Abstract
Saussurea involucrata is a valuable traditional Chinese medicinal herb. This is the first report of a successful genetic transformation protocol for S. involucrata using Agrobacterium tumefaciens. Leaf explants were incubated with A. tumefaciens strain EHA105 harboring the binary vector pCAMBIA 1301, which contains the hpt gene as a selectable marker for hygromycin resistance and an intron-containing β-glucuronidase gene as a reporter gene. Following co-cultivation, about 23.7% of the explants produced hygromycin-resistant calli on MS basal medium (Murashige and Skoog in Physiol Plant 15: 473–497, 1962) supplemented with 1 mg l−1 benzyladenine (BA), 0.1 mg l−1 α-naphthaleneacetic acid (NAA), 0.1 mg l−1 2,4-dichlorophenoxyacetic acid (2,4-D), 20 mg l−1 hygromycin, and 500 mg l−1 cefotaxime. Shoots were regenerated following transfer of the resistant calli to shoot induction medium containing 1.5 mg l−1 BA, 0.1 mg l−1 NAA, 0.25 mg l−1 gibberellic acid (GA3), 20 mg l−1 hygromycin, and 250 mg l−1 cefotaxime, and about 67.5% of the resistant calli differentiated into shoots. Finally, 80% of the hygromycin-resistant shoots rooted on MS media supplemented with 0.2 mg l−1 NAA, 20 mg l−1 hygromycin, and 250 mg l−1 cefotaxime. The transgenic nature of the transformants was demonstrated by detection of β-glucuronidase activity in the primary transformants and by Southern blot hybridization analysis. About 16% of the total inoculated leaf explants produced transgenic plants after approximately 5 months. Using this optimized transformation system, a rice ortholog of the Arabidopsis FLOWERING LOCUS T gene, Hd3a, was transferred into S. involucrata. Introduction of this gene caused an early-flowering phenotype in S. involucrata.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Saussurea involucrata Kar. et Kir. ex Maxim is a valuable traditional Chinese medicinal herb of the family Asteraceae. The plant has long been used under the herbal name “Tianshan snow lotus” for treatment of rheumatoid arthritis, cough with cold, stomachache, dysmenorrhea, and altitude sickness (Encyclopaedia of Traditional Chinese Medicine 1995) and has recently been found to have anti-inflammatory (He et al. 1990; Jia et al. 2005; Chen et al. 2010), antifatigue (Zheng et al. 1993), antitumor (Wu et al. 2009), and anticancer actions (Liu et al. 1985; Han 1995; Way et al. 2010). S. involucrata is a perennial herbaceous plant, a special local plant growing only in the rocky mountains and glaciers near or above the snowline about 4,000 m above sea level in Xinjiang, Tibet, and Yunnan Provinces of China, having the ability to resist harsh climates with severe cold, intense ultraviolet radiation, and low oxygen. The plant begins to blossom around the middle of August in the fifth year of its sexennial growth period. The plant whole height is 14–50 cm, having a dense basal rosette of oblanceolate leaves spiraling up the flowering stem, and a dense woolly head of capitulum with multilayer pale-greenish-yellow phyllaries and purple florets (Chen et al. 1999). Unfortunately, basic biological studies are limited due to the difficulty of reproductive success of this plant under normal growth conditions and the lack of efficient and rapid transformation protocols.
Owing to overexploitation of wild plants and difficulty of cultivation, S. involucrata is now almost extinct in China (Fu 1992). Successful application of plant biotechnology for conservation of rare and endangered plant resources and development of novel germplasm will largely depend on the availability of an efficient plant regeneration and genetic transformation system (Offord and Tyler 2009; Bakhshaie et al. 2010; Irvani et al. 2010; Li et al. 2008a, b). Recently, the potential of biotechnology in S. involucrata conservation and exploitation for commercial purposes has been recognized. Several reports have described plant regeneration from embryos (Wu et al. 2005a, b), leaf explants (Yang and Zou 2005; Lin et al. 2006; Guo et al. 2007; Zhao and Wang 2008), and hairy roots (Fu et al. 2004) of S. involucrata. Regenerated plants successfully survived and blossomed in the third year after transplanting to Taishan Mountain (Zhao and Wang 2008).
As wild S. involucrata grows only in special regions with peculiar climate, and cultivation of the plant in a normal climate has been unsuccessful so far, rapid mass production of S. involucrata for medicinal purposes is limited, and the development and genetic background of this plant are also unknown. Flowering time is very important for reproductive success of higher plants. Therefore, we tried to set up a flower regeneration system in S. involucrata to facilitate basic biological studies of flower development, reproductive success of this plant in a normal climate, rapid mass production of the flowers for medicinal purposes, and the study of bioactive phytochemicals in the flowers.
