Abstract
Both elicitation and precursor feeding are effective strategies for improving secondary metabolite production in plant cell suspension cultures. In this study, cell suspension cultures of Vitis vinifera subjected to methyl jasmonate treatment resulted in a significant increase in levels of anthocyanin production. Moreover, a combination of 5 mg/L phenylalanine and 50 mg/L methyl jasmonate promoted the highest level of anthocyanin biosynthesis, resulting in 4.6- and 3.4-fold increases in anthocyanin content and yield, respectively, over the control. The optimum period for elicitation of anthocyanin synthesis was 4 days following incubation in the presence of elicitors, at the beginning of the exponential growth phase. V. vinifera cell lines of different anthocyanin-producing capabilities responded differently to elicitation and precursor feeding. Anthocyanin production of a low-producing cell line, VV06, could be enhanced with addition of elicitors and precursor feeding. Methyl jasmonate was the only elicitor that increased anthocyanin production of the high-producing cell line VV05, but contributed to moderate enhancement of anthocyanin production compared with VV06. For cell line VV06, synergistic effects were observed for all treatment combinations of methyl jasmonate along with other elicitors and precursors. In addition, 6.1- and 4.6-fold increases in anthocyanin content and yield, respectively, were obtained in the presence of 5 mg/L phenylalanine, 50 mg/L methyl jasmonate, and 1 mg/L dextran. However, none of these treatment combinations exhibited synergistic effects in cell line VV05.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthocyanins are a group of water-soluble pigments visible to the human eye that belong to the widespread class of phenolic compounds collectively named flavonoids (Kong et al. 2003). They play roles as pigments of flowers and fruits to attract insects for pollination and as protectants against UV-B irradiation. Also they exhibit antioxidant properties, free radical scavenging properties and suppress proliferation of human cancer cells (Dai et al. 2007; Kong et al. 2003; Springob et al. 2003; Zhang and Furusaki 1999). Thus, anthocyanins are widely used in food, beverages, cosmetics, pharmaceuticals, among others. In recent years, plant cell and tissue cultures of Vitis vinifera, strawberry (Fragaria sp.), Daucus carota, Perilla frutescens and Aralia cordata have been established and used for the production proanthocyanins (Zhang and Furusaki 1999).
As a promising technology for the production of these bioactive compounds, plant cell cultures offer advantages over extraction of these secondary metabolites from whole plants or via chemical synthesis (Rao and Ravishankar 2002; Verpoorte et al. 1999; Zhang and Furusaki 1999; Zhong 2002). However, commercial applications of plant cell cultures have been met with limited success and with only a few secondary metabolites such as shikonin, ginseng saponins and paclitaxel (Rao and Ravishankar 2002; Roberts and Shuler 1997; Verpoorte et al. 1999; Zhong 2002). Some of the reasons are low product yield, biosynthetic instability and difficulties to scale-up (Bourgaud et al. 2001). Among these, the low or no productivity of desired compounds is a universal phenomenon (Verpoorte et al. 1993; Wiedenfeld et al. 1997). To solve this problem is the key to realizing the commercialization of plant cell culture. In recent years, lots of efforts have been done and a great progress has been made on this. The strategies to improve the productivity include the screening and selection of high-producing cell lines, medium optimization, elicitation, precursor feeding, in situ product removal, immobilization, and so on (Dias et al. 2009; Rhee et al. 2010; Verpoorte et al. 1999; Zhang and Furusaki 1999). Among all these strategies, elicitation is one of the most effective ways (Bonfill et al. 2011; Dass and Ramawat 2009; Frankfater et al. 2009; Ketchum et al. 1999; Kim et al. 2009; Korsangruang et al. 2010; Verpoorte et al. 1999; Wise et al. 2009). Elicitors are defined as molecules that stimulate defense or stress-induced responses in plants, which are classified as biotic or abiotic depending on their origin. The exogenous application of elicitors to in vitro cultures is useful for studying plant responses to potential micro or insect attack as well as for enhancing biosynthesis of valued secondary metabolites in fermentation systems. The synergistic effect will give an unexpected result sometimes. For example, the combination of 50 mg/L chitosan, 10 μM methyl jasmonate and 30 μM Ag+ resulted in 25 mg/L paclitaxel production, being almost 40 times higher than that of the control culture, 10 times higher than that of the culture exposed to Ag+, 6 times higher than that of the culture elicited by chitosan and almost double that of the culture elicited by methyl jasmonate (Zhang et al. 2000). Besides elicitation, precursor feeding is another potent way to improve secondary metabolite production in plant cell culture (Krisa et al. 1999; Whitmer et al. 2002). With continuous precursors feeding, the tryptophan decarboxylase over-expressing transgenic cell line T22 of Catharanthus roseus accumulated 1,250 μmol L−1 (~625 mg L−1) of terpenoid indole alkaloids in which about 45 mg L−1 was ajmalicine and serpentine while the control culture’s accumulation was less than 10 μmol L−1 (Whitmer et al. 2002).
