Abstract
Calophyllum brasiliense (Cambes) produces calanolide secondary metabolites that are active against human immunodeficiency virus type 1 reverse transcriptase. In this study, it was demonstrated that plant tissue culture is a useful technique for producing these metabolites. Different concentrations and combinations of plant growth regulators were tested in leaf and seed explants to establish callus cultures capable of producing calanolides. Highest callus induction (100%) was achieved when seed explants were incubated in a medium consisting of 8.88 μM 6-benzyladenine and 20 μM picloram. Highest callus induction (80.67%) was observed when leaf explants were incubated on a medium consisting of 0.46 μM kinetin and 5.37 μM α-naphthaleneacetic acid. High-performance liquid chromatography quantitative analysis revealed higher calanolide B and calanolide C production in calluses from seed explants than those developed from leaves (309.25 vs. 8.70 mg kg−1 for calanolide B; 117.70 vs. 0.0 mg kg−1 for calanolide C).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
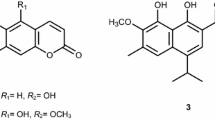
The incidence of human immunodeficiency virus type 1 (HIV-1)-infected people has drastically increased over the last few decades, which has motivated the exploration for new drugs and drug production methods (UNAIDS 2008). Several plant families that produce diverse secondary metabolites which possess activity to arrest the infection caused by HIV-1 have been investigated (Mi-Jeong et al. 2002; Harnett et al. 2005; Ovenden et al. 2004), among them the Calophyllum genus (Clusiaceae) which produce coumarin metabolites with significant activity against HIV-1 reverse transcriptase (RT) (Huerta-Reyes et al. 2004; Kashman et al. 1992; Mckee et al. 1996). In Mexico, only one species of Calophyllum exists, namely C. brasiliense Cambes. This tree shows two chemotypes, i.e., two different chemical compositions in the leaves have been characterized according to its natural distribution. The first chemotype (chemotype 1) grows in Sierra de Santa Marta, State of Veracruz, Mexico, and produces mammea type coumarins with high in vitro cytotoxic activity against human tumor cells (Reyes-Chilpa et al. 2004). The second chemotype (chemotype 2) grows in San Andres Tuxtla, State of Veracruz, Mexico, and produces tetracyclic dipyranocoumarins in low concentrations, such as calanolide A, calanolide B and calanolide C (Fig. 1a–c). Chemotype 2 also produces chromans, such as apetalic acid (Fig. 1d), in larger quantities than the tetracyclic dipyranocoumarins. Calanolide A and calanolide B have been identified as HIV-1 specific and potent RT inhibitors (providing complete protection against HIV-1 replication and cytopathicity), while calanolide C has shown moderate inhibitory HIV-RT properties (Huerta-Reyes et al. 2004; Kashman et al. 1992). Although the calanolide A has been reported as the most outstanding compound, its concentration in leaves is lower (~0.001%) than that of calanolide B (~0.009%) and or calanolide C (~0.003%) (Huerta-Reyes et al. 2004).
Tissue culture is an important tool in plant biotechnology that allows for an increase in biomass or metabolite production by utilizing several techniques in callus or morphogenetic cultures (Ramachandra and Ravishankar 2002; Dornenburg and Knorr 1995). These techniques include bioreactor scale-up, hairy transformed roots, micropropagation, elicitation, precursor compound addition and genetic engineering, among others (Mulabagal and Tsay 2004; Smetanska 2008; Dornenburg and Knorr 1995). Studies using in vitro cultures of the genus Calophyllum also exist. For instance, high in vitro multiplication and successful ex situ survival of micropropagated plants have been reported for C. apetalum (Nair and Seeni 2003) and C. inophyllum (Thengane et al. 2006). There are also reports of anti-HIV dipyranocoumarins production in callus and cell suspension cultures of C. inophyllum (Pawar et al. 2007; Pawar and Thengane 2009). To our knowledge, however, there are no reports using C. brasiliense tissue culture.
