acta Physiol Plant 25:69–75
Suzuki M (1995) Enhancement of anthocyanin accumulation by high osmotic stress and low pH in grape cells (Vitis hybrida). J Plant Physiol 147:152–155
Tholakalabavi A, Zwiazek JJ, Thorpe TA (1997) Osmotically-stressed poplar cell cultures: anthocyanin accumulation, deaminase activity, and solute composition. J Plant Physiol 151:489–496
Vijaya N, Satyanarayana G, Reddy CS (1986) Propagation of rose through tissue culture. Indian Rose Annual 5:136–147
Vitrac X, Larronde F, Krisa S, Decendit A, Deffieux G, Mèrillon JM (2000) Sugar sensing and Ca2+-calmodulin requirement in Vitis vinifera cells producing anthocyanins. Phytochemistry 53:659–665
Wang J, Bao MZ (2007) Plant regeneration of pansy (Viola wittrockiana) ‘Caidie’ via petiole-derived callus. Sci Hortic 111:266–270
Wang S, Jiao H (2000) Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J Agric Food Chem 48:5677–5684
Wrolstad RE, Durst RW, Lee J (2005) Tracking colour and pigment changes in anthocyanin products-review. Trends Food Sci Tech 16:423–428
Yamakawa T, Kato S, Ishida K, Kodama T, Minoda Y (1983) Production of anthocyanins by Vitis sp. cells in suspension culture. Agric Biol Chem 47:2185–2191
Yamamoto Y, Kinoshita Y, Watanabe S, Yamada Y (1981) Anthocyanin production in suspension cultures of high producing cells of Euphorbia millii. Agric Biol Chem 53:417–423
Yi-xun Y, Ling L, Juan-xu L, Jing W (2009) Plant regeneration by Callus-mediated protocorm-like body induction of Anthurium andraeanum Hort. Agric Sci China 8:572–577
Zhang W, Furusaki S (1999) Production of anthocyanins by plant cell cultures. Biotech Bioprocess Eng 4:231–252
Zhong JJ, Yoshida T (1995) High density cultivation of Perilla frutescens cell suspensions for anthocyanin production: effects of sucrose concentration and inoculums size. Enzym Microb Technol 17:1073–1079
Acknowledgments
The senior author acknowledges the grant of Junior Research Fellowship from the Indian Council of Agricultural Research, New Delhi. The authors thank Dr. Vijay Paul and Dr. S. Ramamurthy, IARI, New Delhi for extending their research facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ram, M., Prasad, K.V., Kaur, C. et al. Induction of anthocyanin pigments in callus cultures of Rosa hybrida L. in response to sucrose and ammonical nitrogen levels. Plant Cell Tiss Organ Cult 104, 171–179 (2011). https://doi.org/10.1007/s11240-010-9814-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9814-5