In this study, several elicitors and precursors were used individually or in combinations to enhance anthocyanin production in cell suspension cultures of V. vinifera. At the same time, the effects of elicitation and precursor feeding on high- and low-anthocyanin-producing cell lines were investigated.
Materials and methods
Cell line and culture conditions
The original cell line, capable of anthocyanin accumulation in darkness, was a gift from Dr. Francois Cormier’s group (Quebec, Canada) (Qu et al. 2005). It was developed by Cormier et al., originating from callus established in 1978 from V. vinifera L. cv. Gamay Fréaux var. teinturier berry pulp. VV05 and VV06, representatives of high- and low-anthocyanin-producing cell lines, were established in our lab by screening callus with different pigment intensities. The suspension cultures of VV05 and VV06 had been subcultured for over 1 year. They were subcultured weekly in 250-mL Erlenmeyer flasks enclosed with aluminum foil containing 50 mL maintenance medium which was B5 medium (Gamborg et al. 1968) supplemented with 30 g/L sucrose, 250 mg/L casein hydrolysate, 0.1 mg/L α-naphthaleneacetic acid (NAA) and 0.2 mg/L kinetin (K). The pH was adjusted to 5.7–5.8 before autoclaving. The inoculum size was approximately 5.0 g wet cells prepared by filtering precultured 7-day-old suspension cells with a 50-μm mesh. The subcultures were maintained in darkness on a reciprocating shaker at 100 rpm at 25°C.
Chemicals
Methyl jasmonate (Me-JA) and methyl-β-cyclodextrin were purchased from Sigma. The other chemicals were of analytical grade and obtained commercially.
Preparation of fungal elicitors
Aspergillus niger and Fusarium orthoceras had grown at 30°C on Czapck medium for 4.5 days on a reciprocating shaker at 100 rpm. The fungal mycelia were collected by centrifugation and resuspended in distilled water (50% w/v) and homogenized by the ultrasonator. The homogenates were then autoclaved at 121°C for 1 h to release cell wall fragments, followed by vacuum filtration. The filtrate was passed through a 0.22-μm filter and then autoclaved again at 121°C for 20 min to obtain the crude fungal elicitors.
Elicitation and precursor feeding experiment on a low-anthocyanin producing cell line
Here suspension cultures of VV06 were used for cultivation in 100-mL Erlenmeyer flasks containing 20 mL maintenance medium as above. And the inoculum size was accurately 2.00 g wet cells harvested by filtration of 7-day-old suspension cells with a 50 μm mesh. Then the resulting cultures were incubated at 25°C on a reciprocating shaker at 100 rpm in darkness. Four days after inoculation, the elicitors or precursor were added to the cultures, respectively. And 7 days after inoculation, the cells were harvested for analysis of cell growth and anthocyanin accumulation.