The aim of this study was to evaluate the influence of different combinations and concentrations of plant growth regulators (PGRs) on callus induction from C. brasiliense leaf or seed explants and to partially identify and quantify the tetracyclic dipyranocoumarins and chromans produced by the callus cultures.
Materials and methods
Plant material
Mature seeds were collected in November of 2005 in San Andres Tuxtla, State of Veracruz, Mexico. A voucher of the plant was previously identified and registered as #14425 at the herbarium of the Instituto Mexicano del Seguro Social (IMSS) (Huerta-Reyes et al. 2004). The endocarp and tegument were removed from the seeds, and they were then germinated under shadow conditions in plastic containers filled with agrolite and peat moss (1:1) as a substrate. When seedlings reached 10–15 cm in height (after approximately 3 months), they were transferred to polyethylene bags containing a mix of agrolite, peat moss and soil (1:1:1). Plants were conditioned and grown in a green house located at the Universidad Autonoma Metropolitana-Iztapalapa Campus (UAM-I). One month later, immature leaves of 5–6 cm in length were removed from the plant and used as the source of explants. Leaf or seed explants were disinfected superficially in a soap solution for 5 min, followed by immersion in a 70% (v/v) ethylic alcohol solution for 30 s. With low and constant agitation, leaves were then immersed for 15 min into a 0.6% (v/v) sodium hypochlorite solution supplemented with Tween-20 (three drops per 100 ml of prepared solution). Seed explants were immersed for 1 h in 27% (v/v) tetrachloroisophthalonitrile solution, followed by immersion in 4.2% (v/v) sodium hypochlorite solution for 1 h. Under aseptic conditions, leaves or seeds were rinsed three times with sterilized distilled water. Both disinfected explants were then transferred to Petri dishes containing antioxidant solution (citric acid 100 mg l−1 and ascorbic acid 150 mg l−1). Leaves were cut into 5 mm × 5 mm segments, while disinfected seeds were cut into four equal segments. The segments were then immersed in a new antioxidant solution for 10 min. Finally, three or four explants were placed into jars containing 25 ml of culture medium.
Culture medium and incubation conditions
The basal culture medium consisted of woody plant medium (WPM), 2% (w/v) sucrose, 100 mg l−1 citric acid, 150 mg l−1 ascorbic acid, 250 mg l−1 polyvinylpyrrolidone (PVP) and 0.18% (w/v) phytagel. Callus response in leaf and seeds explants was evaluated using different concentrations of cytokinin and auxin PGRs added to the basal culture medium: (a) kinetin (KIN) (0.00, 0.46, 2.32, 4.65, 6.97, 9.30 and 11.63 μM) and 2,4-dichlorophenoxyacetic acid (2,4-D) (0.00, 0.45, 2.26, 4.53, 6.8, 9.06 and 11.32 μM) or α-naphthaleneacetic acid (NAA) (0.00, 0.53, 2.68, 5.37, 8.05, 11.74 and 13.42 μM); (b) 6-benzyladenine (BA) (0.0, 4.44 and 8.88 μM) and indole-3-butyric acid (IBA) (9.80, 19.60 and 29.40 μM); (c) BA (8.88 μM) and picloram (PIC) (8.28, 16.56 and 24.84 μM) or NAA (10.74, 21.48 and 32.22 μM); and (d) thidiazuron (TDZ) (0.04, 0.45, 4.54, 13.62 and 27.24 μM). Finally, the pH value was adjusted to 5.8, and sterilization (121°C, 15 psi, 18 min) was carried out. Cultures were incubated at 25 ± 2°C in darkness. Three jars were used to evaluate callus induction for each treatment. The percentage of calluses developed in each explant treatment was calculated after 6 weeks. The treatments that induced the highest callus percentages (BA 8.88 μM and PIC 24.84 μM from seed explants and KIN 0.46 μM and NAA 5.37 μM from leaf explant) were selected and subcultured in their respective induction media. Transference was performed every 15 days for the first four subculture cycles, after which transference was done once a month for the next 10 cycles. After the subculture cycles, metabolite production analysis was performed.
