Abstract
The effects of ethylene on growth initiation of tobacco pith tissue in vitro were investigated. Pith explants were incubated on a double inorganic modified White’s media containing 0.2 mg l−1 kinetin with and without indole-3-acetic acid (IAA) and the ethylene synthesis inhibitor aminooxyacetic acid (AOA). The burst of wound ethylene had no effect on growth initiation, was not affected by the AOA, and decreased to its minimum level during the initial 24 h in culture. Tissue growth was initiated after 72 h and continued on IAA-containing media only. A marked increase in ethylene evolution occurred only in tissues subjected to an IAA-containing medium prior to growth initiation. AOA inhibited this ethylene synthesis and the following growth of the tissues. The initial water uptake by the pith explants occurring even in the absence of IAA was also inhibited by AOA. The metabolic indicators for growth initiation such as enhanced respiration, increased activity of nitrate reductase, and initiation of cathodic isoperoxidases were all inhibited by AOA. It was concluded that the primer function of IAA in growth initiation is via inducing the biosynthesis of a marked ethylene signal, which in the absence of which active growth will not occur. The inhibiting effect of AOA is continuous and a transfer of the pith explants to fresh IAA-containing media did not result in a new ethylene burst nor tissue growth induction. The morphological changes in the tissues and cells during the initial stages of their development on the different media are demonstrated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Induction of active growth in either quiescent or stationary tissue involves activation and changes in most metabolic pathways in the cell. Most stationary cells in the plant are components of differentiated tissues and require in most cases dedifferentiation before new growth can be induced. Non-differentiated cells in growing points, cambium, and embryonic tissues are usually not in a fully stationary phase. Pith tissue, on the other hand, which in some plants develops to a sizable volume, is of a low differentiation state and in a stationary stage for long periods. Tobacco pith cells although slowly changing metabolically with age do not change in vivo in their level of differentiation (Lavee and Galston 1968). Thus, pith tissue was widely used for growth and differentiation studies and bioassays for growth regulating substances. Tobacco pith could be easily induced to grow and form an un- or low differentiated tissue mass in vitro in the presence of auxin and cytokinin. However, the extraction of pith from the plant involves injuring a large number of cells causing decompartmentation of metabolites and development of wound ethylene. The duration, persistence, and involvement of this auxin-mediated ethylene burst on the induction of growth of the pith explants and some other tissues are not yet entirely clear both on physiological and molecular basis (Ruzika et al. 2007). Previously, the relationships between auxin and ethylene in plant tissues have been well documented (Burg and Burg 1966; Abeles et al. 1992). More recently, this relationship has been well investigated at the molecular level (Dugardeyn and Van Der Straeten 2008). However, the possible prolonged interaction of ethylene along with growth regulators in the culture medium and their influences on explants cells growth initiation remain unclear. Ethylene inhibition can be induced at different steps in the ethylene biosynthesis pathways (Lin et al. 2009). In order to separate the affects of ethylene released following wounding from that of newly-synthesized ethylene during plant cell growth initiation, inhibitors of the early steps in the ethylene biosynthetic pathway, such as at the S-adenosylmethionine (SAM) or 1-aminocyclo-propane-1-carboxylic acid (ACC), could be used to investigate these effects. Inhibition of ACC synthase activity using aminoethoxy-vinyl-glycine (AVG) or amino-oxyacetic-acid (AOA) preventing the formation of ACC, have been widely used (Yung and Hoffman 1984). This could allow the final stage of ethylene evolution from the ACC in the tissue to be triggered during wounding without further ability to interact with auxin during the growth initiation period. Tobacco pith tissue in the plant is dependent on its neighboring tissue for nutrients and metabolites. Thus, when excised and transferred to in vitro conditions, it is fully dependent on nutrients and metabolites from the media in order to survive, and on exogenous growth regulators for initiation of cell growth and proliferation. The pith tissue is characterized by low metabolic activity in its cells and could be considered as a resting tissue (Lavee and Galston 1968). This, and its low differentiating level, enable the use of the pith in an in vitro system as model tissue to study the transition of a resting to an active growing stage.
