Abstract
Sphaeralcea angustifolia is used in Mexican traditional medicine to treat inflammatory processes. SCopoletin (SC), TOmentin (TO), and sphaeralcic acid (SA) were reported as the main anti-inflammatory compounds in this species. The aim of this study was to establish in vitro conditions for the development of calli and cell suspension cultures that are the producers of these active compounds. Callus cultures of plant leaf explants were set up using different auxin levels of α-naphthalene acetic acid (NAA) in combination with a constant concentration (0.1 mg L−1) of Kinetin (Kn) in Murashige and Skoog (MS) medium. Optimal combinations for callus induction were 1.0 and 2.0 mg L−1 of NAA. SC, TO, and SA were not detected in callus tissues. Employing a 4 % inoculum in fresh biomass, cell suspension was established from friable callus with 1.0 mg L−1 of NAA in combination with 0.1 mg L−1 of Kn in MS liquid medium (27.4 mM nitrate). The cellular suspension synthesized SC and SA, SC was excreted into the culture medium, while SA was excreted into the culture medium and accumulated in biomass. To improve SC and SA production, total nitrate content was reduced in MS medium. On diminishing nitrate content to 2.74 mM, cellular suspension growth was not modified. SC concentration (0.04 %) was 60-fold higher than that detected in the wild plant (0.00067 %), TO was produced (0.096 %), and SA content (0.0036 %) was not improved. SA production in MS medium with 0.274 mM nitrate (0.004 %) was enriched 12-fold (0.0003 %) in relation to that of the wild plant. The anti-inflammatory effects at 5 h of intraperitoneal (i.p.) administration (100 mg per kg BW) of dichloromethane extracts from the medium (42 ± 3 %) and biomass (39 ± 9.3 %) of S. angustifolia cell suspensions cultivated in MS with 2.74 mM nitrate were similar. The effect of the biomass dichloromethane extract was dose dependent with a median Effective Dose (ED50) of 137.63 mg per kg BW.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sphaeralcea angustifolia (Cav.) G. Don is a species of the Malvaceae family. Depending on the region of Mexico where the plant grows, it is denominated “vara de San José” or “yerba del negro” in Spanish, or “tlixihuitl” in the Nahuatl language (Martínez 1979; Calderón Rzedowski and Rzedowski 2001). The fresh aerial parts of this species are employed to treat inflammatory processes and as a wound-healing remedy in Mexican traditional medicine (Aguilar et al. 1994). The anti-inflammatory effect of the chloroform extract from aerial tissues was reported in a preliminary screening study, in which some plant extracts were tested for acute inflammation response in the Carrageenan-induced rat footpad edema (CFE) model (Meckes et al. 2004). Later, it was demonstrated that in rats with chronic inflammation induced by means of complete Freund’s adjuvant (CFA), intraperitoneal (i.p.) administration of the dichloromethane extract at a dose of 100 mg per kg of body weight (BW) per day for 8 days produced sustained and significant inhibition of the edema development (62.6 %). Topical application of the dichloromethane extract produced ear edema reduction (50.6 %) and a protective effect against 12-O-tetradecanoyl phorbol-13-acetate (TPA)-induced mouse ear irritation (García Rodríguez et al. 2012). In the rat CFA-induced arthritis model, i.p. administration of the dichloromethane extract produced significant inhibition of interleukins (IL) IL-1β and IL-6, and tumor necrosis factor alpha (TNF-α) and increased levels of the anti-inflammatory IL-10 (Juárez-Ciriaco et al. 2008). Phytochemical analysis of the extract was carried out to identify the active agents responsible for the anti-inflammatory effect. Active fraction containing β-sitosterol, stigmasterol, α- and β-amyrins, trans-cinnamic acid, and SCopoletin (SC, 7-hydroxy-6-methoxycoumarin) was identified (Fig. 1) as the main active compound (García Rodríguez et al. 2012). Later, TOmentin (TO, 5-hydroxy-6,7-dimethoxycoumarin) and sphaeralcic acid [(SA, 2-(1,8-dihydroxy-4-isopropyl-6-methyl-7-methoxy) naphthoic acid)] were isolated and identified as potent anti-inflammatory compounds (Fig. 1) in this species (Pérez-Hernández et al. 2014). In addition, loliolide, a monoterpene in the methanolic extract of S. angustifolia aerial parts, has also been reported (Osti-Castillo et al. 2010). A gel prepared with the 1 % dichloromethane extract of S. angustifolia aerial tissues standardized in SC demonstrated therapeutic effectiveness and tolerability similar to that with 2 % diclofenac in patients with hand osteoarthritis (Romero-Cerecero et al. 2013).