Preparation of extracts and samples for HPLC analysis
Fresh leaves from seedlings and callus biomass produced by seeds and leaves explants were dried at 60°C in an oven and grounded into fine powders. Five 24-h extraction cycles with hexane at room temperature were performed on the dried samples (500 mg). Extracts were filtered, mixed and concentrated under reduced pressure with a Rotavapor (Buchi RE 111; Buchi Laboratoriums-Tecnick AG, Flawil, Switzerland). Concentrated samples were dried by vaporizing the remaining solvent at room temperature. Due to the scarcity of biological material, we could not isolate calanolide and apetalic acid standards. We were provided with standards calanolide B, calanolide C and apetalic acid by the Institute of Chemistry at UNAM (Huerta-Reyes et al. 2004). So we were not able, against our best wishes, to identify and quantify the production of calanolide A. Previous to HPLC analysis, the dried samples and the calanolide B, calanolide C and apetalic acid standards were diluted with acetonitrile in obtaining 0.75 mg ml−1 solutions, from which calibration curves were obtained by further dilution (10, 25, 50 and 100 μg ml−1).
HPLC analysis
Calanolide B, calanolide C and apetalic acid content was determined with a Waters high-performance liquid chromatography (HPLC) system equipped with 1525 binary pump (Waters Co., MA, USA), a kromasil C18 column (250 × 3 mm, particle size 5 μm) and 2487 dual wavelength absorbance detector (DWAD, Waters Co., MA, USA). The mobile phase was a mixture of acetonitrile (60%) and water (40%), which was pumped isocratically over 45 min at a flow rate of 1 ml min−1. Detection was performed using a wavelength of 284 nm for DWAD. The injection volume consisted of 20 μl. Samples and standards were run in the same way. Each prepared solution was filtered (0.45 μm, nylon filter) before injection. The Breeze 3.3 Waters software was used to process the chromatographic data. For identifying and quantifying calanolides (B and C) and apetalic acid from the samples, the respective retention time (R T ) and peak area data from the calibration curve were used. Each sample was injected three times.
Statistics
SAS 9.0 software (SAS Institute Inc, 2002) was used for statistical analysis. Data from callus induction percentages and calanolides or apetalic acid quantifications were subjected to an analysis of variance (ANOVA) followed by Tukey–Kramer multiple media comparison test. P-values less than 0.05 were considered significant. Each treatment was performed at least three times.
Results and discussion
Induced callus response
Aseptic cultures of leaf and seed explants were evaluated after incubation under conditions of darkness or light in Murashige and Skoog (MS) medium (1962) without PGRs, containing different antioxidant compounds to avoid necrosis of the explants: (a) citric acid 100 mg l−1 and ascorbic acid 150 or 50 mg l−1, (b) activated charcoal 250 or 500 mg l−1, or (c) PVP 250 or 500 mg l−1. The maximum survival percentage of the explants (87%) occurred under dark conditions with 250 mg l−1 of PVP added to the culture medium. These conditions were used in combination with either B5 (Gamborg et al. 1968) or WPM (Lloyd and McCown 1980) for establishing the cultures of C. brasiliense. It is known that optimal growth conditions of in vitro cultures depend on nutritional conditions, which may vary among species (Bhojwani and Razdan 1983). WPM had the best effect on explants growth, which displayed a smooth, soft and elongated appearance. Conversely, a brittle and hard appearance lacking in size increment was observed in explants cultured under B5 or MS culture media. Thus, for PGRs treatments, WPM was selected as the culture medium. These results are similar to those previously reported in C. inophyllum by Pawar et al. (2007), where explants positively responded to WPM.