FLOWERING LOCUS T (FT), a well-known floral integrator gene, plays an important role in controlling flowering time in higher plants (Kardailsky et al. 1999; Kobayashi et al. 1999; Xi and Yu 2009). More recently, FT and its orthologs have been proposed as florigens, or mobile flowering signals, migrating from leaves to the apical meristem to promote floral initiation (Tamaki et al. 2007; Corbesier et al. 2007; Lin et al. 2007). Overexpression of Arabidopsis thalianaFT or ectopic expression of FT orthologs such as CiFT of trifoliate orange (Kobayashi et al. 1999), Hd3a of Oryza sativa L. (Kojima et al. 2002), PtFT1 of Populus trichocarpa (Böhlenius et al. 2006), FT2 of Populus deltoids (Hsu et al. 2006), and OnFT of orchid (Hou and Yang 2009) has been shown to cause an early-flowering phenotype in Arabidopsis. Furthermore, both PtFT1 and FT2 were successfully used to dramatically accelerate flowering in poplar (Böhlenius et al. 2006; Hsu et al. 2006; Zhang et al. 2010), and CiFT was also successfully applied to induce early flowering in trifoliate orange (Endo et al. 2005) and European pear (Matsuda et al. 2009).
In this study, an efficient genetic transformation procedure for S. involucrata using an Agrobacterium tumefaciens-mediated leaf disc transformation method was developed. Based on this transformation protocol, a rice ortholog of the Arabidopsis FLOWERING LOCUS T gene, Hd3a, was transformed into the genome of S. involucrata. Flowering cluster regenerated directly from the callus transformed with Ubiquitin::Hd3a.
Materials and methods
Plant materials and tissue culture conditions
Seeds of Saussurea involucrata Kar. et Kir. ex Maxim were collected from Xinjiang, P.R. China. The seeds were surface-sterilized as previously described (Li et al. 2008a, b) and then placed on Petri dishes containing half-strength Murashige and Skoog (1962) salt (1/2 MS) solid medium containing 100 mg l−1 myo-inositol, 10 mg l−1 thiamine-HCl, 3% (w/v) sucrose, and 0.7% (w/v) agar (Sigma) to germinate. Tissue cultures were incubated at 24 ± 1°C with a 14-h photoperiod under illumination at 35 μmol m−2 s−1 from cool-white fluorescent lamps throughout the experiments, except callus induction, which was conducted under darkness. All plant media were adjusted with 1 N KOH or 1 N HCl to pH 5.8, solidified with 0.7% (w/v) agar (Sigma, St. Louis, MO, USA), and autoclaved at 121°C for 20 min.
Plasmid preparation and bacterial cells
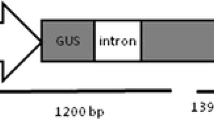
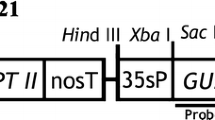
The binary vector pCAMBIA 1301 containing both the reporter gene for β-glucuronidase (uidA) and the selectable marker gene for hygromycin phosphotransferase (hpt) was used for genetic transformation experiments firstly. A HindIII-BamHI fragment containing maize Ubiquitin promoter with Ubiquitin intron from pAHC27 (Takimoto et al. 1994) was cloned into the HindIII-BamHI sites of the binary vector pCAMBIA1390; this vector was named p1390Ubi. The complete open reading frame (ORF) of Hd3a (accession no. Os06g0157700) was amplified from rice leaves by reverse-transcription polymerase chain reaction (RT-PCR) using the following primers: forward 5′-AATGAATTC (EcoRI site) ATGGCCGGAAGTGGCAGGGAC-3′ and reverse 5′-AATGAATTC (EcoRI site) CTAGGGGTAGACCCTCCTG-3′. The fragment was cloned into the EcoRI site of p1390Ubi in the sense orientation (Fig. 1; p1390UbiHd3a). The constructed p1390UbiHd3a was confirmed by sequencing. Both pCAMBIA 1301 and p1390UbiHd3a were then transformed into Agrobacterium tumefaciens strain EHA105 by the liquid-nitrogen freeze–thaw method (Chen et al. 1994), respectively.