For the time-dependent effect of elicitor and precursor on suspension cultures, phenylalanine and methyl jasmonate was added to the cultures 0, 4 and 7 days after inoculation, respectively. And the cells were harvested for analysis on day 0, 4, 7 and 10, respectively.
Effects of elicitation and precursor feeding on high- and low-anthocyanin-producing cell lines of V. vinifera
Here the suspension cultures of VV05 and VV06 were cultured in 100-mL Erlenmeyer flasks containing 20 mL maintenance medium. Accurately 2.00 g wet cells harvested by filtration of 7-day-old suspension cells were inoculated and cultured in the same conditions as above. Four days after inoculation, the precursor or elicitors were added and 7 days after inoculation, the cells were harvested for analysis of cell growth and anthocyanin accumulation.
Analysis of cell growth and anthocyanin accumulation
At the time of sampling, the whole flask was harvested for analysis of cell growth and anthocyanin accumulation (Qu et al. 2005). Firstly, the suspension cultures were collected by vacuum filtration through filter paper, washed with distilled water to remove the residual sucrose and weighed to obtain the fresh cell weight (FCW). Then a certain amount of fresh cells were taken for extraction of anthocyanin. They were extracted with 50% acetic acid solution with a volume equivalent to 20 times the fresh cell weight for 1-h period at room temperature. After filtering through a 0.22-μm filter, the filtrate was diluted 1:4 in McIIvaine’s buffer that contained 14.7 g/L Na2HPO4·12H2O and 16.7 g/L anhydrous citric acid (pH 3.0). The absorbance of the resulting solution was measured at 535 nm by spectrophotometer. Anthocyanin accumulation was represented as color value (CV) which was calculated with the following equation:
In the above-described procedure, the dilution factor was 80. CV allows for the accurate and comparative quantification of anthocyanin produced from a mixture of different pigments, as is the case for many cell cultures.
The rest of the fresh cells was dried at 80°C in an oven overnight and weighed to calculate dry cell weight (DCW). Then according to the relation between fresh cell weight and dry cell weight, the anthocyanin content in terms of CV/g-FCW was converted into CV/g-DCW. Finally, anthocyanin production (CV/L) was calculated from cell growth and anthocyanin content.
Results and discussion
Elicitation experiments by abiotic elicitors
In our preliminary experiments, several abiotic elicitors such as jasmonic acid, methyl jasmonate, salicylic acid, methyl-β-cyclodextrin, chitosan and dextran had been investigated with various concentrations. Here we select the ones (50 mg/L methyl jasmonate, 5 mg/L methyl-β-cyclodextrin and 1 mg/L dextran) presenting more effective effects on anthocyanin accumulation for further investigation.
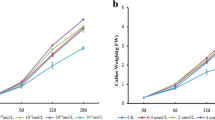
As shown in Fig. 1, neither methyl-β-cyclodextrin nor dextran worked on cell growth and anthocyanin accumulation, while methyl jasmonate had a remarkable effect in improving anthocyanin production whether used in combination with others or not. Methyl jasmonate improved the anthocyanin accumulation clearly but it affected the biomass in an interesting way. That is, it increased the fresh cell weight but decreased the dry cell weight. For example, with the individual employment of methyl jasmonate, the fresh cell weight was 1.1-fold of the control while the dry cell weight only 0.7-fold of the control; anthocyanin content was 2.6-fold and anthocyanin yield was 1.7-fold of the controls. This interesting phenomenon indicated that methyl jasmonate increased the cellular water content.