Initially, KIN and 2,4-D or NAA were selected for developing in vitro C. brasiliense cultures, since they are the more active and are commonly the PGRs employed to establish tissue cultures (Staba 1982). However, callus developed from KIN 0.46 μM and NAA 5.37 μM treatment showed a slow growth when it was subcultured. Furthermore, treatments with KIN and 2,4-D or NAA that induced the highest callus percentages in leaf explants were then tested for seed explants; but no response was observed. It was expected that seed explants would produce significant callus percentages as observed in leaf explants, because the seed cells are less differentiated than those of leaves. Thus, because of the unsatisfactory induction response obtained and because plant material was scarce, no further attempts were made to induce seed explants with KIN and 2,4-D or NAA. In view of these results, cytokinin and auxin PGRs treatments were established, i.e., BA and IBA; BA and PIC or NAA; and TDZ, to test callus induction from seed and leaf explants. It is known that PGRs activity varies depending on the presence of transporter or receptor biosynthesized proteins in the explants, affecting in vitro culture development (Benjamins and Scheres 2008).
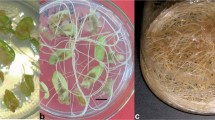
It was observed that callus and root induction on leaf or seeds explants was PGRs-dependent, and contrariwise, the control treatment (i.e., without PGRs) failed to induce any response (Tables 1 and 2). In general terms, the former responses started at the edges or on whole surface of the explants, after culturing the leaves for 40 days or the seeds for 10 days. Different morphologies were developed from the PGRs treatments tested in explants. For leaf explants, treatment with KIN and 2,4-D induced a greenish friable callus (Fig. 2a) that after 2 weeks turned to a yellowish appearance (Fig. 2b). Treatment with KIN and NAA induced the appearance of a white, compact callus (Fig. 2c) or root (Fig. 2d) that completely turned to a brown and friable callus (Fig. 2e) 3 weeks later. Regardless the treatments used for induction, i.e., BA and IBA or PIC or NAA, callus developed from leaves was white and compact, while a brown friable callus was developed from seed explants (Fig. 2f). TDZ, despite its reported activity as an auxin or a cytokinin (Murthy et al. 1998), was the worse PGR treatment as no for callus induction was observed in leaf and seed explants.
Callus and root responses induced in leaf and seed explants of C. brasiliense under PGRs treatments. a Greenish friable callus induced in leaf explants treated with KIN 4.65 μM and 2,4-D 4.53 μM after 40 days of culture; b yellowish callus developed from a 2 weeks later; c white, compact callus; or d root induced in leaf explants treated with KIN 0.46 μM and NAA 5.37 μM after 40 days of culture; e brown friable callus developed from d 3 weeks later; f brown friable callus induced in seed explants with BA 8.88 μM and PIC 24.84 μM after 30 days of culture
When 2,4-D, NAA, IBA or KIN was the sole source of PGRs, callus induction in leaf explants was significant. Concentrations of 0.45, 2.26 and 4.53 μM of 2,4-D, 0.53 or 2.68 and 5.37 μM of NAA, and 0.46, 2.32 and 4.65 μM of KIN, produced callus inducement of 50–67% (Table 1) by the former, and of 29–35.25% (Table 1) by the latter. In comparison, the treatment with 19.6 μM of IBA only produced a 29.17% callus induction (Table 2). However, the combination of auxin and cytokinin was required not only for achieving the highest callus induction in leaf explants (Table 1), but also to induce the callus development from seed explants (Table 2). The highest callus induction (80.67%) in leaf explants was obtained when KIN was combined with NAA at concentrations of 0.46 and 5.37 μM (Table 1), whereas the highest callus induction (100%) occurred in seed explants treated with BA 8.88 μM and PIC 24.84 μM (Table 2). Similar observations were made by Pawar et al. (2007) in Calophyllum inophyllum, who reported callus induction of 86.66% in seed explants when applying similar concentrations of BA and PIC. It is known that plant cells are totipotent and that induction responses depend on the age of the explant (Bhojwani and Razdan 1983). Therefore, the seed explants may produce the highest percentage of calluses.
