Abstract
Somatic embryos directly formed at cut edges or on the surface of leaf explants, around cut ends or along side surfaces of petiole and stem explants of ‘Golden Pothos’ [Epipremnum aureum (Linden & Andre) Bunt.] on Murashige and Skoog (MS) medium supplemented with N-(2-chloro-4-pyridyl)-N′-phenylurea (CPPU) or N-phenyl-N′-1, 2, 3-thiadiazol-5-ylurea (TDZ) with α-naphthalene acetic acid (NAA) and a medium called MK containing MS salts with Kao’s vitamins, supplemented with 2.0 mg/l TDZ and 0.2 mg/l NAA. Somatic embryos were also produced on MS medium containing 2.0 mg/l kinetin (KN) and 0.5 mg/l 2,4-dichlorophenoxyacetic acid (2,4-D) from leaf and petiole explants, MS medium supplemented with 2.0 mg/l CPPU and 0.5 mg/l 2,4-D from petiole and stem explants, and 2.0 mg/l TDZ and 0.2 mg/l or 0.5 mg/l 2,4-D from stem explants. In addition, somatic embryos occurred from stem explants on Chu’s N6 medium containing 2.0 mg/l CPPU and 0.2 mg/l NAA. Somatic embryos matured and grew into multiple buds, shoots, or even plantlets after 2–3 months on the initial culture medium. Germination was optimal on MS medium containing either 2 mg/l 6-benzylaminopurine (BA) and 0.2 mg/l NAA or 2 mg/l zeatin and 0.2 mg/l NAA. Shoots elongated better and roots developed well on MS medium with no growth regulators. Approximately 30–100 plantlets were regenerated from each explant. The regenerated plants grew vigorously after transplanting to a soil-less container substrate in a shaded greenhouse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Epipremnum Schott belongs to the family Araceae Juss. and comprises 15 species (Mayo et al. 1997). All species are herbaceous evergreens native to Southeast Asia and the Solomon Islands (Huxley 1994) where they inhabit shaded forests as root climbing lianes. Among the 15 species, E. aureum (Linden & Andre) Bunt., commonly known as pothos, has been widely grown as an ornamental foliage plant. Because of its climbing habit and tolerance to low light environments, pothos is produced in hanging baskets or on poles as climbers and used primarily for interior decoration (Chen et al. 2005). In the tropics, it may be grown under shade as a landscape plant.
Commercial production of pothos in the United States started in the 1920s (Smith and Scarborough 1981) with a wholesale value of approximately US $17 million in 1998 (USDA 1999). Since pothos easily develops adhesive roots, its propagation has been mainly through eye cuttings, a leaf with a single stem node. Eye cuttings, however, can carry and spread diseases, such as pothos latent virus (Rubino and Russo 1997), Erwina leaf spot, and Pythium root rot (Chase 1997). A Rhizoctonia solani strain, AG-4, was first identified on E. aureum in Buenos Aires, Argentina (Wright et al. 2001). Currently, most eye cuttings are imported from Central and South America. Norman and Yuen (1998) identified a distinct pathotype of Ralstonia solanacearum race 1 that was brought from Costa Rica to Florida through pothos eye cuttings. Wick and Dicklow (2002) found that imported E. aureum from Costa Rica carried Phytophthora capsici to a Massachusetts nursery.
Tissue culture has become an important method of propagating disease-free propagules of ornamental foliage plants (Henny and Chen 2003). Reports on tissue culture of pothos date back to the 1970s when Hosoki (1975) and Miller (1976) propagated pothos lateral buds. Tissue culture methods for pothos propagation were not established until Qu et al. (2000) developed a regeneration method through organogenesis. Propagation through somatic embryogenesis, however, may have advantages over organogenesis, particularly direct somatic embryogenesis, because it can potentially scale-up propagation using bioreactors and produce synthetic seeds (Rani and Raina 2000). Additionally, direct somatic embryogenesis has a lower probability of genetic variation than other propagation methods (Merkle 1997). To date, regeneration from somatic embryogenesis has not been reported in pothos. The objectives of this study were to develop methods of inducing direct somatic embryogenesis and producing disease-free propagules for pothos production.