Effects of abiotic elicitors on cell growth and anthocyanin accumulation in suspension cultures of V. vinifera. Elicitors were added to the suspension cultures 4 days after inoculation. And the whole flasks were harvested 7 days after inoculation. A: 50 mg/L methyl jasmonate; B: 5 mg/L methyl-β-cyclodextrin; C: 1 mg/L dextran; D: 50 mg/L methyl jasmonate + 5 mg/L methyl-β-cyclodextrin; E: 50 mg/L methyl jasmonate + 1 mg/L dextran; F: 5 mg/L methyl-β-cyclodextrin + 1 mg/L dextran; G: 50 mg/L methyl jasmonate + 5 mg/L methyl-β-cyclodextrin + 1 mg/L dextran; H: Control. Data presented as mean ± standard deviation of three replicate samples
Among all the combinations of either two or three of these abiotic elicitors, methyl jasmonate and dextran gave the most significant improvement of anthocyanin accumulation with 3.8-fold anthocyanin content and 3.0-fold anthocyanin yield of the controls. To describe the synergistic effect of different elicitors quantitatively, Yuan et al. introduced a synergistic coefficient (Yuan et al. 2002). Here we defined the synergistic coefficient as the ratio of the anthocyanin yield in the coexistence of two or three elicitors to the summation of the anthocyanin yield in the cases with the individual employment of them. If the synergistic coefficient is bigger than 1, it means the elicitors have synergistic effect on anthocyanin production. The larger synergistic coefficient means the more significant synergistic effect. The synergistic coefficient of methyl jasmonate and dextran is thus calculated as 2,504/(1,450 + 841) = 1.09. Since 1.09 is bigger than 1, it indicates methyl jasmonate and dextran exhibited synergistic effect on anthocyanin production. While the synergistic coefficient of methyl jasmonate and methyl-β-cyclodextrin is calculated as 1,995/(1,450 + 906) = 0.85; the synergistic coefficient of methyl-β-cyclodextrin and dextran is calculated as 881/(906 + 841) = 0.50; and the synergistic coefficient of methyl jasmonate, methyl-β-cyclodextrin and dextran is calculated as 2,174/(1,450 + 906 + 841) = 0.68. All the synergistic coefficients of above three combinations are smaller than 1, so the elicitors used in combinations had no synergistic effect (Fig. 1).
The elicitors acting on different metabolic pathways maybe have a synergistic effect on the biosynthesis of special metabolites, while those acting on the same metabolic pathway maybe not. So learning about elicitation mechanism and biosynthetic pathways of the interested compounds are the keys to guiding the design of rational process to improve secondary metabolite production.
Elicitation experiments by combination of biotic and abiotic elicitors
In the preliminary experiments, two fungal elicitors (A. niger and F. orthoceras) on cell growth and anthocyanin accumulation in suspension cultures of V. vinifera were individually studied. The optimal concentrations of fungal extracts are both 0.025 mL/L. Neither individual use of fungal elicitor had remarkable improvement on anthocyanin production (data not shown). Here we examined whether the combination of biotic and abiotic elicitors could greatly increase anthocyanin production.
As shown in Fig. 2, none of the combinations of fungal elicitors and abiotic elicitors showed an obvious improvement on cell growth and anthocyanin biosynthesis. Additionally, the combination of either fungal elicitor with methyl jasmonate resulted in anthocyanin production decreasing greatly, with 0.7- or 0.6-fold of the controls.
Effects of the combinations of biotic and abiotic elicitors on cell growth and anthocyanin accumulation in suspension cultures of V. vinifera. Elicitors were added to the suspension cultures 4 days after inoculation. And the whole flasks were harvested 7 days after inoculation. A: 0.025 mL/L A. niger + 5 mg/L methyl-β-cyclodextrin; B: 0.025 mL/L A. niger + 1 mg/L dextran; C: 0.025 mL/L A. niger + 50 mg/L methyl jasmonate; D: 0.025 mL/L F. orthoceras + 5 mg/L methyl-β-cyclodextrin; E: 0.025 mL/L F. orthoceras + 1 mg/L dextran; F: 0.025 mL/L F. orthoceras + 50 mg/L methyl jasmonate; G: Control. Data presented as mean ± standard deviation of three replicate samples
Integration of precursor feeding and elicitation
Precursor feeding is another strategy to enhance the yield of secondary metabolite in plant cell culture. In the biosynthetic pathway of polyphenols, l-phenylalanine is the precursor of anthocyanin biosynthesis (Aumont et al. 2004; Zhang et al. 2002). In our preliminary experiment, 5 mg/L phenylalanine was confirmed to be the optimal concentration (data not shown). But its individual use had no remarkable effect on cell growth and anthocyanin accumulation in suspension cultures of V. vinifera. Then the integration of precursor feeding and elicitation was taken into consideration.