The effect of auxins and cytokinins on callus induction on leaf or seed explants was significantly different, with auxins producing a higher induction percentage (P ≤ 0.05). With auxins, the greatest production of calluses in leaf explants occurred with NAA, whereas in seed explants it was with PIC. These results may be due to the fact that auxins are implicated in many aspects of the growth and development process of plants (Benjamins and Scheres 2008; Vanneste and Friml 2009). KIN produced significantly higher callus induction in leaf explants than BA. Explant type did not have a significant effect on callus induction. The highest friable callus production in leaf (FCL) explants occurred with KIN 0.46 μM and NAA 5.37 μM, and in seed (FCS) explants with BA 8.88 μM and PIC 24.84 μM. These were selected and maintained via subculturing. After 1 year of subculturing cycles, FCL and FCS calluses were harvested. The calluses were maintained for 1 year before carrying out secondary metabolite analysis, since somaclonal variation could occur in the callus, affecting secondary metabolite production (Bourgaud et al. 2001).
Calanolides and apetalic acid production
The coumarin production pattern was different for FCS and FCL. It has been shown in cell suspension cultures of C. inophyllum that PGRs affected growth and dipyranocoumarins expression (Pawar and Thengane 2009). Calluses collected from the FCS treatment produced more secondary metabolites than the FCL treatment (Table 3). Calanolide B, calanolide C and apetalic acid were identified from the FCS hexane extract, while only calanolide B was detected in FCL extract (Table 3). Furthermore, a 35.54-fold greater concentration of calanolide B was detected in FCS compared to FCL extract (Table 3). Calanolide B was the major coumarin produced by FCS (309.25 mg kg−1 dry weight (DW)), followed by calanolide C (117.70 mg kg−1 DW) and apetalic acid (30.98 mg kg−1 DW) (Table 3). C. inophyllum callus cultures produced similar concentrations of dipyranocoumarins (405.9 mg kg−1 fresh weight (FW) of inophyllum B and 1413.50 mg kg−1 FW of inophyllum P) (Pawar et al. 2007). Furthermore, other peaks of unidentified compounds were observed in the FCS chromatograms. It is possible that one of those peaks corresponded to calanolide A because of its chemical similarity to calanolide B and calanolide C (Huerta-Reyes et al. 2004). Also, those peaks might be associated with other related calanolide derivatives, which may potentially represent novel anti-HIV coumarin class of compounds. Mckee et al. (1996) and Kashman et al. (1992) isolated and identified new anti-HIV coumarin compounds from C. lanigerum and C. teysmanniil. Calanolide A was not evaluated in this work for a lack of a standard. This standard does not exist commercially, and because of the scarcity of the plant material available, we could not isolate calanolide A for obtaining a standard.
Additionally, chromatographical analysis was performed on hexane extracts from greenhouse plants leaves, contaminated by fungi or healthy. Higher concentrations of calanolide B and apetalic acid were produced in the fungal-stressed leaves than in the healthy leaves (Table 3). Calanolide B production was 10.25-fold greater, and apetalic acid production 6.9-fold greater in the fungal-stressed leaves. This higher coumarins production by the stressed leaves could be associated with secondary production induced by abiotic and biotic stress conditions (Ramachandra and Ravishankar 2002; Taiz and Zeiger 2006). Thus, it is likely that calanolide B and apetalic acid acted as phytoalexins in C. brasiliense (Whitehead and Threlfall 1992). Hay et al. (2003) reported several chromans, to which apetalic acid belongs, as antifungal compounds. These authors emphasized that an ongoing research effort drive exists for finding compounds with biological activities not only against HIV-1 but also against human pathogenic bacteria or fungi, particularly in immunocompromised patients.