The collection of S. angustifolia plants from its natural habitat has been restricted by the Mexican Ministry of the Environment and Natural Resources (SEMARNAT, NOM-059-ECOL-1994) due to it being considered a species at risk of extinction. Consequently, the search for alternatives in preserving S. angustifolia medicinal plants is mandatory. Biotechnological approaches exhibit a potential for bioactive compound production and industrial exploitation by means of cell suspension cultures (Zhong 2001; Stafford 2002; Marja et al. 2004; Vanisree et al. 2004; Smetanska 2008). Moreover, under controlled culture conditions, such as nitrate restriction, it is possible to modulate cell suspension growth, and the secondary metabolism can be controlled to yield carbon-rich metabolites, such as phenolic compounds (Urbanczyk-Wochniak and Fernie 2005; Fritz et al. 2006; Zhou and Zhong 2009; Mora-Izquierdo et al. 2011; Nicasio-Torres et al. 2012). The aim of the present study was to evaluate the influence of plant growth regulators for inducing callus formation in S. angustifolia leaf explants, and subsequently, creating a cell suspension culture system to increase bioactive compound production by nitrate restriction in Murashige and Skoog (MS) culture medium (Murashige and Skoog 1962).
Materials and methods
Plant material Foliage and fruits of “vara de San José” plants were collected in Hidalgo State, Mexico, in June 2005. Three plant samples were authenticated by Abigail Aguilar, M.Sc., Head of the Herbarium at the Instituto Mexicano del Seguro Social in Mexico City [IMSSM] as S. angustifolia, and vouchers were stored for reference under #14294.
In vitro cultures
Axenic culture
Ten S. angustifolia seeds were transferred into glass bottles provided with cotton balls moistened with water and the containers were incubated at 26 ± 2 °C during a light:dark (16 h:8 h) photoperiod under 50 μM m−2 s−1 warm white-fluorescent light intensity. After 10 weeks, the growing seedlings were transplanted into foam potting cups prepared with a commercial substrate [Sunshine Fine mixture (70–80 %) Canadian peat moss, vermiculite, ground lime (chalk), and a moisturizing agent] and preserved under incubation conditions.
Young leaves from 6-month-old S. angustifolia plants were removed and disinfected by several passages through soap solution (2 %), ethanol (70 %) for 3 min, and 0.3 % sodium hypochlorite commercial solution with Tween-20 (0.4 %) for 10 min; finally, the leaves were rinsed with sterile distilled water. The disinfected organs were then excised into 0.5-cm2 sections and placed into glass Petri dishes containing MS liquid medium with 100.0 mg L−1 of cysteine (Sigma) to prevent phenolic compound oxidation and harm to the explants. After a period of 10 min, the excess of the medium on the explants was removed onto a sterile filter paper; then, explants were transferred into glass containers with 40.0 mL of half-strength MS medium with Chloramphenicol (50.0 mg L−1) and Amphotericin B (5.0 mg L−1) antibiotics for microbial growth inhibition (Debergh and Zimmerman 1993; Nicasio-Torres et al. 2012). The MS medium was supplied with 30.0 g L−1 of sucrose, adjusted to pH 5.7, 3.0 g L−1 of PhytaGel (Sigma), and autoclaved at 1 kg cm−2 for 18 min at 120 °C. Explants in glass containers were incubated at 26 ± 2 °C during a light:dark (16 h:8 h) photoperiod under 50 μM m−2 sec−1 warm white-fluorescent light intensity.