Materials and methods
Plant materials
Shoot tips (about 8 cm long) including the youngest leaf and attached stem were excised from ‘Golden Pothos’ [E. aureum (Linden & Andre) Bunt.] stock plants grown in a shaded greenhouse under a maximum photosynthetically photon flux density of 300 μmol/m2/s at the Mid-Florida Research and Education Center (University of Florida, Apopka, Fla.). The shoot tips were washed with tap water followed by a spray of the tissue surface with 70% ethanol after which the tips were separated according to leaf, petiole, and stem in a laminar flow hood. The separated leaves, petioles, and stems were sterilized by putting them into separate bottles containing 20% Clorox (1.2% NaOCl) solution and a few drops of Tween 20 for 20 min with occasional agitation. After pouring out the Clorox solution, leaves, petioles, and stems were rinsed three times with sterile distilled water.
Leaves were cut into 1.2–1.5 cm squares, and petioles and stems were cut into 1 cm long segments in sterile Petri dishes. The explants were transferred onto Petri dishes containing 20 ml of MS (Murashige and Skoog 1962) medium, or other media as indicated below, with six pieces or segments per dish. Leaf explants were placed with the adaxial surface up, and petiole and stem segments were placed horizontally.
Media and environmental conditions
Basal media consisted of (1) MS medium [MS mineral salts and vitamins, 80 mg/l adenine, 100 mg/l MES, 0.1% banana powder (Sigma, St Louis, Mo.), 2.5% (w/v) sucrose, and 0.6% (w/v) agar (Sigma,)]; (2) Chu’s N6 basal medium (Chu et al. 1975); and (3) a mix of MS and KM (Kao 1977), called MK including all the ingredients of MS except that the vitamins were replaced by KM vitamins. The pH was adjusted to 5.8 with 1 M KOH before autoclaving at 121°C for 25 min. The MS or KM vitamins and different plant growth regulator solutions were filter-sterilized and added to the autoclaved basal media when the medium temperature dropped to about 50°C.
Initially, the following combinations of growth regulators were prepared for the basal media MS, N6, and KM, respectively: (1) N-(2-chloro-4-pyridyl)-N′-phenylurea (CPPU) at 0, 1, 2, and 5 mg/l at each level was mixed with α-naphthalene acetic acid (NAA) at 0, 0.2, 0.5, and 1 mg/l, or 2,4-dichlorophenoxyacetic acid (2,4-D) at 0, 0.2, 0.5, 1, and 2 mg/l, respectively; (2) kinetin (KN) at 0, 1, 2, and 5 mg/l at each level with NAA at 0, 0.2, 0.5, and 1 mg/l, or 2,4-D at 0, 0.2, 0.5, 1, and 2 mg/l, respectively; and (3) N-phenyl-N′-1,2,3-thiadiazol-5-ylurea (TDZ) at 0, 1, 2, 2.5, and 5 mg/l, at each level with NAA at 0, 0.2, 0.5, and 1 mg/l, or 2,4-D at 0, 0.2, 0.5, 1, and 2 mg/l, respectively. Leaf, petiole, and stem explants, each with three replications, were cultured as a preliminary screening of culture media. Based on the occurrence of direct somatic embryogenesis when cultured under the following environmental conditions, only those media listed in Tables 1, 2, and 3 were selected and used for the experiment reported here.
Cultures were first maintained in darkness at 25°C for 5–12 weeks and then placed under a 16-h photoperiod provided by cool-white fluorescent lamps at a photon flux density of 8 μmol m−2 s−1. Germinated embryos and their subsequent growth took place under a 16-h photoperiod at a photon flux density of 124 μmol m−2 s−1.
Each Petri dish was considered an experimental unit. Depending on the availability of plant material, the number of Petri dishes for each medium and explant varied (Tables 1, 2, 3). Explants that responded to the induction were recorded at 4–10 weeks after culture of petiole and stem explants, and at 4–12 weeks for leaf explants. Means and standard errors for the frequencies of explants that produced somatic embryos and whose embryos germinated from initial media were calculated. Subsequently, the explants with direct somatic embryos were subcultured on MS medium containing 2 mg/l 6-benzylaminopurine (BA) and 0.2 mg/l NAA or 2 mg/l zeatin with 0.2 mg/l NAA for more germination and shoot development. Clumps with small shoots (up to 1.5 cm) were transferred to MS medium without growth regulators for better rooting and continuous growth.
Plantlets were then separated and planted into a soil-less substrate (60% Canadian peat, 20% vermiculite, and 20% perlite based on volume and supplemented with 4 kg/m3 dolomite) after washing off the medium with tap water. Potted plants were directly grown in a shaded greenhouse under a maximum photosynthetically photon flux density of 200 μmol m−2 s−1, temperature range of 20–28°C, and relative humidity of 70–100%.