T-DNA region of the constructed p1390UbiHd3a. The full-length complementary DNA (cDNA) encoding the rice Hd3a gene (539 bp) was inserted into the EcoRI site of p1390Ubi in the sense orientation. RB right border of the T-DNA, LB left border of the T-DNA, 35S pro 35S promoter from cauliflower mosaic virus, Ubi pro Ubiquitin promoter with Ubiquitin intron from maize, CA CaMV35S polyA, NA nopaline synthase polyA, hpt hygromycin phosphate transferase
For plant genetic transformation, a single colony of the bacteria was inoculated in YEP medium supplemented with kanamycin (50 mg l−1) and rifampicin (50 mg l−1) and cultured overnight at 28°C. Bacterial cells were collected by centrifugation at 10,000g for 30 s at 25°C and resuspended in 30 ml (OD600 = 0.4–0.5) liquid MS medium (pH 5.2) supplemented with 20 mg l−1 acetosyringone (AS). After incubation on a shaker (160 rpm) for 3 h at 28°C, the Agrobacterium was used to infect the explants.
Genetic transformation of S. involucrata
Leaves from 1-month-old seedlings were cut into 0.5 × 0.5 cm2 and inoculated with Agrobacterium cells harboring pCAMBIA 1301 in 30 ml liquid MS medium supplemented with 20 mg l−1 AS for 15 min. Subsequently, the explants were blotted on sterile filter paper to remove most of the liquid medium, and transferred to agar-solidified (0.7% w/v) callus induction medium, which is MS basal medium supplemented with 1 mg l−1 benzyladenine (BA), 0.1 mg l−1 α-naphthaleneacetic acid (NAA), 0.1 mg l−1 2,4-dichlorophenoxyacetic acid (2,4-D) (Zhao and Wang 2008), and 20 mg l−1 AS for co-cultivation. After 3 days, the explants were washed with sterile water containing 500 mg l−1 cefotaxime to remove Agrobacterium cells. Subsequently, the explants were transferred onto selection medium, which is callus induction medium supplemented with 20 mg l−1 hygromycin and 500 mg l−1 cefotaxime. Eighteen days later, the hygromycin-resistant calli were transferred onto hygromycin-resistant shoot regeneration medium, which is MS basal medium supplemented with 1.5 mg l−1 BA, 0.1 mg l−1 NAA, 0.25 mg l−1 gibberellic acid (GA3), 20 mg l−1 hygromycin, and 250 mg l−1 cefotaxime and incubated in the light. Transfer onto the same fresh hygromycin-resistant shoot regeneration media was carried out at 3-week intervals. After another 8 weeks, hygromycin-resistant shoots (4–5 cm in height) were separated and transferred to MS basal medium supplemented with 0.2 mg l−1 NAA (Zhao and Wang 2008), 20 mg l−1 hygromycin, and 250 mg l−1 cefotaxime for rooting. After 4 weeks of growth, the regenerated plants were repotted into plastic pots containing autoclaved vermiculite and sand (1/1, v/v) for further aseptic growth under plant growth chamber conditions (17 ± 1°C in a 14-h photoperiod under illumination at 35 μmol m−2 s−1 from cool-white fluorescent lamps).
For genetic transformation of Hd3a into the genome of S. involucrata, leaf explants were inoculated with Agrobacterium cells harboring p1390UbiHd3a, and the subsequent tissue culture was performed as described above.
Southern and Northern bolt analyses
Genomic DNA was isolated from leaves of nontransformed and transformed plants as well as from transformed flowers using the cetyl trimethylammonium bromide (CTAB) method (Muhammad et al. 1994). Firstly, to examine whether any residual Agrobacterium existed in the regenerated plants, Agrobacterium VirB1 gene-specific primers VBFw (5′-GAA GGC AAC AGG GCC GCT GTC-3′) and VBRe (5′-TCC GCC CTC CGG GGA ACG ACG C-3′) were used to amplify the 630-bp fragment from VirB1. The reaction mixture for PCR of hpt was incubated in a DNA thermal cycler under the following conditions: 94°C for 5 min, followed by 30 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min, with a final 10 min extension at 72°C; for PCR of Hd3a, the annealing temperature was 55°C. For Southern blot analysis, 5 μg genomic DNA was digested with the restriction enzyme HindIII, separated on 0.8% (w/v) agarose gel, and transferred to Hybond N+ nylon membrane. The hpt probe was labeled with digoxigenin (DIG) by PCR method using the primers HFw (5′-CGA TCT TAG CCA GAC GAG CGG GTT C-3′) and HRe (5′-GCT GGG GCG TCG GTT TCC ACT ATC GG-3′), while the Hd3a probe amplification used the primers Hd3aFw (5′-ATG GCC GGA AGT GGC AGG GAC-3′) and Hd3aRe (5′-CTA GGG GTA GAC CCT CCT G-3′). Prehybridization, washing, and chemiluminescent detection of the blots were performed according to the manufacturer’s instructions (Roche Diagnostics GmbH, Mannheim, Germany).