Callus cultures
After 10 days, visually non-contaminated leaf explants were transferred into glass bottles containing 40 mL of whole MS medium for callus induction. The MS medium was supplied with 30.0 g L−1 of sucrose, adjusted to pH 5.7, 3.0 g L−1 of PhytaGel (Sigma), and autoclaved. Different treatments resulting from several combinations of growth regulator α-naphthalene acetic acid (NAA) at 0, 0.5, 1.0, and 2.0 mg L−1 were mixed with an unvarying concentration of Kinetin (Kn) 0.1 mg L−1 in MS medium. The explants in glass containers were incubated under the same conditions as described previously. Explants were transferred into new medium every 4 weeks, and the percentages of explants with evidence of callus were scored for all treatments. After 20 weeks in culture, callus biomasses from each treatment were dried for extraction and chemical analyses.
Cell suspension cultures
Sphaeralcea angustifolia cell suspension in batch cultures were started with 3.2 g of friable callus (inoculum 4 %, w/v) developed with 1.0 mg L−1 of NAA in combination with Kn (0.1 mg L−1) in 80 mL of liquid MS medium (27.4 mM of total nitrate: NH4NO3, 15.9 mM and KNO3, 11.5 mM) supplied with 30.0 g L−1 of sucrose, adjusted to pH 5.7, and autoclaved. Flasks of cell suspensions were placed in an orbital shaker at 110 rpm (New Brunswick Scientific Co.) and incubated under the same conditions employed for axenic and callus cultures. Successful S. angustifolia cell suspension was changed to new medium under sterile conditions every 16 days, utilizing the same inoculum.
Growth and production kinetics
Nitrate concentrations in MS medium
Cell suspensions were cultivated into whole MS medium (27.4 mM total nitrate concentration) and into MS medium with total nitrate concentration reduced to 2.74 and 0.274 mM for the development of three independent experiments (Fritz et al. 2006; Mora-Izquierdo et al. 2011; Nicasio-Torres et al. 2012). Growth curves, under each nutrient condition, were obtained by registration of the dry weights (DW) of filtered biomasses from three flasks, every other day during a period of 23 days. The Growth Index (GI) was calculated considering maximal biomass obtained with a reduction of the inoculum and divided by the inoculum; the growth rate (µ) was calculated by means of a semi-log calculation of exponential phase and time (graph not shown); doubling time of the equation was Dt = ln 2/µmax, and biomass produced according to the sucrose content was determined based on the theoretical value (Y = 0.5 g of biomass/g of sucrose) reported for plants (Quintero 1981). Active compound curves were obtained by quantification of SC, TO, and SA in the dichloromethane extracts from the biomasses and the culture media of these batch cultures.
Chemical analyses
Extract preparation
Dry biomasses of calli and cell suspensions were extracted at room temperature three times by maceration (24 h for each procedure) with reactive-grade dichloromethane (Merck, Mexico). The dichloromethane extracts obtained for each sample were filtered through filter paper (Whatman No. 1), pooled, and concentrated to dryness under reduced pressure (Meckes et al. 2004). Culture media were partitioned three times with dichloromethane; extracts from each medium were pooled and concentrated to dryness (Filippini et al. 1998).
Dry aerial tissues of wild S. angustifolia plants were finely ground and three samples were separately extracted at room temperature three times by maceration (24 h for each procedure) with hexane (1:20 w/v) and subsequently extracted three times with dichloromethane (1:20 w/v). The dichloromethane extracts obtained for each sample were filtered through filter paper (Whatman No. 1), pooled, and concentrated to dryness under reduced pressure, and concentrated to dryness (Meckes et al. 2004). Dichloromethane extracts from calli, cell suspension biomass, culture media, and aerial tissues of wild plants were dissolved in high-purity methanol (Merck, Mexico) for their chromatographic analysis.