In this study, the interaction between ethylene and auxin along with durations of these initiations in initiating growth of excised stationary tobacco pith tissues in vitro were investigated. Morphological changes and some metabolic indicators such as respiration, induction of cathodic isoperoxidases, and nitrate reductase activity related to growth initiation were determined.
Materials and methods
Tobacco plants of cv. W-38 were grown in a greenhouse and harvested before flowering after developing 30–35 leaves. The pith from the stem region between leaves 15–25, reaching a diameter of 12–15 mm, was used for the various experiments. The pith cylinders were extracted from the stem using a sterile cork borer with a diameter of 5 mm, and divided into 4-mm sections in a laminar flow hood. The explants were planted individually in 30-ml tubes containing 10 ml culture medium.
The basal medium used in all experiments consisted of Double Inorganic Modified White’s (2I-MW) medium containing 2% sucrose, 1% agar, and 0.2 mg l−1 kinetin, and pH of the medium adjusted to 6.3. Auxin, 2.0 mg l−1 IAA (indole-3-acetic acid) and the ethylene synthesis inhibitor AOA (amino-oxyacetic acid) at a concentration of 10−3 M were added to the media according to the different experiments (IAA, AOA, and Kinetin were supplied by Sigma Chemical, St. Louis, MO, USA). The cultures were kept in a growth chamber with a constant temperature of 26–28°C and fluorescent light at an intensity of 3 μmol m−2 s−1. Humidity in the chamber was not controlled.
Explants growth on basis of fresh and dry weight were determined at 24-h intervals for 7 days. Cell development was monitored in a 0.5-mm-thick slice of the upper surface of the explants using a binocular at an actual magnification of ×15 showing the whole surface of the explants on a non-transparent background and a ×96 magnification to view the cells with the tissue slice placed on a light transparent base.
Fifteen tubes, each with a single pith tissue, were used for each treatment, and each experiment was repeated four times. Mean values of 15 repetitions in each of the four experiments were calculated. Ethylene release from tissues was determined by enclosing the tubes under sterile conditions for 1 h, extracting 3 ml air from each tub, and injecting either 1- or 2-ml samples into a Packard 840 gas chromatograph (Packard instruments, Downers Grove, IL. USA), with a Flame Ionization Detector (FID). A 1.5-m-long Alumina-Alcoa column was used with a nitrogen carrier gas at a flow rate of 50 ml/min. Injection port temperature was 100°C, column temperature 120°C, and detector temperature 140°C. Ethylene gas with 7.6 ml/l served as standard. Ethylene evolution was measured every 24 h starting at culture establishment for 168 h. After each sampling, the cultures were flashed with fresh sterile air and returned to the culture chamber.
Tissue respiration was determined on basis of CO2 evolution parallel to the ethylene measurements. From each extracted air sample, 1 ml was injected into a Gow Mac 20 gas chromatograph (Gow Mac Instrument, Bethlehem, PA, USA) with helium as carrier gas. The data per gram tissue were calculated from the CO2 evolution from the tissue divided by the weight data at each sampling date.
Isoperoxidases were determined using starch gel electrophoresis. Glass plates measuring 160 × 130 mm covered with a layer of 6-mm starch gel were used. The gel was prepared with 10% starch in a pH 9 borate buffer heated for about 1 min up to boiling level for polymerization. Air bubbles were removed by a by weak vacuum before pouring the plates. Cell sap squeezed from the tissues was absorbed in 4 × 6 mm Whatmann #3 squares and introduced in the middle of the starch plate. A borate buffer, pH 8.3, was used as bridge buffer for the electrophoresis. Current of 100 V was used for 30 min then stopped the paper squares with the samples removed and a current of 250 V applied for another 2 h. Thereafter, the gels were sliced in the middle and washed with a phosphate buffer containing guaiacol and H2O2 (Lavee and Galston 1968). The developing anodic and cathodic peroxidase bands were photographed and recorded.
The level of nitrate reductase activity in the tissues was determined calorimetrically. The tissues were weight and incubated for 2 h in the dark, at room temperature, in a 0.25 M KNO3 solution buffered to pH-7.8 with 0.05 M tris–HCl. The activity of the enzyme was determined by the amount of NO2 which developed based on colorimetric absorption at wave length 470 nm.
