Abstracts
Primulina tabacum is a rare and endangered species that is endemic to China. Establishing an efficient regeneration system is necessary for its conservation and reintroduction. In this study, when leaf explants collected from plants grown in four ecotypes in China are incubated on Murashige and Skoog (MS) medium containing 5.0 μM thidiazuron (TDZ) for 30 days, then transferred to medium containing 5.0 μM 6-benzyladenine (BA), adventitious shoots are then observed. Conversely, when leaf explants are incubated on medium containing 5.0 μM BA for 30 days, then transferred to medium containing 5.0 μM TDZ, somatic embryogenesis is induced. This indicates that somatic embryogenesis and shoot organogenesis could be switched simply by changing the order of two cytokinins supplemented in the culture medium. Histological investigation has revealed that embryogenic cells are induced within 30 days following incubation of explants in medium containing TDZ. Only if embryogenic cells were induced, TDZ could enhance somatic embryogenesis and BA could stimulate shoot organogenesis. When comparing explants from different ecotypes, leaf explants from Zixiadong in Hunan Province could induce low numbers (1–2) of either somatic embryos or adventitious shoots on medium containing either 5.0 μM TDZ or 5.0 μM BA, respectively. Whereas, leaf explants from plants collected from the other three ecological habitats could induce 50–70 somatic embryos/adventitious shoots per explant. Moreover, somatic embryos could induce secondary somatic embryogenesis and adventitious shoots on different media. All regenerated shoots developed adventitious roots when these are transferred to rooting medium, and over 95% of plantlets have survived following acclimatization and transfer to a potting mixture (1:1, sand:vermiculite).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The species Primulina tabacum Hance (Gesneriaceae) was first discovered in the Lianjiang limestone drainage areas in Lianzhou, North Guangdong, China in 1881 (Hance 1883), followed shortly thereafter by its disappearance for more than 120 years. Its recent rediscovery has important scientific value in the study of both ancient and recent climate, soil and co-evolutionary biology of animals and plants in South China in the Five Ridges Region (Ni et al. 2006; Wang et al. 2009; Liang et al. 2010). Now the species P. tabacum has been listed as a ‘first grade’ critically endangered species in China (Peng and Cheng 2002; He and Li 2005). In order to preserve and utilize this rare and endangered plant species, it is absolutely necessary to establish a secure in situ environment and it is prudent to establish an efficient propagation and plant regeneration system in the event of sudden deterioration or loss of the natural environment. We have reported on the micropropagation of P. tabacum and also its reintroduction to its natural habitats (Ma et al. 2010; Ren et al. 2010a, b). Those are the first reports on the reintroduction of a rare and endangered plant species via biotechnology in China. Some other scientists have also used biotechnology to conserve and reintroduce rare and endangered plants back into their natural environments (Nikabadi et al. 2010; Bunn et al. 2011). However, micropropagation and reintroduction of micropropagated material could undoubtedly decrease the genetic diversity of plant species which would not benefit its long-term reproduction and conservation. To avoid this, it is necessary to increase the number of plants that are inoculated and extend exploration of its original habitats.
Somatic embryogenesis and shoot organogenesis are different pathways encompassed within in vitro morphogenesis. Different species, genotypes or explants may need different culture media or conditions to induce somatic embryogenesis or shoot organogenesis (Gregory 2004). In P. tabacum, somatic embryogenesis and shoot organogenesis have both been successfully induced on different induction media which contained one of the two different cytokinins: thidiazuron (TDZ) and 6-benzyladenine (BA) (Ma et al. 2010). In this report, we increased the number of ecotypes from which explants were derived and investigated some differences related to the induction of somatic embryogenesis and shoot organogenesis from these four habitats. Secondary somatic embryogenesis and adventitious shoot formation were also explored.