Results
Leaves
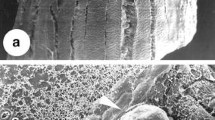
Leaf explants expanded and curved after 2 weeks on the media listed in Table 1. Somatic embryos formed directly at cut edges of explants (Fig. 1a), at the base near the midrib, or on the leaf surface (Fig. 1b). Embryos were white or green, small, and globular (Fig. 1c), appearing individually (Fig. 1d) or in clusters (Fig. 1e). Somatic embryogenesis was asynchronous, occurring anywhere between 5 weeks of initial culture to about 4 months. Some embryos were able to germinate on the initiation medium (Fig. 1f) or proliferated into larger embryo masses by producing secondary embryos when subcultured onto the same fresh medium. Shoots always appeared first from germinated embryos on either the initial culture medium or MS medium containing 2 mg/l BA and 0.2 mg/l NAA or 2.0 mg/l zeatin and 0.2 mg/l NAA. This phenomenon concurs with somatic embryo development of Anthurium andraeanum Linden ex Andre, another species from the family Araceae, where development of the root pole was delayed relative to the shoot pole (Matsumoto et al. 1996). Many leaf explants cultured on the MS medium containing KN and 2,4-D produced a few isolated somatic embryos at their edges or on their surfaces, which germinated with both shoots and roots on this medium (Fig. 1g).
Somatic embryos of Epipremnum aureum ‘Golden Pothos’ directly formed at cut edges (a) or on the surface (b) of leaf explants on Murashige-Skoog (MS) medium containing 2.0 mg/l N-(2-chloro-4-pyridyl)-N′-phenylurea (CPPU) with 0.2 mg/l α-naphthalene acetic acid (NAA). Globular embryos were white or green (c), appearing individually (d) or in clusters (e). Somatic embryos were able to germinate on the initial medium (f). Leaf explants cultured on MS medium containing 2.0 mg/l kinetin (KN) and 0.5 mg/l 2,4-dichlorophenoxyacetic acid (2,4-D) produced a few isolated somatic embryos, which germinated directly on the surface of leaf explant with both shoots and roots (g)
Among the media tested, the best for direct somatic embryogenesis of leaf explants was MS medium containing 2.0 mg/l CPPU with 0.2 mg/l NAA, since 91% of cultured explants produced embryos and 89% of explants with embryos germinated on this medium (Table 1). The next best medium was MS containing 2.0 mg/l TDZ with 0.2 mg/l NAA, where 71% explants produced embryos and 72% explants with embryos germinated. The frequencies of explants cultured and explants with embryos germinated on the other media listed in Table 1 ranged from 42% to 57% and 44% to 71%, respectively.
Petioles
Somatic embryos were observed from petiole explants 4–6 weeks after culture on the MS and MK media listed in Table 2. White, round somatic embryo clusters formed at the cut edges of explants (Fig. 2a), along the surface of the two sides; later embryos also appeared from upper surfaces of the explants (Fig. 2b). These embryos were well-developed structures and easy to separate; an assortment of embryos including a germinated embryo with first leaf and radicle are shown in Fig. 2c. Somatic embryos germinated and developed into multiple buds or shoots and simultaneously produced secondary embryo clumps in 2–3 months on initial MS medium containing 2.0 mg/l CPPU with 0.2 mg/l NAA or 2.5 mg/l TDZ with 0.5 mg/l NAA, or MK medium containing 2.0 mg/l TDZ and 0.2 mg/l NAA (Fig. 2d). Shoots developed into plantlets on MS medium without growth regulators (Fig. 2e).
Somatic embryos of E. aureum ‘Golden Pothos’ directly appeared from cut edges (a), along the surfaces of the two sides, and later from the upper surfaces of petiole explants (b). Embryos were well-developed structures and easy to separate; an assortment of embryos including a germinated embryo with the first leaf and radicle are shown (c). Somatic embryos germinated and developed into multiple buds or shoots and simultaneously produced secondary embryo clumps (d). Shoots developed into plantlets with roots on MS medium without growth regulators (e)
The frequencies of somatic embryos produced by petiole explants on all media listed in Table 2 were above 90% except for MS containing 2.0 mg/l KN and 0.5 mg/l 2,4-D and MK containing 2.0 mg/l TDZ and 0.2 mg/l NAA, which had frequencies of 36% and 75%, respectively. The frequencies of explants with embryos that germinated on MS medium containing 2.0 mg/l CPPU with 0.2 mg/l NAA, 2.0 mg/l TDZ with 0.2 mg/l NAA, and 2.5 mg/l TDZ with 0.5 mg/l NAA were 88, 79, and 89%, respectively. Embryo germination on the initial MS medium supplemented with 2.0 mg/l CPPU and 0.5 mg/l 2,4-D was only 37%. The germination frequency of the remaining MS and MK media were 50% and 45%, respectively. However, all embryos germinated after they were transferred to MS medium containing 2.0 mg/l BA with 0.2 mg/l NAA or 2.0 mg/l zeatin and 0.2 mg/l NAA.