All the results are based on mean values of 15 independent tissues for each treatment repeated in each of four independent experiments. All data underwent an analysis of variance using ANOVA JMP 5.0.1 software (SAS Institute, Cary, NC, USA) for each parameter measured. Standard error (SE) or standard deviation (SD) for each measured criteria is indicated by vertical bars in the figures. Differences larger than 2.5× SE were considered significant at the t = 0.05 level.
Results
The growth of pith tissue during the first 7 days after planting was followed daily on fresh and dry weight basis in the presence and absence of IAA in the culture medium. During the first 24 h after excision and placing the explants on the media, an increase of 15–20% of the tissues fresh weight was apparent. This increase in tissues fresh weight occurred equally in the presence an absence of IAA in the culture medium. In the following 48 h, no further increase in the tissues fresh weight occurred. At 72 h, a rapid increase in fresh weight was recorded in the tissues cultured on the IAA-containing media. After 168 h in culture, these tissues reached a mean weight of 275 mg about tripling their initial size. A limited non-significant but consistent increase of weight also started in the tissues cultured for 96 h on the IAA-deficient medium, about 24 h after active growth initiation in tissues cultured on the IAA-containing media (Fig. 1). The increase in dry weight of the tissues showed a similar tendency though the increase was only of 25% during the first week of culture. A difference in dry weight between tissues cultured on an IAA-containing and -deficient media started to be apparent only after the first 7 days in culture.
The release of ethylene and its relationship to plant cell growth was determined daily. An initial burst of ethylene reaching 110 nl g−1 of fresh tissue occurred within the first 2 h in culture due to the massive wounding of the pith tissue during its excision from the tobacco plants. This massive burst of ethylene, regardless of the media used, diminished thereafter to a low level of less than 20 nl g−1 fresh tissue 24 h after excision. The level of ethylene evolution from the tissues remained low for another 48 h until the beginning of active tissue growth on IAA-containing medium. At that stage (after 72 h in culture)- another major burst of ethylene reaching 95 nl g−1 fresh tissue occurred. By 96 h in culture, while the tissue was actively growing, ethylene evolution from the tissues receded to 35 nl g−1 h−1. Thereafter, although the tissues continued to grow linearly (Fig. 1), ethylene evolution was reduced to the basic level of less than 20 nl g−1 h−1 measured prior to the ethylene burst at 72 h and the active growth initiation (Fig. 2). These results for tissues cultured on IAA-containing medium, were highly significant and the SD did not accede 3% of the amount measured at each date.
The significance of the short ethylene burst from the pith tissues at about 72 h of culturing was determined by adding 10−3 M of the ethylene synthesis inhibitor AOA to the IAA-containing culture medium. While on the IAA-containing media, cell swelling occurred after 48 h and the first cell divisions could be noticed 24 h thereafter; on media with the addition of AOA, the pith remained in its stationary phase. A complete growth inhibition prevailed throughout the culture period and the explants remained similar to those planted on an IAA-deficient medium. After 144 h in culture, while new callus started to cover the sides of the explants growing on the IAA-containing medium and their weight doubled, the tissues on the same medium containing AOA remained unchanged. The AOA caused a complete growth inhibition of the pith tissues keeping them in a non-growing stationary phase similar to the tissues placed on an IAA-deficient medium (Fig. 3). The developmental inhibition of tobacco pith tissue by the AOA was even more pronounced than that in the tissues placed on the IAA-deficient medium. In the tissues inhibited due to the lack of IAA in the medium, some cell swelling occurred after 96 h in culture even though growth was not induced. In the AOA-inhibited tissues, even that cell swelling, although IAA was present in the medium, did not occur.