Materials and methods
Somatic embryogenesis and shoot organogenesis from leaf explants in vivo
Plants of P. tabacum (approximately 10–15 cm high) were harvested in 2007–2008 from four different ecotypes: Dixiahe (Fig. 1a) and Qingjiang (Fig. 1b) in Lianzhou region of the Guangdong Province as well as Xiaguan (Fig. 1c) and Zixiadong (Fig. 1d) in Jiunishan region of the Hunan Province. Among these, plants collected from Dixiahe were the earliest to be discovered in the 1880s by Hance (1883); moreover, plants collected from Zixiadong were the most remote ecotypes from Dixiahe.
Healthy leaves were surface sterilized in 70% (v/v) alcohol for 10 s and 0.1% (w/v) mercuric chloride for 8 min, rinsed with sterile distilled water 3 times, then cut into 1.0 cm2 explants and placed adaxial surface down on MS basal media (Murashige and Skoog 1962) supplemented with 5.0 μM TDZ for inducing somatic embryogenesis and 5.0 μM BA for inducing shoot organogenesis. All the media contained 30 g l−1 sucrose and were adjusted to pH 6.5 and solidified with 0.6% agar (Ma et al. 2010). Culture jars are maintained in a controlled environment growth room at 26 ± 2°C and a 12-h photoperiod with low diffuse light (PPFD <10 μmol m−2 s−1). The number of induced somatic embryos or adventitious shoots was counted after culture for 80 days (Table 1).
Proliferation of shoots
Clumps of adventitious shoots with 8–10 shoots each were transferred to MS medium containing 5.0 μM BA and 0.5 μM α-naphthaleneacetic acid (NAA) and cultured under a 12-h photoperiod with PPFD <10 μmol m−2 s−1. The adventitious shoots were subcultured on the same medium every 40 days.
Induction of secondary somatic embryogenesis and shoot organogenesis
Primary somatic embryos, and in vitro leaves and petioles from the above culture were isolated and inoculated on different induction media, cultured in the dark for 30 days then incubated under dim light (PPFD <10 μmol m−2 s−1). After culturing for a total of 60 days, the number of somatic embryos or shoots per explant was recorded. The experiment was repeated once and data was analyzed using the LSD test (P = 0.05).
Effect of cytokinins on induction media and duration of the induction period on somatic embryogenesis and shoot organogenesis
In vitro leaves 0.6 cm2 in size were used as explants and cultured on induction media in the dark. The induction media contained 5.0 μM BA and 5.0 μM TDZ, for induction of shoot organogenesis and somatic embryogenesis, respectively. After culturing for 15, 30 and 45 days in darkness, the leaf explants (or callus clumps) were transferred to other media containing 5.0 μM TDZ, 5.0 μM BA or free of plant growth regulators for further culture with a 12-h photoperiod at low light intensity (PPFD <10 μmol m−2 s−1). After culturing for a total of 60–80 days, the number of induced somatic embryos or adventitious shoots were counted. For each treatment the number of explants was 70–100 and all experiments were repeated twice (Table 2). All data were statistically analyzed by one-way AVOVA with a post hoc test (PLSD) used to separate treatment means (P ≤ 0.05).
Plantlet root formation and transplanting
Single shoots were isolated from multiple shoot clusters and transferred to rooting medium containing 0.5 μM indole-3-butyric acid (IBA) and 0.2% activated carbon for root formation. After 50 days of incubation in light at PPFD = 50 μmol m−2 s−1, plantlets were removed from the jars and roots were washed to free agar. Rooted shoots were transferred to sand:vermiculite (1:1) and acclimatized in a shaded glasshouse at 25 ± 3°C and 90 ± 5% relative humidity. Survival rate and growth were monitored. Three months later, single plantlets were transferred to plastic pots 12 cm high with a potting mixture of humus and pond sludge (1:1) for continuous culture under the same conditions as for initial stages of acclimatization.