Stems
Somatic embryos generally appeared from cut ends of stem explants that touched MS medium supplemented with 2.5 mg/l or 5 mg/l TDZ with 0.5 mg/l NAA or 2 mg/l CPPU with 0.2 mg/l NAA or with 0.5 mg/l 2,4-D (Fig. 3a) or along two sides that touched the medium. Some somatic embryos formed and germinated from the upper surface explants (Fig. 3b). Embryos were semi-transparent, occurring separately or as fused embryo cluster. On N6 medium containing 2 mg/l CPPU and 0.2 mg/l NAA, stem explants formed large white, non-transparent somatic embryos at both ends (Fig. 3c). Somatic embryos clustered underneath explants that touched the culture medium became larger, white or light yellow nodular particles. These nodules developed into buds or formed secondary somatic embryos at their surface. The embryos, nodular particles, and buds were in a mixed mass often seen in cultures of stem sections. Subsequently, embryo clusters developed into multiple shoots with roots (Fig. 3d). Individual plantlets germinated from somatic embryos could be easily separated (Fig. 3e).
Somatic embryos of E. aureum ‘Golden Pothos’ formed directly from cut end of stem explants that touched MS medium containing 2.0 mg/l CPPU with 0.2 mg/l NAA (a). Some somatic embryos formed and germinated from the upper surface explants (b). On N6 medium containing 2 mg/l CPPU and 0.2 mg/l NAA, stem explants formed large white, non-transparent somatic embryos (c). Embryo clusters developed into multiple shoots with roots (d). Individual plantlets could be easily separated (e)
Again, the best medium for somatic embryogenesis of stem explants appeared to be MS containing 2.0 mg/l CPPU with 0.2 mg/l NAA since the frequencies of both explants with embryo formation and germination were up to 90% (Table 3). In general, the rates of explants with embryo production and germination in stem explants were slightly higher than leaf or petiole explants cultured on the same medium. The frequencies of explants with embryo formation ranged from 71% to 94% and embryo germination varied from 50% to 90%, respectively (Table 3).
Shoot elongation and plant growth
Somatic embryos induced from leaf, petiole, and stem explants were generally able to mature, germinate, and develop into shoots on the initial culture medium. For example, embryos from leaf, petiole, and stem explants developed into shoots after 2–3 months on the initial MS medium containing 2.0 mg/l CPPU and 0.2 mg/l NAA. Approximately 30–100 plantlets could be produced from a single cultured explant (Figs. 2e, 3d). The germination rate of somatic embryos induced from petiole explants was lower than that of stem explants on MS medium containing 2 mg/l CPPU and 0.5 mg/l 2,4-D. If such embryo-occurring explants were transferred to germination medium (MS containing 2 mg/l BA with 0.2 mg/l NAA, or 2 mg/l zeatin with 0.2 mg/l NAA), all embryos germinated and developed into plantlets (Fig. 4a). The optimal medium for shoot elongation and root development was the MS without growth regulators. Plants with well-developed roots grew vigorously after transplanting into soil-less substrate in the shaded greenhouse (Fig. 4b). Several thousand regenerated plants were produced and grown in the shaded greenhouse.
Discussion
As far as is known, zygotic embryogenesis has not been described in pothos because Epipremnum seldom flowers. This is the first report of direct somatic embryogenesis and subsequent plant regeneration of Epipremnum. Somatic embryos formed directly at cut edges or on the surfaces of leaf sections, around cut ends, or along the sides of petiole and stem explants. Somatic embryos were able to germinate directly on the initial induction medium; however, complete embryo germination and shoot development occurred in MS medium containing 2.0 mg/l BA and 0.2 mg/l NAA or 2.0 mg/l zeatin and 0.2 mg/l NAA. Shoots elongated, roots developed on MS medium without growth regulators, and plantlets grew well in a commercial soil-less substrate. Regenerated plants exhibited golden blotched foliar variegation in the shaded greenhouse. Some plants initially began with three or four solid green leaves but produced variegated leaves thereafter. A few others were maintained as green-leaved plants.