HPLC conditions
High-performance liquid chromatography (HPLC) analyses were carried out in a Waters system (2695 Separation Module) coupled with a diode array detector (2996) with a 190–600-nm detection range, and operated by the Manager Millennium software system (Empower 1; Waters Corporation, México). Extracts were eluted at a 1.2-mL min−1 flow rate with (A) high-purity H2O (H3PO4-1.0 %) and (B) high-purity CH3CN-gradient mobile phases (Merck, México) in a Spherisorb® RP-18 column (250 × 4.6 mm, 5 µm; Waters Corporation, México) and the compounds were identified at λ 340 nm and λ 357 nm. The gradient system was initiated with water (100 %) for 1 min and solvent B was gradually incorporated at 15 % (at 1 min), at 37 % (at 10 min), at 85 % (at 6 min), and at 100 % (at 2 min). This final proportion was maintained for 3 min and the mobile phase returned to initial conditions at 3 min. Identification of SC (99 %; Sigma-Aldrich Química, México), TO (93 %), and SA (95 %) was performed by comparing their retention times (TO-10.963 min, SC-11.286 min, and SA-17.374 min) and absorbance spectra. TO and SA compounds were isolated and purified at our Laboratory from S. angustifolia cell suspensions, according to the procedure already reported. The structures of both compounds were confirmed by comparison of spectroscopic data of 1H NMR (Pérez-Hernández et al. 2014). Calibration curves were constructed with standard solutions of 20, 40, 80, 160, and 320 μg mL−1; SC presented a regression equation of Y = 21,985(X)-117,889 and R 2 = 0.9997, while these for TO were Y = 46,018(X)-394,683 and R 2 = 0.9992, and for SA, these were Y = 2576(X)-23,696 and R 2 = 0.9996.
Anti-inflammatory activity
Animals
Male albino ICR mice weighting around 35 g (Harlan, Mexico City) were housed with eight animals per cage. Mice were maintained under laboratory conditions at 25 °C, 12-h light/12-h dark cycles with lights turned on at 07:00 a.m., and free access to water and standard food pellets (Harlan). The mice had at least 3 weeks for adaptation to the laboratory environment prior to initiating the experiment. All experimental procedures were carried out according to a protocol approved by the Institutional Research Committee in compliance with the Official Mexican Regulation dating from 1999 (NOM-062-ZOO-1999). Minimal number of animals (n = 7) and requisite duration of observation were employed to obtain consistent data. The local Ethics Committee approved the experimental protocol on January 1, 2011 with registration number R-2011-1701-3.
Carrageenan footpad edema (CFE)
The mice were divided into nine groups with eight mice each. Thirty minutes prior to λ-carrageenan injection (Sigma-Aldrich Química, México), the animals were injected intraperitoneally (i.p.), according to previous reports (Juárez-Ciriaco et al. 2008; García Rodríguez et al. 2012), with 100 mg per kg BW of dichloromethane extracts from the aerial tissues of the wild plant (Group 1), from cell biomasses (Groups 2, 3, and 4), from culture media (Groups 5, 6, and 7), vehicle (Tween 20 solution at 2 %; Merck, México) (Group 8), and with 45 mg per kg BW of Indomethacin (99 % purity; Sigma-Aldrich Química, México) (Group 9). The λ-carrageenan (1 %) was applied to the mouse’s right paw (20 µL) to induce acute inflammation (Morris 2003; Boeris et al. 2004; Gupta et al. 2005). Footpad sizes were measured before (time = 0) and after (at 1, 3, 5, and 7 h) λ-carrageenan injection using a digital micrometer (Micrometric Digimatic Calibration MDC-1″-SB; Mitutoyo Products, Mexico City, México). Groups 10 and 11 were injected i.p. with 50 and 200 mg per BW of biomass dichloromethane extract from cell suspension cultivated in MS medium with 2.74 mM nitrate for median Effective Dose (ED50) determination. CFE in controls and treated groups was determined with respect to footpad volume at initial time (T = 0), and the percentage of inhibition of edema development was calculated utilizing the following expression:
Statistical analysis
Each of the calculated growth parameters of cell suspensions (GI, µ, Dt, maximal biomasses, and biomass yields) was compared by means of Analysis of Variance (ANOVA) followed by Tukey’s multiple range tests; values of p ≤ 0.05 were considered statistically significant (SAS ver. 9.1 statistical software; SAS Institute, Inc.). Data obtained from CFE (volume and inhibition %) were analyzed by one-way ANOVA, and p values of ≤0.05 were considered significant. Significant differences among treatment means were calculated by the Tukey0.05 test.