Histological investigation of somatic embryogenesis of in vitro-grown leaf explants
To study the ontogeny and development of embryogenic cell masses, somatic embryos and adventitious shoots, leaf explants incubated on induction medium containing 5.0 μM TDZ and 5.0 μM BA, were subjected to histological analysis. The culture jars were cultured in the dark for 30 days then transferred to low-light condition (<10 μmol m−2 s−1). After culturing for 0, 10, 20, 30, 45 and 60 days, the leaves (or callus clumps) were fixed in FAA (1:1:18, formaldehyde: glacial acetic acid: 70% alcohol) solution and maintained at room temperature for one week, then transferred to 70% alcohol for storage until required for analysis. The samples were stained with hematoxylin at first, then dehydrated in an increasing alcohol series (35%, 50%, 75%, 85%, 95% and pure alcohol), and embedded in paraffin. Transverse sections 8 μm thick were made with a paraffin-compatible microtome (Fourth Shanghai Medicine Manufacturing Co.) and then laid on filter paper with a brush. The sections were placed in parallel on a slide with 0.1% formalin at 40–45°C to allow the sections to stretch. Slides were then placed in a warm box at 60°C. The sections were dewaxed with xylene three times and finally covered with neutral balsam. Sections were observed under a microscope (Olympus SZX12).
Results
Somatic embryogenesis and shoot organogenesis from leaf explants in vivo
Leaf explants seemed not to respond at first when placed on medium supplemented with 5.0 μM TDZ. As the culture time progressed to 60 days, globular-like protuberances protruded from the leaf surface in some of the cultured explants and generally formed somatic embryos (Fig. 2a). As the culture time progressed, more and more somatic embryos were induced on the leaf surface or on leaf edges. Somatic embryos appeared to be globular when first observed then progressed to more mature stages over time. Finally, shoot buds developed from apex of the somatic embryo, but could not develop into seedlings on the same induction medium (Fig. 2a).
Somatic embryogenesis and shoot organogenesis in P. tabacum (Bar = 2 mm). a Primary somatic embryogenesis induced from a leaf explant (white arrows indicate shoot buds developing at the top of somatic embryos) on the induction medium supplemented with 5.0 μM TDZ; b adventitious shoots formation induced from one leaf explant on the induction medium supplemented with 5.0 μM BA; c multiple shoot propagation on the medium supplemented with 5.0 μM BA and 0.5 μM NAA; d secondary somatic embryogenesis induced from a primary somatic embryo on induction medium supplemented with 5.0 μM TDZ; e adventitious shoots formation induced from one leaf stalk in vitro on the induction medium supplemented with 5.0 μM BA; f Adventitious shoot formation induced from one primary somatic embryo; g adventitious shoot formation induced from one leaf explant in vitro on the induction medium supplemented with 5.0 μM BA; h Both somatic embryos (white arrows) and adventitious shoots (black arrows)) were induced on the same leaf explant on the induction medium supplemented with 5.0 μM TDZ for 30 days then transferred to the medium containing 5.0 μM BA for continue culture; i plantlets in a plastic tray with sand:vermiculite (1:1) potting mixture for 2 months after transplanting from in vitro conditions. j flowering of a transplanted plant after 1 year
When leaf explants were cultured on medium supplemented with 5.0 μM BA for 60 days, many bud-like protuberances were induced on the leaf surface or leaf edge (Fig. 2b). As the culture incubation period increased more and more adventitious shoot buds were induced and some of these developed multiple shoots. The maximum number of adventitious shoots/explant observed exceeded 100 on one leaf explant.
Among the four habitats, leaf explants in three habitats (Fig. 1a, b, c) could induce averagely 50–70 somatic embryos or adventitious shoots (Table 1; Fig. 2a, b). However, only the leaf explants from habitat Zixiadong (Fig. 1d) of Hunan Province could induce very few somatic embryos or adventitious shoots (1–2). Due to its low index of propagation, we could not establish an efficient propagation system for the latter ecotype on the above media.
Proliferation of shoots
When adventitious shoot clumps were transferred to propagation medium containing 5.0 μM BA and 0.5 μM NAA, they developed into multiple shoot clumps. As the culture period was extended to 2 months, some adventitious roots became visible at the base of shoots (Fig. 2c).