As shown in Fig. 3, among the combinations of the precursor with biotic or abiotic elicitors, phenylalanine and methyl jasmonate had an outstanding effect. The anthocyanin content was 5.6-fold and the anthocyanin yield was 4.4-fold of the controls. While the individual use of phenylalanine or methyl jasmonate had no such a significant increase in anthocyanin biosynthesis. For example, phenylalanine could only make the anthocyanin yield 0.7-fold of the control and methyl jasmonate 1.7-fold of the control. So phenylalanine and methyl jasmonate had a significant synergistic effect on anthocyanin accumulation in suspension cultures of V. vinifera. None of the other combinations of phenylalanine and elicitor had such an obvious effect.
Effects of precursor feeding and elicitation on cell growth and anthocyanin accumulation in suspension cultures of V. vinifera. Elicitors or precursor were added to the suspension cultures 4 days after inoculation. And the whole flasks were harvested 7 days after inoculation. A: 5 mg/L phenylalanine + 5 mg/L methyl-β-cyclodextrin; B: 5 mg/L phenylalanine + 1 mg/L dextran; C: 5 mg/L phenylalanine + 50 mg/L methyl jasmonate; D: 5 mg/L phenylalanine + 0.025 mL/L A. niger; E: 5 mg/L phenylalanine + 0.025 mL/L F. orthoceras; F: 5 mg/L phenylalanine; G: Control. Data presented as mean ± standard deviation of three replicate samples
Effects of the addition time of phenylalanine and methyl jasmonate on suspension cultures of V. vinifera
As shown in Fig. 4, the addition time of phenylalanine and methyl jasmonate to the cultures had a great impact on anthocyanin accumulation in suspension cultures of V. vinifera. When phenylalanine and methyl jasmonate were added on day 0, cells died immediately. When they were added on day 4, the fresh cell weight increased a little firstly and then decreased dramatically, while the dry cell weight decreased as soon as phenylalanine and methyl jasmonate were added. This also indicated that the cellular water content increased at the beginning of phenylalanine and methyl jasmonate were added. The anthocyanin content and yield had remarkable improvements with the addition time of day 4. While when phenylalanine and methyl jasmonate were added on day 7, cell growth and anthocyanin accumulation were both inhibited immediately. So the optimum time for the addition of phenylalanine and methyl jasmonate was 4 days after inoculation, at the beginning of the exponential growth phase.
Effects of the addition time of phenylalanine and methyl jasmonate on cell growth and anthocyanin accumulation in suspension cultures of V. vinifera. 5 mg/L phenylalanine and 50 mg/L methyl jasmonate were added to the suspension cultures 0, 4 and 7 days after inoculation, respectively. And the whole flasks were harvested 4, 7, 10 and 14 days after inoculation, respectively. Data presented as mean ± standard deviation of three replicate samples
The cellular water content increased with the elicitor treatment and precursor feeding. Especially, the cellular water content increased significantly in the conditions that improved the anthocyanin production greatly (Figs. 1, 3). This indicated that elicitation and precursor feeding made the extracellular osmotic potential lower than the control. While Naill et al. reported that the addition of methyl jasmonate made the cellular water content lower and extracellular osmotic potential higher than unelicited cultures of Taxus cuspidata (Sakuta et al. 1994). We can see from the cell culture kinetics in our experiment that the cellular water content was the highest in the stationary phase when the anthocyanin production was also the highest during the culture time. This indicates that there may be some interesting relations between anthocyanin production and cellular water content in suspension culture of V. vinifera.