For Northern blot analysis, RNA was extracted from leaves of nontransformed and transformed plants as well as from transformed flowers according to the Trizol method (Invitrogen, Carlsbad, CA, USA), and 10 μg RNA from each line was used for running on a formaldehyde gel and then blotted to a Hybond N+ membrane. The same Hd3a probe was used to detect the transcript of Hd3a.
Assay for uidA expression in transgenic plantlet
Histochemical staining of β-glucuronidase (GUS) activity was performed according to Jefferson (1987). Hygromycin-resistant callus, transformed buds, and nontransformed buds (wild type) were submerged in a solution containing 5-bromo-4-chloro-3-indolyl glucuronide (X-Gluc) at 37°C for 3 h and then destained in 70% alcohol.
Results
Establishment of an efficient Agrobacterium-mediated leaf disc transformation method
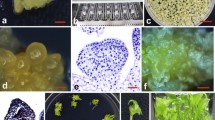
We selected hygromycin as the selection reagent in genetic transformation of Saussurea involucrata Kar. et Kir. ex Maxim according to Li et al. (2006), and demonstrated that all leaves turned brown quickly and failed to produce callus in 2 weeks, and that all buds gradually became brown and died in 2–3 weeks under the selection pressure of 20 mg l−1 hygromycin (Fig. 2a). The Agrobacterium strain EHA105 harboring pCAMBIA1301 vector, which contains the hpt gene and uidA gene, was used to infect the leaf explants. Highly efficient transient GUS expression was observed after 3 days of co-cultivation (Table 1). Hygromycin-resistant callus began to be observed on the surface of the infected brown leaves after 10 days of selection. After 18 days of selection culture, the frequency of resistant calli was about 23.7%. Because of bacterial overgrowth on the brown leaf explants, the calli were divided from the brown explants and transferred to hygromycin-resistant shoot regeneration medium (Fig. 2b). Shoot regeneration began to be observed on the surface of the callus after 35 days of culture, and about 67.5% of the hygromycin-resistant calli could differentiate into shoots (Table 1). Subsequently, all the shoots could further develop on the resistant shoot selected medium (Fig. 2c), and 80% of the hygromycin-resistant shoots rooted on the rooted medium successfully (Fig. 2d).
Regeneration of transgenic plants from leaf explants in Saussurea involucrata Kar. et Kir. ex Maxim. a, c Non-transformed buds (a) and transformed buds (c) after a 20-day culture on hygromycin-resistant shoot regeneration medium. b Hygromycin-resistant calli after a 6-day culture in the light on hygromycin-resistant shoot regeneration MS medium containing 1.5 mg l−1 BA, 0.1 mg l−1 NAA, 0.25 mg l−1 GA3, 20 mg l−1 hygromycin, and 250 mg l−1 cefotaxime. d Regenerated plantlet after a 28-day culture on rooting medium. e, f, g GUS assay of transformed callus derived from leaf explant (e), bud (f), and non-transformed bud (g). h Regenerated plants grown in plant growth chamber after a 45-day culture
Histochemical GUS assay showed that the transgene was expressed in resistant calli (Fig. 2e) and resistant buds (Fig. 2f), while there was no GUS staining in buds of nontransformed plant control (Fig. 2g).
Southern blot analysis was further used to confirm the presence of T-DNA in the S. involucrata genome. Firstly, the VirB1-specific primers were also used to examine the presence of residual Agrobacterium in the regenerated buds. Amplification of the genomic DNA from Agrobacterium produced the 630-bp band, while amplification of DNAs from different transgenic buds did not give this band (data not shown). Southern blot analysis using the hpt probe was performed on randomly selected transgenic lines (T1, T2, T3, and T4). The results showed that all the selected lines had T-DNA integrated in their genome. As the genomic DNA was restricted with HindIII, which cuts only once inside the T-DNA region in pCAMBIA 1301, the number of hybridization bands reflects the copy number of the T-DNA integrated into the S. involucrata genome. The T-DNA copies integrated into the transgenic lines ranged from one to two (Fig. 3). No hybridization signal was detected in samples of nontransformed plants. These results indicated that all the transgenic plants were from independent transgenic events, as they had different copy numbers of T-DNA integrated into different positions of the genome.
Totally, 27 independent transgenic lines were regenerated from 169 leaf explants in three independent experiments. On average, 16 transgenic lines were obtained from every 100 leaf explants after approximately 5 months (Table 1). The rooted transgenic plantlets were repotted in a plant growth chamber at 17 ± 1°C with a 14-h photoperiod under illumination at 35 μmol m−2 s−1 from cool-white fluorescent lamps; about 75% of the transgenic plants survived under these conditions after 45 days of transplanting (Fig. 2h).