Induction of secondary somatic embryogenesis and shoot organogenesis
Primary somatic embryos, leaves and leaf stalks, all from an in vitro source, could be used as explants and cultured on induction media producing almost the same results as previous experiments using leaf explants, i.e. TDZ-induced secondary somatic embryos from primary somatic embryos (Fig. 2d). Adventitious shoots induced by BA could form from primary somatic embryos, leaves and petioles (Fig. 2e–g). However, the number of somatic embryos or adventitious shoots produced was observed to be less than with primary explants sourced from in vivo tissue (Table 1). This result may be related to the size and thickness of explants because explants produced in vitro were much smaller than primary leaf explants sourced from in planta tissues.
Effect of cytokinins on the induction media and the duration of the induction period on somatic embryogenesis and shoot organogenesis
As the leaf explants in vitro were cultured on medium containing 5.0 μM TDZ for 15 days and then transferred to medium containing 5.0 μM BA for further culture, some globular somatic embryos became visible within 60 days. As the culture was transferred to medium containing no plant growth regulators, neither somatic embryos nor adventitious shoots could be observed (Table 2). When cultured for 30 days on TDZ-containing medium, some somatic embryos were observed on the leaf explants while after 45 days, both some somatic embryos and adventitious shoots were visible (Table 2).
Similarly, when leaf explants in vitro were cultured on medium containing 5.0 μM BA for 15 days and then transferred to medium containing 5.0 μM TDZ for further culture, some adventitious shoots became visible within 60 days (Fig. 2h). Similarly, as the culture was transferred to medium containing no plant growth regulators, neither somatic embryos nor adventitious shoots were observed on leaf explants (Table 2). When cultured for 30 days on BA-containing medium, some adventitious shoots were observed on the leaf explants while after 45 days, more adventitious shoots were visible (Table 2).
Plantlet root formation and transplanting
Single shoots were isolated from multiple shoot clusters and transferred to rooting media. Roots formed fastest within 20 days of continuous culture in the light. After incubation for a total of 50 days, rooted shoots were transferred to plastic trays containing sand:vermiculite (1:1). More than 95% of the plantlets could survive and no obvious morphological variation was observed (Fig. 2i). After plantlets were transferred to plastic pots, they could flower normally only after 1 year (Fig. 2j).
Histology of somatic embryogenesis from leaves in vitro
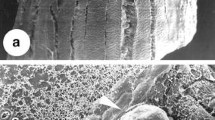
Histological examination showed only 3–4 layers of mesophyll cells in fresh leaves (Fig. 3a). On induction medium with 5.0 μM TDZ or 5.0 μM BA, even when cultured for 10–20 days, there were no embryogenic cell clusters and leaf explants did not become swollen since the layers of mesophyll cells increased (Fig. 3b). After culturing for 30 days, some embryogenic cell masses, darkly stained, were observed (Fig. 3c–e). As culture time was prolonged on induction medium, some globular somatic embryos were observed on the surface of one side of leaf explants when cultured for 40–50 days (Fig. 3f). In a few cases, both sides of leaf explants could induce somatic embryos. As culture time was extended to 50–60 days on induction medium, globular-shaped structures, the somatic embryos, generally developed into heart- or torpedo-shaped somatic embryos (Fig. 3g). The induced somatic embryos could develop into shoot buds when left on the same induction medium (Fig. 3h). After leaf explants were cultured on medium containing 5.0 μM BA for 50–60 days, some adventitious shoots formed (Fig. 3i).