Results and discussion
Callus cultures
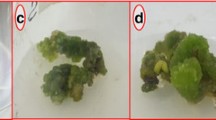
Within 10 days of inoculation into MS medium plus 0.5, 1.0, and 2.0 mg L−1 of NAA in combination with 0.1 mg L−1 of Kn, the leaf segments developed hard and pale callus granules. This process was preceded by the thickening of explants as a result of intensive cell proliferation, which led to callus formation with a different behavior notably on the area of the excised surface. In the three NAA concentrations evaluated, some explants did not exhibit callus growth, because the explants usually underwent browning and died due to phenolic oxidation. High NAA concentrations were necessary for survival of the explants and for callus growth (Table 1). After 8 weeks in culture (second subculture), some callus biomasses exhibited morphogenic characteristics with the three hormonal combinations (Table 1). The optimal hormonal combinations for callus induction in MS medium were 1.0 and 2.0 mg L−1 of NAA with 0.1 mg L−1 of Kn. Characteristics of the callus mass varied according to the NAA concentration: callus biomasses obtained with 0.5 mg L−1 of NAA were pale yellow with the presence of a red pigmentation, with 1.0 mg L−1 of NAA, callus biomasses were green in color, and with 2.0 mg L−1 of NAA, these were brownish-yellow in color (Table 1).
Cell suspension culture
The cellular proliferation of the S. angustifolia cell suspension in batch culture set into full MS medium (27.4 mM of nitrate) constituted of free cells with green pigmentation, and retained the characteristics of the cell suspension throughout the entire time in culture. The kinetics demonstrated a sigmoid growth pattern (Fig. 2): the lag phase lasted 4 days; thereafter, the logarithmic growth phase began, obtaining maximal biomass (15.42 g L−1) at day 16 followed by the stationary phase. The kinetic constants (Table 2), such as µ and Dt, as well as cellular biomass produced with respect to carbon source (sucrose), are found within the parameters already reported for plants (Quintero 1981; Hurtado and Merino 2001; Zhong 2001; Nicasio-Torres et al. 2012; Osuna et al. 2014; Tapia et al. 2013). To modify phenolic compound biosynthesis, cell suspensions were cultivated in MS medium with total nitrate concentration reduced to 2.74 mM (tenfold) and 0.274 mM (100-fold); under these conditions, the initial green cellular biomass formed small cell clusters and became brownish-yellow in color due to phenolic oxidation after 3 days in culture. Growth curves exhibited lag phases of 2 days, the time when the logarithmic growth phases began, reaching maximal biomasses on day 16 of cultivation (Fig. 2; Table 2). Under the three nitrate content conditions in the MS media assayed, there were no statistically significant differences in the kinetic constants µ and Dt; however, GI (F 0.05 = 11.66; p = 0.003; Tukey0.05 = 4.64), maximal biomasses (F 0.05 = 68.92; p = 0.001; Tukey0.05 = 6.07), and biomass produced per gram of sucrose added to MS medium (F 0.05 = 68.92; p = 0.001; Tukey0.05 = 6.07) diminished significantly when the nitrate concentration was reduced to 0.274 mM (Table 2).
SCopoletin (SC), TOmentin (TO), and Sphaeralcic Acid (SA) content
SC, TO, and SA were not detected in the dichloromethane extracts from callus biomasses of S. angustifolia under the established HPLC method, with a lowest detectable concentration of 5 µg mL−1.
Analyses of dichloromethane extracts of biomass and culture media of cell suspensions developed in full MS medium (27.4 mM nitrate) indicated that SC (107.35 μg L−1) was mainly released into the culture medium on day 9 (Fig. 3a). In cultures in MS medium with 2.74 mM nitrate, maximal SC excretion (Fig. 3a) was identified at day 2 of culture (999 μg L−1), remaining at low levels until the end of culture (97.55–116.57 μg L−1); SC accumulation in biomass was favored at the end of the stationary phase (day 23; 26.14 μg L−1). In MS medium with 0.274 mM nitrate, SC was accumulated in the stationary phase, and maximal concentration (19.9 μg L−1) was obtained at the time of maximal biomass (16 day) (Fig. 3a).