Ectopic expression of Hd3a causes early flowering
To explore whether Hd3a from rice is able to induce early flowering in S. involucrata, Hd3a driven by the maize Ubiquitin promoter with Ubiquitin intron was transformed into S. involucrata genome for functional analysis using the transformation method described above. The first flower-like structure initiated directly from the hygromycin-resistant callus after 12 days on the shoot regeneration medium (Fig. 4a, b), compared with 28 days for regenerated shoot, while in wild-type S. involucrata, it takes 5–6 years to blossom in special regions with peculiar climate (Chen et al. 1999). Twenty independent Ubiquitin::Hd3a transgenic S. involucrata lines were obtained. Fifteen lines showed identical phenotypes, which were flower structures initiated directly from callus without any leaves or buds (Fig. 4c, d, e), one line with obvious stem blossomed in 45 days after transfer onto shoot regeneration medium (Fig. 4f), another line blossomed in 28 days after transfer onto rooting medium (Fig. 4g), whereas three lines were phenotypically indistinguishable from untransformed wild-type plants, one blossoming in 20 days after transplanting to the plant growth chamber (Fig. 4h).
Hd3a overexpression induces early flowering in Saussurea involucrata Kar. et Kir. ex Maxim. a, c Overview of a tissue culture plate with hygromycin-resistant calli transformed with Ubiquitin::Hd3a after 18-day culture (a) and 28-day culture (c) on shoot regeneration medium. b, d Close-up view of a developing flower initiated directly from callus (b) and a section of a flower (d). e Flowering cluster initiated directly from callus after 35 days on shoot regeneration medium. f Flowering stem after 45 days on shoot regeneration medium. g Regenerated flowering plant after 28-day culture on rooting medium. h Regenerated plant blossomed in plant growth chamber after 20-day culture
Southern blot analysis was used to confirm the presence of Hd3a in the transgenic S. involucrata genome, as well as the copy number integrated. As the genomic DNA was restricted with HindIII, which is the only restriction site in T-DNA region, the number of hybridization bands reflected the copy number of the T-DNA integrated into the S. involucrata genome (Fig. 1). Southern blotting using Hd3a as a probe detected hybridization signal in nontransformed plants, indicating that S. involucrata contains Hd3a-homologous sequences. We observed hybridization signals in samples from transgenic lines (lines 1 and 2 are flower clusters; lines 3 and 4 are flowering plants) that differed from those observed in nontransformed samples (Fig. 5a). Comparing the band patterns between WT and Hd3a transgenic lines, we concluded that one T-DNA copy had integrated into transgenic line 4, while lines 1, 2, and 3 had two copies (Fig. 1b). These results also indicated that lines 1, 2, 3, and 4 derived from independent transgenic events.
Detection of a foreign gene Hd3a in transgenic lines by Southern blot analysis (a) and expression of Hd3a in transgenic lines by Northern blot analysis (b). P, constructed p1390UbiHd3a. WT, regenerated nontransformed plants. 1 and 2, flowering cluster initiated directly from callus. 3 and 4, leaves from different Hd3a transgenic flowering plants. Arrow indicates Hd3a-homologous sequence in S. involucrata
Northern blot analysis of transgenic lines with Hd3a probe showed the presence of a single, expected 539-bp transcript in transformed lines (Fig. 5b). A hybridization signal also appeared in nontransformed plant, indicating that an ortholog of Hd3a is expressed in leaves of S. involucrata. Compared with the intensity of the hybridization signal of WT, the transgenic lines showed higher intensity of hybridization signals (Fig. 5b). These results obviously indicated that the early-flowering phenotype in the Ubiquitin::Hd3a transgenic S. involucrata was due to overexpression of the rice Hd3a gene.
Discussion
Fu et al. (2004, 2005b) first reported a hairy root system for S. involucrata using an A. rhizogenes-mediated transformation method, and successfully transferred chalcone isomerase (CHI) from S. medusa into the genome of S. involucrata to produce higher levels of flavonoids from hairy roots (Li et al. 2006). Intact plantlets could be regenerated from hairy roots; however, further molecular evidence confirming integration of target gene in the genome of the regenerated plants has not been described (Fu et al. 2004). Our transformation method is based on an Agrobacterium-mediated leaf disc transformation. Starting from the original seeds, it is possible to obtain transgenic plants growing on soil within 6 months. Since S. involucrata is a rare plant that lives under specific environmental conditions, its seeds are naturally scarce. Leaves of the regenerated shoots could be also used as explants for genetic transformation using our transformation method, the transformation frequency being the same as when using leaf explants from seedlings. On the other hand, about 20% brown leaf explants caused by hygromycin selection were lost because of bacteria overgrowth. To improve the transformation efficiency, it is better to transfer hygromycin-resistant calli from the brown explants to fresh media as soon as possible.