Effects of elicitation and precursor feeding on high- and low-anthocyanin-producing cell lines of V. Vinifera
The suspension cultures of VV05 and VV06 are representatives of high- and low-anthocyanin-producing cell lines of V. vinifera which were obtained by cell line selection. The cells of VV05 were deep red colored and the cells of VV06 were pink when observed with naked eye. Besides, the aggregates of VV05 were relatively bigger than that of VV06 in the long term suspension cultures. Figure 5 showed the culture kinetics of VV05 and VV06. The two cell lines had similar time courses of cell growth and anthocyanin accumulation except for their anthocyanin-producing-abilities.
Figure 6 and Table 1 showed the cell growth and anthocyanin accumulation of VV05 and VV06 in the presence of different elicitors and precursor. The data in Table 1 were ratios of experimental results of elicitation and precursor feeding to their corresponding controls. Anthocyanin production of low-anthocyanin-producing cell line VV06 could be improved by all the elicitors and precursor tested. Among all these, methyl jasmonate had an outstanding effect. For high-anthocyanin-producing cell line VV05, methyl jasmonate was the only one increasing its anthocyanin production but with a moderate improvement.
The cell growth and anthocyanin accumulation of high- and low-anthocyanin-producing cell lines of V. vinifera with elicitation or precursor feeding. The elicitors or precursor were added to the suspension cultures 4 days after inoculation. And the whole flasks were harvested 7 days after inoculation. Data presented as mean ± standard deviation of three replicate samples. A: Control; B: 5 mg/L phenylalanine; C: 50 mg/L methyl jasmonate; D: 1 mg/L dextran; E: 5 mg/L phenylalanine + 50 mg/L methyl jasmonate; F: 5 mg/L phenylalanine + 1 mg/L dextran; G: 50 mg/L methyl jasmonate + 1 mg/L dextran; H: 5 mg/L phenylalanine + 50 mg/L methyl jasmonate + 1 mg/L dextran
In total, the anthocyanin production increased more significantly when elicitors and precursor used in combination than used individually. Synergistic effects were observed for all combinations of methyl jasmonate with other elicitors and precursor for cell line VV06. But for cell line VV05, none of the combinations showed a synergistic effect on anthocyanin production. With the combination of 5 mg/L phenylalanine, 50 mg/L methyl jasmonate and 1 mg/L dextran, anthocyanin content and yield of VV06 were 7.1- and 5.6-fold of the controls, respectively. As for cell line VV05, 5 mg/L phenylalanine plus 50 mg/L methyl jasmonate was the most effective combination among all treatments, which only resulted in anthocyanin content and yield 2.8- and 2.3-fold of the controls.
All these indicated that low-producing cell line had much more potentialities to improve the secondary metabolite productivity than high-one. In despite of this, in our experiment, the highest anthocyanin production was 2,332 CV/L for VV05, while 2,194 CV/L for VV06. The low-producing cell line (VV06) may have much more potentialities in improving the secondary metabolite productivity, but it doesn’t mean it will always give a final higher production than the high-producing cell line (VV05). Therefore, it will always be an important and necessary work to select cell lines in plant cell culture.
In the previous studies, a process that combined 20 μM jasmonic acid treatment and continuous light irradiation of 8,000–8,300 lux resulted in the maximum anthocyanin production of 2,200 CV/L in Vitis vinifera suspension cultures (Zhang et al. 2002). In our experiment, the combination of 5 mg/L phenylalanine and 50 mg/L methyl jasmonate resulted in the anthocyanin production of 2332 CV/L for high-anthocyanin-producing cell lines of VV05 and 2,194 CV/L for low-anthocyanin-producing cell lines of VV06. It indicated that the combination of elicitation and precursor feeding was an effective way to enhancing the anthocyanin biosynthesis in Vitis vinifera suspension cultures.