Our results showed that C. brasiliense callus cultures may represent a feasible bio-source for producing calanolides. Production of calanolide B and calanolide C by FCS were 3.04- and 2.92-fold higher than that obtained from the healthy greenhouse plants leaves (Table 3). Also, FCS calanolide B and calanolide C production was ~3.43- and ~3.9-fold greater than that reported for wild plants leaves (Huerta-Reyes et al. 2004). In short, it is likely that biotic elicitation represents a suitable option to increase calanolides content. Another feasible strategy for increasing calanolides production could be the establishment of cell suspension cultures, which, besides of all, allow for an enhanced process control as stated by Pawar et al. (2007, 2009). Higher concentrations of several secondary metabolites had been obtained from cell suspension cultures compared to those from wild plants (Mulabagal and Tsay 2004).
Concluding, the highest percentage induction of callus from C. brasiliense was achieved in seed explants (100%) treated with BA 8.88 μM and PIC 24.84 μM (FCS); whereas for leaf explants (80.67%) was achieved in KIN 0.46 μM and NAA 5.37 μM treatment (FCL). Calluses that developed from these treatments showed better growth after a year of maintenance. Treatment with FCS was better for the production of coumarins compared to FCL treatment, producing 309.25 mg kg−1 DW of calanolide B, 117.70 mg kg−1 DW of calanolide C, and 30.98 mg kg−1 DW of apetalic acid. The FCS treatment is a viable alternative for the production of secondary metabolites, which offers the possibility of applying other techniques to increase the accumulation of these important compounds. Moreover, this work is the first one dealing with the production of calanolide B and calanolide C in tissue cultures from C. brasiliense.
Abbreviations
- ANOVA:
-
Analysis of variance
- BA:
-
6-Benzyladenine
- DWAD:
-
Dual wavelength absorbance detector
- DW:
-
Dry weight
- HIV-1 RT:
-
Human immunodeficiency virus type 1 reverse transcriptase
- HPLC:
-
High-performance liquid chromatography
- IBA:
-
Indole-3-butyric acid
- KIN:
-
Kinetin
- NAA:
-
α-Naphthaleneacetic acid
- PGR(s):
-
Plant growth regulator(s)
- PIC:
-
Picloram
- PVP:
-
Polyvinylpyrrolidone
- RT :
-
Retention time
- TDZ:
-
Thidiazuron
- WPM:
-
Woody plant medium
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
References
Benjamins R, Scheres B (2008) Auxin: the looping star in plant development. Annu Rev Plant Biol 59:443–465
Bhojwani SS, Razdan MK (1983) Plant tissue culture. Elsevier Science, Amsterdam
Bourgaud F, Gravot A, Milesi S, Gontier E (2001) Production of plant secondary metabolites: a historical perspective. Plant Sci 161:839–851
Dornenburg H, Knorr D (1995) Strategies for the improvement of secondary metabolite production in plant cell cultures. Enzyme Microb Tech 17:674–684
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Harnett SM, Oosthuizen V, Van de Venter M (2005) Anti-HIV activities of organic and aqueous extracts of Sutherlandia frutescens and Lobostemon trigonus. J Ethnopharmacol 96:113–119
Hay AE, Guilet D, Morel C, Larcher G, Macherel D, Le Ray AM, Litaudon M, Richomme P (2003) Antifungal chromans inhibiting the mitochondrial respiratory chain of pea seeds and new xanthones from Calophyllum caledonicum. Planta Med 69:1130–1135
Huerta-Reyes M, Basualdo MC, Abe F, Jiménez-Estrada M, Soler C, Reyes-Chilpa R (2004) HIV-1 Inhibitory compounds from Calophyllum brasiliense leaves. Biol Pharm Bull 27:1471–1475