As wild S. involucrata grows only in Alpine regions, near or above the snowline, cultivation of this species in a normal climate has not been reported so far. More recently, Zhao and Wang (2008) reported that regenerated plants could successfully survive and blossom in the third year after transplanting to Taishan Mountain; however, the cultivation conditions have not been described. We tried to plant the transgenic plantlets in the plant growth chamber at 17 ± 1°C; about 75% of the transgenic plants could survive under these conditions after 45 days of transplanting. Optimization of cultivation conditions for S. involucrata is now under further investigation.
S. involucrata is a valuable medicinal herb, well known for its flavonoids (Zhao et al. 2001; Fu et al. 2005a; Chu et al. 2006) and high tolerance to harsh, very cold Alpine climate with extremely high ultraviolet radiation and drought. The efficiency of this transformation protocol will allow us to easily generate mutants up- or downregulated for various key enzymes involved in flavonoid biosynthetic pathways (Cheng et al. 2007) and abiotic stress response pathways, and to understand the regulation or accumulation of specific flavonoid metabolites and its abiotic stress tolerance. A new set of mutants has recently been produced via dominant negative suppression of a rice MutL homolog, OsPMS1, using this transformation method.
Basic biological studies of S. involucrata are limited due to the difficulty of the reproductive success of this valuable plant under normal growth conditions. However, in flowering plants, the flower is the most important plant part for reproduction. An efficient flower regeneration protocol for S. involucrata is presented herein for the first time, with modified Hd3a expression under the control of the maize Ubiquitin promoter using our transformation method.
More recently, Hd3a and its orthologs have been proposed as florigens, or mobile flowering signals (Corbesier et al. 2007; Lin et al. 2007; Tamaki et al. 2007; Notaguchi et al. 2008; Xi and Yu 2009). Overexpression of Hd3a or its ortholog has been shown to cause an early-flowering phenotype in Arabidopsis, poplar (Böhlenius et al. 2006; Hsu et al. 2006; Zhang et al. 2010), rice (Tamaki et al. 2007), trifoliate orange (Endo et al. 2005), and European pear (Matsuda et al. 2009). Flower-like structures initiated directly from the stem explant transformed with 35S::PtFT1 within 4 weeks (Böhlenius et al. 2006), and flowers appeared early in transgenic plants (Kobayashi et al. 1999; Kojima et al. 2002; Endo et al. 2005; Böhlenius et al. 2006; Hsu et al. 2006; Tamaki et al. 2007; Matsuda et al. 2009). Our results also showed that overexpression of Hd3a accelerated flowering in S. involucrata. In addition to its early-flowering phenotype, the transgenic Hd3a callus could differentiate directly into flower cluster, and it took the callus less time to differentiate into flower than to differentiate into buds. However, these phenomena have not been described in plants overexpressing FT or its ortholog (Kobayashi et al. 1999; Kojima et al. 2002; Endo et al. 2005; Böhlenius et al. 2006; Hsu et al. 2006; Tamaki et al. 2007; Hou and Yang 2009; Matsuda et al. 2009).
The flower of S. involucrata is a precious source of medicines and plays an important role in reproduction. The efficiency of our flower regeneration system will allow us to easily generate flower in vitro for biological studies of flower development, bioactive phytochemicals in flower, and rapid mass production of flowers for medicinal purposes. Since the Ubiquitin promoter-driven Hd3a expression is so strong in S. involucrata, causing flowers to initiate directly from callus, we are now producing transgenic plants with modified Hd3a expression under an abiotic stress-inducible promoter, rd29A.