Light microscopic sections of somatic embryogenesis and shoot organogenesis from leaf explant in vitro in P. tabacum (Bar = 0.2 mm). a Fresh leaf section; b leaf explant after culturing for 20 days on the induction medium supplemented with 5.0 μM TDZ or 5.0 μM BA; c leaf explant culturing for 30 days showing primordial somatic embryo cell clumps on the induction medium supplemented with 5.0 μM TDZ or 5.0 μM BA; d, e primordial somatic embryo cell clumps were more visible on the leaf after culturing for 40 days on the induction medium supplemented with 5.0 μM TDZ; f globular somatic embryo was visible on the surface of leaf after culturing for 50 days on the induction medium supplemented with 5.0 μM TDZ; g heart-stage somatic embryos were observed on both sides of a leaf after culturing for 60 days on the induction medium supplemented with 5.0 μM TDZ; h germination of a somatic embryos after culturing for 70 days on the induction medium supplemented with 5.0 μM TDZ; i adventitious shoots were visible after culturing for 60–70 days on the induction medium supplemented with 5.0 μM BA
Discussion
For most crops, different subspecies, genotypes or cultivars have obvious and different features and, during morphological induction, they usually show different responses to the same induction medium. In recent years, P. tabacum has spread naturally from Guangdong province to neighboring provinces (i.e., Guangxi and Hunan) showing an increasing expansion (Wu et al. 2010). For the wild species, P. tabacum, there is no concept of subspecies, genotype or cultivar. In the process of systematic evolution and expansion, they usually varied to adapt to new environments, particularly the plants in Zixiadong (Fig. 1d), which have almost the same leaf morphology, flower color, and ability to grow in a moist limestone drainage environment (and other characteristics) as plants in Dixiahe (Fig. 1a). P. tabacum is thus considered as the same taxonomic species (Ni et al. 2006; Liang et al. 2010; Ren et al. 2010a, b). In this tissue culture experiment, P. tabacum plants from Zixiadong showed much more differences during induction and propagation than plants from the other three habitats. We thus suggest that P. tabacum plants in Zixiadong be classed as different wild ecotypes. This is the first report identifying a new ecotypes via biotechnology and may be a reason why the species has very high levels of genetic differentiation among populations since its populations are very small (Ni et al. 2006; Liang et al. 2010).
In the family Gesneriaceae, somatic embryogenesis or shoot organogenesis have been successfully induced in some species: Leaf explants of Chirita longgangensis could directly induce somatic embryos along cut edges on induction medium (MS basal) supplemented with 0.5 mg l−1 BA and 0.1 mg l−1 NAA (Tang et al. 2007). In Chirita medica, adventitious buds were induced from leaf explants on MS basal medium with 0.1 mg l−1 NAA and 0.1 mg l−1 BA (Li et al. 2009). In Aeschynanthus radicans, leaf explants produced somatic embryos when induction medium (MS) contained 6.81 μM TDZ and 2.68 μM 2,4-D (Cui et al. 2009). In Sinningia speciosa, leaf explants were cultured on MS medium containing 2.0 mg l−1 BA and 0.2 mg l−1 NAA, producing small green calli, after which adventitious buds formed (Xu et al. 2009). In Metabriggsia ovalifolia, high activity of cytokinins (TDZ or BA) combined with low activity of auxins (NAA, IAA or IBA) could induce shoot organogenesis from leaves (Ma et al. 2011a). In African violet (Saintpaulia ionantha), a high concentration of TDZ (5–10 μM) induce somatic embryogenesis while a low concentration (2.5 μM) induce shoot organogenesis (Mithila et al. 2003; Taha et al. 2009). For P. tabacum, 5.0 μM TDZ induced somatic embryogenesis and 5.0 μM BA induced shoot organogenesis (Ma et al. 2010). As expected, all of these studies indicate that different species within the Gesneriaceae need different combinations and concentrations of PGRs for successful organogenesis.
Auxins usually induce somatic embryogenesis. TDZ could also induce somatic embryogenesis in many species indicating that TDZ has a similar effect as auxin with respect to somatic embryogenesis in these species (Ipekci and Gozukirmizi 2003; Zhang et al. 2005; Khan et al. 2006; Kumari et al. 2008). In some cases, TDZ also induce shoot organogenesis (Hosseini-Nasr and Rashid 2002; Feyissa et al. 2005; Ma et al. 2011b, c). In other cases, TDZ induced somatic embryogenesis at high concentrations and induced shoot organogenesis at low concentrations (Dolendro et al. 2003; Mithila et al. 2003; Ma et al. 2011c). Previous studies indicated that TDZ may act by modulating PGRs, either directly or as a result of induced stress. Other possibilities include the modification of cell membranes, energy levels, nutrient uptake, or nutrient assimilation (Murthy et al. 1998). In all cases, the regeneration pathway maybe either somatic embryogenesis or shoot organogenesis, or both. TDZ is able to alter the pathway of morphogenesis from shoot organogenesis to somatic embryogenesis through a simple change in TDZ concentration or PGR types (Mithila et al. 2003; Ma et al. 2011c). Our results with P. tabacum support the observation that TDZ plays a different role compared with BA. From the results of our histological experiments, during short culture period (less than 30 days), both TDZ and BA could induce were embryogenic cells within 30 days. Only if embryogenic cells were induced, TDZ could enhance somatic embryogenesis and BA could stimulate shoot organogenesis. The embryogenic cells would develop somatic embryos directly as culture time increased. However, once the somatic embryos had been induced, TDZ inhibited their further development into shoots except for a long period culture. During the long culture period (cultured for 30 days or more), once the embryogenic cells were induced, they would develop somatic embryos or adventitious shoots depending on the PGR combination. When leaf explants were cultured on TDZ-contained medium for 30 days, and then transferred to BA-containing media, some somatic embryos and adventitious shoots could both be induced; and in the process of germination and regeneration of somatic embryos, BA also enhanced shoot formation.