View of tobacco cv. W-38 pith explants and their cross-sections grown on 2I-MW media containing IAA and AOA, IAA alone and without IAA, from planting (T-0) to 144 h in culture. The level of magnification for the pith explants was ×15 and of the cross-sections ×96. The 2I-MW media contained 2% sucrose, 1% agar, 0.2% kinetin, pH adjusted to 6.3 cultured at 27°C
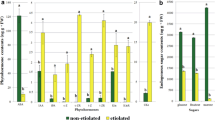
The evolution of ethylene from the growing tissues after the peak at 72 h on the IAA-containing medium remained significantly higher than the evolution from inhibited tissues cultured on AOA or lack of IAA-containing media. It should be noted that the non-growing pith cultured on the auxin deficient medium showed a low peak of about 25 nl g−1 h−1 of ethylene evolution after 72 h in culture. Such a peak of ethylene evolution did not occur in the AOA inhibited cultures (Fig. 4a). The difference between ethylene evolution from growing and non-growing tobacco pith tissue increased with time on per tissue basis due the increase in cell number and size of the growing tissues (Fig. 4b).
Similar to ethylene, the respiration of the growing tissues also increased considerably between 48 and 72 h in culture. The rate of CO2 evolution increased during that culture period from 75 to 60 μl g−1 h−1. Thereafter, the rate of respiration declined stabilizing after 144 h at a level of 94 μl g−1 h−1 only slightly higher than that of the non-growing tissues (Fig. 5a). Still, as expected, the CO2 evolution of the whole growing tissues increased continuously while that of the non-growing tissues remained low (Fig. 5b). It should be noted that the respiration rate of the AOA-inhibited tissues was lower during most of the culture period than the respiration of the non-growing tissues inhibited due to the absence of IAA in culture medium. The initial high respiration level of the wounded pith cylinders, regardless of the culture media used, was similar to the enhanced ethylene evolution due to the wounding. The respiration of the tissues reached their basal low level after 48 h while ethylene had already reached its basic low level after 24 h. However, both increased after 72 h with the initiation of active growth (Figs. 4 and 5).
The inhibitory effect of AOA seems to be of a permanent nature at least for a culturing period of 25 days. Placing the tissue on an IAA-deficient medium for 8 days and transferring it thereafter to an IAA-containing media resulted in an active growth initiation, at the normal rate after the transfer. On the other hand, tissues planted on a medium containing IAA and AOA for 8 days and thereafter transferred to an IAA-containing medium without the ethylene inhibitor remained inhibited and no growth was initiated during the following 17 days on the inhibitor-free IAA-containing medium (Fig. 6).
Two additional metabolic parameters for growth initiation were determined in the pith explants cultures on the three different media. Increased activity of nitrate reductase is one of the early indicators of growth initiation. The activity of this enzyme was determined at 24-h intervals in the tissues from culture initiation up to 168 h thereafter on the media with and without IAA and with IAA + AOA. During the first 72 h in culture, no change from the initial basic activity of the nitrate reductase occurred in the tissues. By 92 h, an increase in activity was found both in cultures on media containing or lacking IAA. On the IAA-deficient medium which induced only some tissue swelling, but no real growth, the enzyme activity leveled off after 96 h and even slightly declined. The activity of nitrate reductase in the tissues growing on the IAA-containing medium continued to increase linearly reaching after 168 h a level 4× higher per gram tissue than the initial basic activity. In the parallel cultures with medium containing both IAA and AOA which inhibited even cell swelling, the activity of nitrate reductase remained low and unchanged throughout the 168 h recorded (Fig. 7). Thus, the full growth inhibition inflicted by the AOA also prevented metabolic activation in the tissue.
A similar metabolic inhibition was also apparent during the induction of cathodic isoperoxidases involved in growth initiation of tobacco pith under in vitro conditions. The peroxidase activity in stationary non-growing intact pith tissue is due solely to anodic isozymes. With the transfer to culture media, new cathodic isoperoxidases are induced both on IAA-containing and -deficient media. However, in tissues which are actually growing, an additional cathodic peroxidase is developing. This occurred in the present study after 120 h and only on the IAA-containing medium. The addition of the ethylene synthesis inhibitor, AOA, to the IAA-containing medium inhibited the appearance of all cathodic isoperoxidases including those not connected to the active growth which are also induced in the tissues placed on IAA-deficient media (Fig. 8). A reduction in the activity of the initial anodic isoperoxidases was noted with growth initiation after 72 h in culture but only in tissues induced to grow in culture on the IAA-containing medium without the AOA ethylene synthesis inhibitor. In non-growing tissues planted on nutrient medium without an active growth-inducing auxin, the level of the anodic isoperoxidases remained constant and no change in their activity was noted.