Kashman Y, Gustafson KR, Fuller RW, Cardellina IIJH, McMahon JB, Currens MJ, Buckheit RW, Hughes SH, Cragg GM, Boyd MR (1992) The calanolides, a novel HIV inhibitory class of coumarin derivatives from the tropical rainforest tree, Calophyllum lanigerum. J Med Chem 35:2735–2743
Lloyd G, McCown B (1980) Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. Comb Proc Int Plant Prop Soc 30:421–427
McKee TC, Fuller RW, Covington CD, Cardellina JH, Gulakowski RJ, Krepps BL, McMahon JB, Boyd MR (1996) New Pyranocoumarins Isolated from Calophyllum lanigerum and Calophyllum teysmanniil. J Nat Prod 59:754–758
Mi-Jeong A, Kee-Dong Y, Chul YK, So-Young M, Yong-ung K, Hyun JK, Jeong HK, Cha-Gyun S, Chong-Kyo L, Tae GK, Seung HK, Hoon H, Jinwoong K (2002) Inhibition of HIV-1 reverse transcriptase and HIV-1 integrase and antiviral activity of Korean seaweed extracts. J Appl Phycol 14:325–329
Mulabagal V, Tsay HS (2004) Plant cell cultures - an alternative and efficient source for the production of biologically important secondary metabolites. Int J Appl Sci Eng 2:29–48
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plantarum 15:473–497
Murthy BNS, Murch SJ, Saxena PK (1998) Thidiazuron: a potent regulator of in vitro plant morphogenesis. In Vitro Cell Dev Biol-Plant 34:267–275
Nair LG, Seeni S (2003) In vitro multiplication of Calophyllum apetalum (Clusiaceae), an endemic medicinal tree of the western ghats. Plant Cell Tiss Organ Cult 75:169–174
Ovenden SPB, Yu J, Wan SS, Sberna G, Murray TR, Rhodes D, Cox S, Coates J, Neville GW, Meurer-Grimes BM (2004) Globoidnan A: a lignan from Eucalyptus globoidea inhibits HIV integrase. Phytochemistry 65:3255–3259
Pawar KD, Thengane SR (2009) Influence of hormones and medium components on expression of dipyranocoumarins in cell suspension cultures of Calophyllum inophyllum L. Process Biochem 44:916–922
Pawar KD, Joshi SP, Bhide SR, Thengane SR (2007) Pattern of anti HIV dypiranocoumarin expression in callus cultures of Calophyllum inophyllum Linn. J Biotechnol 130:346–353
Ramachandra RS, Ravishankar GA (2002) Plant cell culture: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153
Reyes-Chilpa R, Estrada ME, Ramírez AT, Amekraz B, Aumelas A, Jankowski CK, Vázquez TM (2004) Cytotoxic effects of mammea type coumarins from Calophyllum brasilense. Life Sci 75:1635–1647
Smetanska I (2008) Production of secondary metabolites using plant cell cultures. Adv Biochem Eng Biotechnol 111:187–228
Staba EJ (1982) Plant tissue culture as a source of biochemicals. CRC Press, Florida
Taiz L, Zeiger E (2006) Plant physiology. Sinauer Associated, USA
Thengane SR, Bhosle SV, Deodhar SR, Pawar KD, Kulkarni DK (2006) Micropropagation on Indian laurel (Calophyllum inophyllum), a source of anti-HIV compounds. Curr Sci 90:1393–1397
UNAIDS (2008) Report on the global AIDS epidemic. Available via http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp. Accessed 10 March 2009
Vanneste S, Friml J (2009) Auxin: trigger for change in plant development. Cell 136:1005–1016
Whitehead IM, Threlfall DR (1992) Production of phytoalexins by plant tissue cultures. J Biotechnol 26:63–81
Acknowledgments
The authors thank Dr. Marius Mumbai Massip from Universidad of Barcelona, Spain, and Dr. Arturo Navarro Ocaña from UNAM for their assistance with the HPLC analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bernabé-Antonio, A., Estrada-Zúñiga, M.E., Buendía-González, L. et al. Production of anti-HIV-1 calanolides in a callus culture of Calophyllum brasiliense (Cambes). Plant Cell Tiss Organ Cult 103, 33–40 (2010). https://doi.org/10.1007/s11240-010-9750-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9750-4