Abbreviations
- BA:
-
6-benzyladenine
- TDZ:
-
Thidiazuron
- NAA:
-
α-naphthalene acetic acid
- KIN:
-
Kinetin
- IBA:
-
3-indolebutyric acid
References
Bunn E, Turner SR, Dixon KW (2011) Biotechnology for saving rare and threatened flora in a biodiversity hotspot. In Vitro Cell Dev Biol- Plant 47:188–200
Cui J, Chen JJ, Henny R (2009) Regeneration of Aeschynanthus radicans via direct somatic embryogenesis and analysis of regenerants with flow cytometry. In Vitro Cell Dev Biol- Plant 45:34–43
Dolendro SN, Sahoo L, Sarini NB, Jaiwal PK (2003) The effect of TDZ on organogenesis and somatic embryogenesis in pigeonpea (Cajanus cajan (L.) Mill. sp. Plant Sci 164:341–347
Feyissa T, Welander M, Negash L (2005) In vitro regeneration of Hagenia abyssinica (Bruce) JF Gmel. (Rosaceae) from leaf explants. Plant Cell Rep 24:392–400
Gregory CP (2004) In vitro morphogenesis in plants: recent advances. In Vitro Cell Dev Biol- Plant 40:342–345
Hance HF (1883) Primulina tabacum Hance. J Bot (Lond) 21:169
He KJ, Li YD (2005) Plant resources of national protection grade I in Guangdong province. J Trop Subtrop Bot 13:519–525 (In Chinese)
Hosseini-Nasr M, Rashid A (2002) Thidiazuron-induced shoot-bud formation on root segments of Albizzia julibrissin is an apex-controlled, light-independent and calcium-mediated response. Plant Grow Regul 36:81–85
Ipekci Z, Gozukirmizi N (2003) Direct somatic embryogenesis and synthetic seed production from Paulownia elongate. Plant Cell Rep 22:16–24
Khan H, Siddique I, Anis M (2006) Thidiazuron induced somatic embryogenesis and plant regeneration in Capsicum annuum. Biol Plant 50:789–792
Kumari GK, Ganesan M, Jayabalan N (2008) Somatic organogenesis and plant regeneration in Ricinus communis. Biol Plant 52:17–25
Li J, Xing Q, Chen WL, Guo DH, Shi L (2009) Tissue culture and rapid propagation of Chirita medica D Fang ex WT Wang. Prop Ornam Plant 9:97–101
Liang KM, Lin ZF, Ren H, Liu N, Zhang QM, Wang J, Wang ZF, Guan LL (2010) Characteristics of sun- and shade-adapted populations of an endangered plant Primulina tabacum Hance. Photosynth 48:494–506
Ma GH, He CX, Ren H, Zhang QM, Li SJ, Zhang XH, Bunn E (2010) Direct somatic embryogenesis and shoot organogenesis from leaf explants of Primulina tabacum Hance. Biol Plant 54:361–365
Ma GH, Teixeira da Silva JA, Lü JF, Zhang XH, Zhao JT (2011a) Shoot organogenesis and plant regeneration in Metabriggsia ovalifolia. Plant Cell Tiss Org Cult 105:355–361
Ma GH, Teixeira da Silva JA, Wu GJ (2011b) Direct adventitious shoot formation from apical shoot explants of Euphorbia tirucalli. J Plant Grow Regul 30:114–116
Ma GH, Lü JF, Teixeira da Silva JA, Zhang XH, Zhao JT (2011c) Somatic embryogenesis and shoot organogenesis from leaf and shoot explants of Ochna integerrima (Lour). Plant Cell Tiss Organ Cult 104:157–162
Mithila J, Hall JC, Victor JMR, Saxena PK (2003) Thidiazuron induces shoot organogenesis at low concentrations and somatic embryogenesis at high concentrations on leaf and petiole explants of African violet (Saintpaulia ionantha Wendl.). Plant Cell Rep 21:408–414