Discussion
The tobacco pith tissue is characterized by the homogeneity of the tissue and its low metabolic activity. Pith transferred to nutrient media starts to increase in fresh weight after about 72 h. The initial increase in weight is due to water uptake only and occurs both in the presence of a growth-inducing auxin and somewhat later and to a lesser extent also in the absence of auxin in the medium. This initial increase in fresh weight of the pith in culture was blocked by adding the ethylene inhibitor AOA to the media. From these data, it could be concluded that the process of water uptake is a metabolic process controlled by IAA and ethylene preceding cell division and, thus, can be shut down by a metabolic inhibitor. The involvement of ethylene in growth initiation is limited, however, to de novo synthesized ethylene as the burst of ethylene induced by wounding the pith during its removal from the plant had no effect on the following induction of growth. Furthermore, the rapid short burst of wound ethylene was not affected by the presence of AOA or auxin in the media. After the burst of the wound ethylene, the level of ethylene in the tissue reduced reaching a minimum level regardless of the presence or absence of an auxin and the ethylene inhibitor in the media. On the other hand, a major new burst of ethylene from the pith was recorded after 72 h but only on the IAA-containing medium leading to active tissue growth. A similar increase in ethylene evolution was reported in explants of bean leaves 96 h after excision (Jackson and Osborne 1970). The slight increase in de novo ethylene evolution on IAA-deficient media can be attributed to the low levels of endogenous ethylene in the pith tissue. This peak of ethylene evolution was blocked by the AOA and no following growth of the tissue occurred. The level of respiration of the tissue as expressed by CO2 evolution followed a similar pattern though less extreme. The increase in respiration was clearly noticeable on basis of the cumulative CO2 from the whole tissue. The level of CO2 evolution from the tissues in the absence of IAA in the medium was low but somewhat higher than in the presence of the AOA. As the initial growth or water uptake was inhibited in the presence of AOA, it could be concluded that a metabolic ethylene signal is obligatory for the induction of active growth. On the other hand, the ethylene signal does not appear in the absence of IAA. Thus, a functional auxin ethylene interaction is required for growth initiation which in tobacco pith is apparently obligatory. The mode of action involved is rather unclear as, while IAA enhances quantitatively ethylene biosynthesis (Abeles and Rubinstein 1964; Tsuchisaka and Theologis 2004), ethylene at the same time was shown to enhance habituation of tobacco tissue to auxin (Koves and Szabo 1987), enhance IAA oxidase activity (Morgan et al. 1968), and affect auxin biosynthesis (Ruzika et al. 2007). This initial required interaction is even further confusing as, during the active growth of the tobacco pith, IAA is obligatory for its proliferation while the levels of ethylene released from the tissues are very low. The obligatory requirement of an auxin for active growth of tobacco pith has been well known for many years but not the joint auxin ethylene signal required for the initiation of the metabolic processes involved in active growth. This was demonstrated via the activity of nitrate reductase essential during growth initiation (Hahlbrock, 1974). The activity of this enzyme was completely blocked by the AOA ethylene synthesis inhibitor together with blocking the growth in spite of the presence of IAA in the medium. Thus, by blocking the initial signal for growth initiation by inhibiting ethylene synthesis, the following metabolic activities required for growth and controlled by IAA remain blocked and do not allow even delayed tissue growth to occur. On media containing IAA without the inhibitor, the activity of the nitrate reductase increased parallel with the cell proliferation and growth. Furthermore, tissues placed on an auxin-deficient media undergoing an initial slight growth also showed a slight increase in nitrate reductase activity which declined again with the cessation of growth of those tissues due to the lack of IAA. Similarly, the AOA also completely inhibited the synthesis of cathodic isoperoxidases which were shown to be related to the transition from resting pith tissues to active growth under in vitro conditions (Galston et al. 1968; Miller and Crawford 1985).