Embryogenesis was slower and asynchronous in leaf explants. Small, round, individual somatic embryos appeared 5 weeks to 3 or 4 months after culture. The formation of somatic embryos around petiole segments was faster and more synchronous; embryos matured and germinated with buds or shoots in 2 months, resulting in plantlets in 3–4 months. Somatic embryos were produced from stem explants in areas that touched the culture medium, and those embryos were aggregated with secondary embryos, which germinated and developed into shoots. Among the three basal media tested, more explants produced somatic embryos on MS medium than on MK or N6 media.
CPPU—a synthetic compound with cytokine-like activity—has been used to delay fruit maturity and increase fruit size and yield in several fruit crops (Reynolds et al. 1992). Reports of using CPPU in tissue culture have been few. It was shown that CPPU improved efficiency of shoot formation in raspberry (Rubus idaeus subsp. vulgatus Arrhen.) (Millan-Mendoza 1998) and lavender (Lavandula vera DC) (Tsuro et al. 1999), induced embryonic callus in grape (Vitis labrusca) (Nakajima et al. 2000) and Citrus (Fiore et al. 2002), and stimulated somatic embryogenesis in peanut seedlings (Murthy and Saxena 1994). There have been no reports of using CPPU for inducing somatic embryos of ornamental plants. This study demonstrated that somatic embryogenesis occurred in leaf, petiole, and stem explants of pothos on MS medium supplemented with CPPU and NAA, which extends the potential applications of CPPU in ornamental plants.
In the family Araceae, embryogenesis has been reported in Pinellia pedatisecta (Wu et al. 1996), Spathiphyllum (Werbrouck et al. 2000), Typhonium trilobatum (Das et al. 1999), Xanthosoma sagittifolium (Gomez et al. 1992), and three varieties of Anthurium (Kuehnle et al. 1992; Matsumoto and Kuehnle 1997) by using BA or KN with 2,4-D, and KN or TDZ with NAA. It has also been reported that 1–2 mg/l TDZ with NAA promoted adventitious shoot regeneration of green pothos (Qu et al. 2000). In this study, the most effective medium for direct somatic embryo formation and regeneration was MS containing CPPU or TDZ with NAA. Thus, disease-free propagules of pothos can be produced from clean stocks for commercial production.
Abbreviations
- BA:
-
6-Benzylaminopurine
- CPPU:
-
N-(2-Chloro-4-pyridyl)-N′-phenylurea
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- KM:
-
Kao’s medium
- KN:
-
Kinetin
- MES:
-
2-(N-Morpholino) ethane-sulfonic acid
- MS:
-
Murashige and Skoog’s medium
- N6:
-
Chu’s (N6) medium
- NAA:
-
α-Naphthalene acetic acid
- TDZ:
-
N-Phenyl-N′-1,2,3-thiadiazol-5-ylurea
References
Chase AR (1997) Foliage plant diseases: diagnosis and control. American Phytopathological Society, St Paul
Chen J, McConnell DB, Henny RJ, Norman DJ (2005) The foliage plant industry. Hortic Rev 31:47–112
Chu CC, Wang CC, Sun CS, Hus C, Yin KC, Chu CY, Bi FY (1975) Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Sci Sin 18:659–668
Das P, Palai SK, Patra A, Samantaray S, Rout GR (1999) In vitro somatic embryogenesis in Typhonium trilobatum Schott. Plant Growth Regul 27:193–197
Fiore S, Pasquale FD, Carimi F, Maurizio S (2002) Effect of 2,4-D and 4-CPPU on somatic embryogenesis from stigma and style transverse thin cell layer of Citrus. Plant Cell Tissue Organ Cult 68:57–63
Gomez L, Valverde R, Arias O, Thorpe T (1992) Regeneration of Xanthosoma sagittifolium by somatic embryogenesis. Agron Costarricense 16:219–223
Henny RJ, Chen J (2003) Foliage plant cultivar development. Plant Breed Rev 23:245–290
Hosoki T (1975) Propagation of tropical plants by tissue culture. Ph.D. Thesis, University of Hawaii, Honolulu
Huxley A (1994) The new royal horticultural society dictionary of gardening. Macmillan, London
Kao KN (1977) Chromosomal behavior in somatic hybrids of soybean-Nicotiana glauca. Mol Gen Genet 150:225–230
Kuehnle AR, Chen FC, Sugii N (1992) Somatic embryogenesis and plant regeneration in Anthurium andraeanum hybrids. Plant Cell Rep 11:438–442
Matsumoto TK, Kuehnle AR (1997) Micropropagation of Anthurium. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 40. High-tech and micropropagation VI. Springer, Berlin Heidelberg New York, pp 14–29
Matsumoto TK, Webb DT, Kuehnle AR (1996) Histology and origin of somatic embryos derived from Anthurium andraeanum Linden ex Andre lamina. J Am Soc Hortic Sci 121:404–407
Mayo SJ, Bogner J, Boyce PC (1997) The genera of Araceae. Royal Botanical Gardens, Kew
Merkle SA (1997) Somatic embryogenesis in ornamentals. In: Geneve RL, Preece JE, Merkle SA (eds) Biotechnology of ornamental plants. CAB International, Wallingford, pp 13–33
Millan-Mendoza BM (1998) Regeneration of Rubus in vitro using forchlorfenuron (CPPU). Rev Fac Agron (LUZ) 15:242–248
Miller LR (1976) Tissue culture propagation of some tropical foliage plants. M.Sc. Thesis, University of California, Riverside, Calif.