Conclusions
It was confirmed that methyl jasmonate could elicit a significant increase in anthocyanin production whether used alone or in combination with other elicitors or precursor in suspension cultures of V. vinifera. The combination of 5 mg/L phenylalanine with 50 mg/L methyl jasmonate showed the best improvement in anthocyanin biosynthesis. The optimum time for their addition was 4 days after inoculation, at the beginning of the exponential growth phase. To the same elicitor treatment and precursor feeding, high- and low-anthocyanin-producing cell lines showed different responses. Synergistic effects were observed for all combinations of methyl jasmonate with others for the low-producing cell line, but none for the high-producing cell line.So when use the strategies of elicitation or precursor feeding to improve secondary metabolites production in plant cell culture, different cell lines and their characteristics must be individually taken into consideration, which has been approved in our previous work (Qu et al. 2006). There is not a general application in elicitation or other technique in plant cell and tissue culture.
Abbreviations
- NAA:
-
α-Naphthaleneacetic acid
- K:
-
Kinetin
- Me-JA:
-
Methyl jasmonate
- FCW:
-
Fresh cell weight
- DCW:
-
Dry cell weight
- CV:
-
Color value
References
Aumont V, Larronde F, Richard T, Budzinski H, Decendit A, Deffieux G, Krisa S, Mérillon JM (2004) Production of highly 13C-labeled polyphenols in Vitis vinifera cell bioreactor cultures. J Biotechnol 109:287–294
Bonfill M, Mangas S, Moyano E, Cusido RM, Palazón J (2011) Production of centellosides and phytosterols in cell suspension cultures of Centella asiatica. Plant Cell Tiss Organ Cult 104:61–67
Bourgaud F, Gravot A, Milesi S, Gontier E (2001) Production of plant secondary metabolites: a historical perspective. Plant Sci 161:839–851
Dai J, Patel JD, Mumper RJ (2007) Characterization of blackberry extract and its antiproliferative and anti-inflammatory properties. J Med Food 10(2):258–265
Dass S, Ramawat KG (2009) Elicitation of guggulsterone production in cell cultures of Commiphora wightii by plant gums. Plant Cell Tiss Organ Cult 96:349–353
Dias LLC, Santa-Catarina C, Ribeiro DM, Barros RS, Floh EIS, Otoni WC (2009) Ethylene and polyamine production patterns during in vitro shoot organogenesis of two passion fruit species as affected by polyamines and their inhibitor. Plant Cell Tiss Organ Cult 99:199–208
Frankfater CR, Dowd MK, Triplett BA (2009) Effect of elicitors on the production of gossypol and methylated gossypol in cotton hairy roots. Plant Cell Tiss Organ Cult 98:341–349
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–156
Ketchum REB, Gibson DM, Croteau RB, Shuler ML (1999) The kinetics of taxoid accumulation in cell suspension cultures of Taxus following elicitation with methyl jasmonate. Biotechnol Bioeng 62:97–105
Kim OT, Bang KH, Kim YC, Hyun DY, Kim MY, Cha SW (2009) Upregulation of ginsenoside and gene expression related to triterpene biosynthesis in ginseng hairy root cultures elicited by methyl jasmonate. Plant Cell Tiss Organ Cult 98:25–33
Kong JM, Chia LS, Goh NK, Chia TF, Brouillard R (2003) Analysis and biological activities of anthocyanins. Phytochemistry 64:923–933
Korsangruang S, Soonthornchareonnon N, Chintapakorn Y, Saralamp P, Prathanturarug S (2010) Effects of abiotic and biotic elicitors on growth and isoflavonoid accumulation in Pueraria candollei var. candollei and P. candollei var. mirifica cell suspension cultures. Plant Cell Tiss Organ Cult 103:333–342