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Murthy BNS, Murch SJ, Saxena PK (1998) Thidiazuron: a potent regulator of in vitro plant morphogenesis. In Vitro Cell Dev Biol- Plant 34:267–275
Ni XW, Huang YL, Wu L, Zhou RC, Deng SL, Wu DR, Wang B, Su G, Tang T, Shi S (2006) Genetic diversity of the endangered Chinese endemic herb Primulina tabacum (Gesneriaceae) revealed by amplified fragment length polymorphism (AFLP). Genet 127:177–183
Nikabadi S, Bunn E, Turner S, Stevens J, Dixon K (2010) Development of an in vitro propagation protocol for ex situ conservation of two critically endangered species of Commersonia (Malvaceae) from Western Australia. Aust J Bot 7:565–574
Peng SL, Cheng WC (2002) Rare and endangered plants in Guangdong. Science Press, Beijing (In Chinese)
Ren H, Ma GH, Zhang QM, Guo QF, Wang J, Wang ZF (2010a) Moss is a key nurse plant for reintroduction of the endangered herb, Primulina tabacum Hance. Plant Ecol 209:313–320
Ren H, Zhang QM, Wang ZF, Guo QF, Liang KM (2010b) Conservation and possible reintroduction of an endangered plant based on an analysis of community ecology: a case study of Primulina tabacum Hance in China. Plant Spec Biol 25:43–50
Taha RM, Daud N, Hasbullah NA, Awal A (2009) Somatic embryogenesis and production of artificial seeds in Saintpaulia ionantha Wendl. Acta Horticul 829:331–336
Tang ZH, Lin HH, Shi L, Chen WL (2007) Rapid in vitro multiplication of Chirita longgangensis WT Wang: an endemic and endangered Gesneriaceae species in China. HortSci 42:638–641
Wang ZF, Ren H, Zhang QM, Ye WH, Liang KM, Li ZC (2009) Isolation and characterization of microsatellite markers for Primulina tabacum, a critically endangered perennial herb. Conserv Genet 10:1433–1435
Wu WH, Jiang RH, Nong DX, Xu WB (2010) Primulina Hance, a newly recorded genus of Gesneriaceae from Guangxi China. Guihaia 30:290–291 (Chinese)
Xu QL, Hu Z, Li CY, Wang XY, Wang CY (2009) Tissue culture of Sinningia speciosa and analysis of the in vitro-generated tricussate whorled phyllotaxis (twp) variant. In Vitro Cell Dev Biol- Plant 45:583–590
Zhang Q, Chen J, Henny RJ (2005) Direct somatic embryogenesis and plant regeneration from leaf, petiole, and stem explants of Golden Pothos. Plant Cell Rep 23:587–595
Acknowledgments
Supports from the Corporation Program of National Basic Research Program of China (973 Program) (2007CB411600), Guangdong Scientific Program (2007A060306011) and the National Natural Science Foundation of China (30671711; 30972295) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, X., Lü, J., da Silva, J.A.T. et al. Somatic embryogenesis and shoot organogenesis from leaf explants of Primulina tabacum . Plant Cell Tiss Organ Cult 109, 213–221 (2012). https://doi.org/10.1007/s11240-011-0087-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-011-0087-4