Murthy BNS, Saxena PK (1994) Somatic embryogenesis in peanut (Arachis hypogaea L.): stimulation of direct differentiation of somatic embryos by forchlorfenuron (CPPU). Plant Cell Rep 14:145–150
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–479
Nakajima I, Kobayashi S, Nakamura Y (2000) Embryogenic callus induction and plant regeneration from unfertilized ovule of ‘Kyoho’ grape. J Jpn Soc Hortic Sci 69:186–188
Norman DJ, Yuen JMF (1998) A distinct pathotype of Ralstonia (Pseudomonas) solanacearum race 1, biovar 1 entering Florida in pothos (Epipremnum aureum) cuttings. Can J Plant Pathol 20:171–175
Qu L, Chen J, Henny RJ, Huang YF, Caldwell RD, Robinson CA (2000) Thidiazuron promotes adventitious shoot regeneration from pothos (Epipremnum Aureum) leaf and petiole explants. In Vitro Cell Dev Biol Plant 38:268–271
Rani V, Raina SN (2000) Genetic fidelity of organized meristem-derived micropropagated plants: a critical reappraisal. In Vitro Cell Dev Biol Plant 36:319–330
Reynolds AG, Nardle DA, Zuruwski C, Looney NE (1992) Phenylureas CPPU and thidiazuron affect yield components, fruit composition and storage potential of four seedless grape selections. J Am Soc Hortic Sci 117:85–89
Rubino L, Russo M (1997) Molecular analysis of the pothos latent virus genome. J Gen Virol 78:1219–1226
Smith CN, Scarborough EF (1981) Status and development of foliage plant industries. In: Joiner J (ed) Foliage plant production. Prentice-Hall, Englewood Cliffs, pp 1–39
Tsuro M, Koda M, Inoue M (1999) Comparative effect of different type of cytokinin for shoot formation and plant regeneration in leaf-derived callus of lavender (Lavandula vera DC). Sci Hortic 81:331–336
USDA (1999) 1998 census of horticultural specialties. United States Department of Agriculture, Washington
Werbrouck SPO, Eeckhaut TGR, Debergh PC (2000) Induction and conversion of somatic embryogenesis on the anther filament of Spathiphyllum schott. Acta Hortic 520:263–269
Wick RL, Dicklow MB (2002) Epipremnum, a new host for Phytophthora capsici. Plant Dis 86:1050
Wright ER, Grijalba PE, Gasoni L (2001) First report of Rhizoctonia solani AG-4 on Epipremnum aureum in Buenos Aires, Argentina. Plant Dis 85:96
Wu AM, Zhu BC, Li QY (1996) Studies on somatic embryogenesis of Pinellia pedatisecta Schott. Acta Agron Sin 22:66–71
Acknowledgements
We thank Dr. Dennis J. Gray for providing photographic equipment used in this study and for his valuable comments on this manuscript’s revision, and Kelly Everitt for critical reading of this manuscript. This research was supported in part by the Gloeckner Foundation, Inc., New York and USDA-TSTAR, and approved for publication as journal series R-10158 by the Florida Agricultural Experiment Station.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K.K. Kamo
Rights and permissions
About this article
Cite this article
Zhang, Q., Chen, J. & Henny, R.J. Direct somatic embryogenesis and plant regeneration from leaf, petiole, and stem explants of Golden Pothos. Plant Cell Rep 23, 587–595 (2005). https://doi.org/10.1007/s00299-004-0882-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-004-0882-z