Krisa S, Téguo PW, Decendit A, Deffieux JV, Mérillon JM (1999) Production of 13C-labelled anthocyanins by Vitis vinifera cell suspension cultures. Phytochemistry 51:651–656
Qu JG, Zhang W, Yu XJ, Jin MF (2005) Instability of anthocyanin accumulation in Vitis vinifera L. var Gamay Fréaux suspension cultures. Biotechnol Bioprocess Eng 10:155–161
Qu JG, Zhang W, Jin MF, Yu XJ (2006) Effect of Homogeneity on Cell Growth and Anthocyanin Biosynthesis in Suspension Cultures of Vitis vinifera. Chin J Biotechnol 22(5):805–810
Rao SR, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153
Rhee HS, Cho HY, Son SY, Yoon SYH, Park JM (2010) Enhanced accumulation of decursin and decursinol angelate in root cultures and intact roots of Angelica gigas Nakai following elicitation. Plant Cell Tiss Organ Cult 101:295–302
Roberts SC, Shuler ML (1997) Large-scale plant cell culture. Current Opinions Biotechnol 8:154–159
Sakuta M, Hirano H, Kakegawa K, Suda J, Hirose M, Joy RW IV, Sugiyama M, Komamine A (1994) Regulatory mechanisms of biosynthesis of betacyanin and anthocyanin in relation to cell division activity in suspension cultures. Plant Cell Tiss Org Cult 38:167–169
Springob K, Nakajima J, Yamazaki M, Saito K (2003) Recent advances in the biosynthesis and accumulation of anthocyanins. Nat Prod Rep 20:288–303
Verpoorte R, Van der Heijden R, Schripsema J, Hoge JHC, Ten Hoopen HJG (1993) Plant cell biotechnology for the production of alkaloids: present status and prospects. J Nat Prod 56:186–207
Verpoorte R, Van der Heijden R, ten Hoopen HJG, Memelink J (1999) Metabolic engineering of plant secondary metabolite pathways for the production of fine chemicals. Biotechnol Lett 21:467–479
Whitmer S, Van der Heijden R, Verpoorte R (2002) Effect of precursor feeding on alkaloid accumulation by a tryptophan decarboxylase over-expressing transgenic cell line T22 of Catharanthus roseus. J Biotechnol 96:193–203
Wiedenfeld H, Furmanowa M, Roeder E, Guzewska J, Gustowski W (1997) Camptothecin and 10-hydroxycamptothecin in callus and plantlets of Camptotheca acuminata. Plant Cell Tiss Org Cult 49:213–218
Wise ML, Sreenath HK, Skadsen RW, Kaeppler HK (2009) Biosynthesis of avenanthramides in suspension cultures of oat (Avena sativa). Plant Cell Tiss Organ Cult 97:81–90
Yuan YJ, Wei ZJ, Miao ZQ, Wu JC (2002) Acting paths of elicitors on Taxol biosynthesis pathway and their synergistic effect. Biochem Eng J 10:77–83
Zhang W, Furusaki S (1999) Production of anthocyanins by plant cell cultures. Biotechnol Bioprocess Eng 4:231–252
Zhang CH, Mei XG, Liu L, LJ YU (2000) Enhanced pacilitaxel production induced by the combination of elicitors in cell suspension cultures of Taxus chinensis. Biotechnol Lett 22:1561–1564
Zhang W, Curtin C, Kikuchi M, Franco C (2002) Integration of jasmonic acid and light irradiation for enhancement of anthocyanin biosynthesis in Vitis vinifera suspension cultures. Plant Sci 162:459–468
Zhong JJ (2002) Plant cell culture for production of paclitaxel and other taxanes. J Biosci Bioeng 94(6):591–599
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (No. 20176058).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qu, J., Zhang, W. & Yu, X. A combination of elicitation and precursor feeding leads to increased anthocyanin synthesis in cell suspension cultures of Vitis vinifera . Plant Cell Tiss Organ Cult 107, 261–269 (2011). https://doi.org/10.1007/s11240-011-9977-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-011-9977-8