The AOA inhibition of the synthesis of the ethylene required for growth induction was found to be of a long-term nature and could not be reversed during any stage of the experimental culture period of 25 days. Pith explants subjected to a growth-inhibiting medium containing both IAA and AOA when transferred to an inductive medium with IAA only, remained inhibited and no growth of the tissues was initiated. The synthesis of ethylene also remained inhibited and no burst of ethylene from the tissues was detected at any time after their removal from the AOA-containing medium and transfer to a growth inductive IAA-containing medium. On the other hand, the non-growing tobacco pith due to lack of auxin in the medium could be induced to grow once transferred to an IAA-containing medium. A clear ethylene peak was detected from those tissues before the initiation of growth (data not shown).
In conclusion, it was established many years ago that an auxin is essential for the initiation and control of active growth from the stationary tobacco pith tissue in culture. In the present study, we established that the initial effect of IAA in inducing active growth is linked to a de novo synthesis of ethylene in the tissue in the absence of which no active growth could be induced.
Abbreviations
- IAA:
-
Indole-3-acetic acid
- AOA:
-
Aminooxyacetic acid
- ACC:
-
1-Aminocyclo-propane-1-carboxylic acid
- AVG:
-
Aminoethoxy-vinyl-glycine
- SAM:
-
S-Adenosylmethionine
- FID:
-
Flame ionization detector
- 2I-MW:
-
Double Inorganic Modified White’s medium
References
Abeles FB, Rubinstein B (1964) Regulation of ethylene evolution and leaf abscission by auxin. Plant Physiol 39:963–969
Abeles FB, Morgan PW, Saltveit ME (1992) Ethylene in plant biology. Academic, San Diego
Burg SP, Burg EA (1966) The interaction between auxin and ethylene and its role in plant growth. Proc Natl Acad Sci USA 55:262–269
Dugardeyn J, Van Der Straeten D (2008) Ethylene: fine-tuning plant growth and development by stimulation and inhibition elongation. Plant Sci 175:59–70
Galston AW, Lavee S, Siegel BZ (1968) The induction and repression of peroxidase isosymes by indol-3-acetic acid. In: Wightman F, Setterfield G (eds) Biochemistry and physiology of plant growth substances. Runge, Ottawa, pp 455–472
Hahlbrock K (1974) Correlation between nitrate uptake, growth and changes in metabolic activities of cultured plant cells. In: Street HE (ed) Tissue culture and plant science. Academic, London, pp 264–378
Jackson MB, Osborne DJ (1970) Ethylene, the natural regulator of leaf abscission. Nature 225:1019–1022
Koves E, Szabo M (1987) Ethylene production in habituated and auxin-requiring tobacco callus cultures. Does ethylene play a role in the habituation? Physiol Plant 69:351–355
Lavee S, Galston AW (1968) Structural, physiological and biochemical gradients in tobacco pith tissue. Plant Physiol 43:1760–1768
Lin Z, Zong S, Grieson D (2009) Recent advances in ethylene research. J Exp Bot 60:3311–3336
Miller AR, Crawford DL (1985) Lignification and xylogenesis in Lactuca pith explants cultured in vitro in the presence of auxin and cytokinin: a role for endogenous ethylene. J Exp Bot 36:110–118
Morgan PW, Beyer E, Gausman HW (1968) Ethylene effects on auxin physiology. In: Wightman F, Setterfield G (eds) Biochemistry and physiology of plant growth substances. Runge, Ottawa, pp 1255–1274
Ruzika K, Ljung K, Vaneste S, Podhorska R, Beeckman T, Friml J, Bekova A (2007) Ethylene regulates root growth through auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19:2197–2212
Tsuchisaka A, Theologis A (2004) Unique and overlapping expression patterns among Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiol 136:2982–3000
Yung SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35:155–189
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lavee, S., Parnes, A. & Avidan, N. Involvement of ethylene in growth induction of stationary tobacco pith tissue in vitro. Plant Cell Tiss Organ Cult 103, 123–129 (2010). https://doi.org/10.1007/s11240-010-9761-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9761-1