References
Bakhshaie M, Babalar M, Mirmasoumi M, Khalighi A (2010) Somatic embryogenesis and plant regeneration of Lilium ledebourii (Baker) Boiss., an endangered species. Plant Cell Tiss Organ Cult 102:229–235
Böhlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss SH, Nilsson O (2006) CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312:1040–1043
Chen H, Nelson RS, Sherwood JL (1994) Enhanced recovery of transformants of Agrobacterium tumefaciens after freeze–thaw transformation and drug selection. Biotech 16:664–670
Chen FJ, Yang YG, Zhao DX, Gui YL, Guo ZC (1999) Advances in studies of species, habitats, distribution and chemical composition of snow lotuses (Saussurea) in China. Chinese Bull Bot 16:561–566
Chen W-Q, Yang Z-M, Hu T-P, Lin Y-Z, Huang L-T, Wang D-N (2010) Study of the effects of the ethanol extraction of cultured Saussurea on anti-inflammation. China Prac Med 5(4):12–14
Cheng L, Xu Y, Grotewold E, Jin Z, Wu F, Fu C, Zhao D (2007) Characterization of Anthocyanidin Synthase (ANS) gene and anthocyanidin in rare medicinal plant-Saussurea medusa. Plant Cell Tiss Organ Cult 89:63–73
Chu QC, Fu L, Cao YH, Ye JN (2006) Electrochemical profiles of herba Saussureae involucratae by capillary electrophoresis with electrochemical detection. Phytochem Anal 17:176–183
Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, Coupland G (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316:1030–1033
Encyclopaedia of Traditional Chinese Medicine (1995) Shanghai Science and Technology Press, Shanghai, pp 2087–2088
Endo T, Shimada T, Fujii H, Kobayashi Y, Araki T, Omura M (2005) Ectopic expression of an FT homolog from Citrus confers an early flowering phenotype on trifoliate orange (Poncirus trifoliata L. Raf.). Transgenic Res 14:703–712
Fu LG (1992) China plant red data book-rare and endangered plants, vol 1. Chinese Science, Beijing, pp 234–235
Fu CX, Jin ZP, Yang R, Wu FY, Zhao DX (2004) Establishment of Saussurea involucrata hairy roots culture and plantlet regeneration. Chin J Biotech 20:366–371
Fu CX, Xu YJ, Zhao DX, Ma FS (2005a) A comparison between hairy root cultures and wild plants of Saussurea involucrata in phenylpropanoids production. Plant Cell Rep 24:750–754
Fu CX, Zhao DX, Xue XF, Jin ZP, Ma F (2005b) Transformation of Saussurea involucrata by Agrobacterium rhizogenes: hairy root induction and syringin production. Process Biochem 40:3789–3794
Guo B, Gao M, Liu C-Z (2007) In vitro propagation of an endangered medicinal plant Saussurea involucrata Kar. et Kir. Plant Cell Rep 26:261–265
Han SL (1995) Study on the effect of four chemical constituents against cancer of Saussurea involucrata. Carcinogen Teratogen Mutagen 7:80–83
He X, Li GH, Chen HY (1990) Studies on the antiphlogistic effect and mechanism of the flavones from S. involucrata Kar. et Kir. J Northwest Pharm 5:17–19
Hou C-J, Yang C-H (2009) Functional analysis of FT and TFL1 orthologs from orchid (Oncidium Gower Ramsey) that regulate the vegetative to reproductive transition. Plant Cell Physiol 50(8):1544–1557
Hsu C-Y, Liu Y, Luthe DS, Yuceer C (2006) Poplar FT2 shortens the juvenile phase and promotes seasonal flowering. Plant Cell 18:1846–1861
Irvani N, Solouki M, Omidi M, Zare AR, Shahnazi S (2010) Callus induction and plant regeneration in Dorem ammoniacum D., an endangered medicinal plant. Plant Cell Tiss Organ Cult 100:293–299
Jefferson RA (1987) Assaying chimeric genes in plants: the Gus gene fusion system. Plant Mol Biol Rep 5:387–405
Jia JM, Wu CF, Liu W, Yu H, Hao Y, Zheng JH, Ji YR (2005) Antiinflammatory and analgesic activities of the tissue culture of Saussurea involucrata. Biol Pharm Bull 28(9):1612–1614
Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D (1999) Activation tagging of the floral inducer FT. Science 286:1962–1965
Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T (1999) A pair of related genes with antagonistic roles in mediating flowering signals. Science 286:1960–1962
Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, Yano M (2002) Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol 43:1096–1105
Li FX, Jin ZP, Zhao DX, Cheng LQ, Fu CX, Ma F (2006) Overexpression of the Saussurea medusa chalcone isomerase gene in S. involucrata hairy root cultures enhances their biosynthesis of apigenin. Phytochemistry 67:553–560
Li M, Li H, Jiang H, Pan X, Wu G (2008a) Establishment of an Agrobacteriuim-mediated cotyledon disc transformation method for Jatropha curcas. Plant Cell Tiss Org Cult 92:173–181
Li M, Li H, Jiang H, Wu G (2008b) Establishment of a highly efficient Agrobacterium tumefaciens-mediated leaf disc transformation method for Broussonetia papyrifera. Plant Cell Tiss Org Cult 93:249–255
Lin K, Wang X-J, Zhao M-A, Liu M, Bo L-T (2006) Induction and differentiation of somatic embryos of Saussurea involucrata. Acta Bot Boreal-Occident Sin 26(7):1351–1354
Lin MK, Belanger H, Lee YJ, Varkonyi-Gasic E, Taoka K, Miura E, Xoconostle-Cázares B, Gendler K, Jorgensen RA, Phinney B, Lough TJ, Lucas WJ (2007) FLOWERING LOCUS T protein may act as the long distance florigenic signal in the cucurbits. Plant Cell 19:1488–1506
Liu LS, Xiao XH, Zhang LD (1985) Effect of the flavonoids from Saussurea involucrata on DNA synthesis of cancer cells. J Lanzhou Univ Nat Sci 4:80–83
Matsuda N, Ileda K, Kurosaka M, Takashina T, Isuzugawa K, Endo T, Omura M (2009) Early flowering phenotype in transgenic pears (Pyrus communis L.) expressing the CiFT gene. J Japan Soc Hort Sci 78(4):410–416
Muhammad AL, Ye GN, Weeden NF, Reisch BI (1994) A simple and efficient method for DNA extraction from grapevine cultivars, Vitis species and Ampelopsis. Plant Mol Biol Rep 12:6–13
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Notaguchi M, Abe M, Kimura T, Daimon Y, Kobayashi T, Yamaguchi A, Tomita Y, Dohi K, Mori M, Araki T (2008) Long-distance, graft-tramsmissible action of Arabidopsis FLOWERING LOCUS T protein to promote flowering. Plant Cell Physiol 49:1645–1658
Offord CA, Tyler JL (2009) In vitro propagation of Pimelea spicata R.Br (Thymelaeaceae), an endangered species of the Sydney region, Australia. Plant Cell Tiss Organ Cult 98:19–23
Takimoto I, Christensen AH, Quail PH, Uchimiya H, Toki S (1994) Non-systemic expression of a stress-responsive maize polyubiquitin gene (Ubi-1) in transgenic rice plants. Plant Mol Biol 26:1007–1012
Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K (2007) Hd3a protein is a mobile flowering signal in rice. Science 316:1033–1036
Way TD, Lee JC, Kuo DH, Fan LL, Huang CH, Lin HY, Shieh PC, Kuo PT, Liao CF, Liu H, Kao JY (2010) Inhibition of epidermal growth factor receptor signaling by Saussurea involucrata, a rare traditional Chinese medicinal herb, in human hormone-resistant prostate cancer PC-3 cells. J Agric Food Chem 58(6):3356–3365
Wu LQ, Guo SX, Xiao PG (2005a) Tissue culture and plantlet regeneration from embryo of Saussurea involucrata. China J Chin Materia Medica 30(11):814–816
Wu LQ, Guo SX, Xiao PG (2005b) Cell suspension culture and flavonoids production in Saussurea involucrata. China J Chin Materia Medica 30(13):965–968
Wu W, Qu Y, Gao H, Yang J, Xu J, Wu L (2009) Novel ceramides from aerial parts of Saussurea involucrata Kar. et. Kir. Arch Pharm Res 32(9):1221–1225
Xi W, Yu H (2009) An expanding list: another flowering time gene, FLOWERING LOCUS T, regulates flower development. Plant Signal Behav 4(12):1142–1144
Yang L, Zou JH (2005) In vitro tissue culture and plantlet regeneration of Saussurea involucrata Kar. et Kir. Physiol Comm 41(4):553
Zhang H, Harry DE, Ma C, Yuceer C, Hsu C-Y, Vikram V, Shevchenko O, Etherington E, Strauss SH (2010) Precocious flowering in trees: the FLOWERING LOCUS T gene as a research and breeding tool in Populus. J Exp Bot 61(10):2549–2560
Zhao H-Q, Wang X-J (2008) Establishment of rapid propagation technique in tissue culture of Saussurea involucrata Kar. et Kir. fromTianshan Mountain. J Anhui Agri Sci 36(7):2743–2744–2746
Zhao D, Xing J, Li M, Lu D, Zhao Q (2001) Optimization of growth and jaceosidin production in callus and cell suspension cultures of Saussurea medusa. Plant Cell Tiss Organ Cult 67:227–234
Zheng RL, Liu GS, Xing GX, Jia ZJ, Du M, Tan LQ (1993) Free radical scavenging and antifatigue activities of Saussurea involucrata polysaccharides. Acta Pharmacol Sin 14(Suppl):47–49
Acknowledgments
This research was supported by the CAS/SAFEA International Partnership Program for Creative Research Teams and the Funds for Guangdong Provincial Key Laboratory of Biotechnology for Plant Development, South China Normal University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, M., Li, H., Hu, X. et al. Genetic transformation and overexpression of a rice Hd3a induces early flowering in Saussurea involucrata Kar. et Kir. ex Maxim. Plant Cell Tiss Organ Cult 106, 363–371 (2011). https://doi.org/10.1007/s11240-011-9927-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-011-9927-5