SCopoletin (a SC), TOmentin (b TO) and Sphaeralcic Acid (c, SA) excretion, as well as SA accumulation (d), in cell suspension cultures in batch from Sphaeralcea angustifolia developed in Murashige and Skoog (MS) medium complemented with different nitrate concentrations. Mean ± Standard error of the mean (SEM); n = 3
In cell suspension cultivated in MS medium with 2.74 mM nitrate, TO was only detected in the culture medium (Fig. 3b) during the stationary phase (day 16, 952.88 μg L−1, and day 18, 867.03 μg L−1), with a similar values than that obtained for SC at day 2 of culture (Fig. 3a). In cultures grown in MS medium with 0.274 mM nitrate, TO was also accumulated in biomass at the end of the adaptation phase (day 2, 17.46 μg L−1); its release into the culture medium occurred at the beginning of the logarithmic phase (day 4, 5.44 μg L−1). The concentrations detected in biomass were lower than those reported previously in the biomass of cell suspensions from S. angustifolia cultivated under the same conditions; however, excretion of TO was not assessed (Pérez-Hernández et al. 2014).
In cell suspensions cultivated in MS medium with 27.4 and 2.74 mM nitrate content, SA was excreted (39.0 μg L−1) and its levels were similar during the culture period. When nitrate was reduced to 0.274 mM, SA excretion was increased (day 2, 246.31 μg L−1), but it decreased 2 days later at a similar level to those detected in MS media with 27.4 and with 2.74 mM nitrate (Fig. 3c). Accumulation of SA in biomass was preserved in similar and constant concentrations during cultivation (38.92–59.22 μg L−1) in suspensions developed with complete MS medium (Fig. 3d). By decreasing the nitrate concentration to 2.74 mM in MS medium, SA production increased from day 2 onward, reaching its maximal production level at 11 days (112.04 μg L−1), this concentration remained during the stationary growth phase. SA concentration was twofold higher than that detected in the whole MS medium. In cultures with greater nitrate restriction (0.274 mM), a similar effect was observed: maximal production (244.80 μg L−1) was obtained on day 16, which was fourfold higher than that obtained in whole MS medium (Fig. 3d). SA concentrations in the biomasses of S. angustifolia cell suspensions cultivated under the same conditions were lower than those reported previously. In this report, SA excretion was not assessed (Pérez-Hernández et al. 2014).
Yield of SC production per gram of vegetal tissue was 5.7-fold higher (0.038 mg g−1 of biomass) in cellular suspension than that quantified for the S. angustifolia wild plant (0.0067 mg g−1 of aerial parts). It has been reported that in cell suspension cultures, 90 % of the coumarins produced are excreted into the culture medium (Alami et al. 1998; Sharan et al. 1998; Taguchi et al. 2001; Ryabushkina 2005; Gnonlonfin et al. 2011); this is a phenomenon that maintains a certain analogy with the effect observed in S. angustifolia cell suspensions.
It is well known that phytoalexins, such as the coumarins, are solely accumulated in plants under abiotic stress conditions or by pathogen attack (biotic stress) as a first mechanism of defense (Kai et al. 2006). Production of this type of compounds in cell suspension cultures in some cases is null or achieved at low yields. Biotic stimulation (fungi and bacteria) has not been very useful in some cases (Fliniaux et al. 1997).
Modification of nutritional factors has comprised one of the more effective strategies adopted to increase phenolic compound production in cell suspension cultures, such as flavonoids, phenylpropanoids, and coumarins (Urbanczyk-Wochniak and Farnie 2005; Fritz et al. 2006). To date, the effect of nitrate content on SC production has solely been evaluated in Nicotiana tabacum plants; SC levels were increased in leaves, stems, and roots (Armstrong et al. 1970). In S. angustifolia cell cultures developed in MS medium with nitrate restriction (2.74 mM), SC production (0.4 mg g−1 per g of biomass) was tenfold higher than that obtained in cell suspension cultures grown in whole MS medium (27.4 mM nitrate), and 60-fold higher than that obtained in aerial parts of the wild plant. Nitrate restriction in S. angustifolia cell suspensions was more effective for increasing SC production in comparison with N. tabacum cultures (SC level was doubled: 0.004 mg g−1) stimulated with methyl jasmonate (Sharan et al. 1998).
The cell suspension of S. angustifolia developed in complete MS medium produced fivefold higher levels of SA (0.0144 mg g−1) than that quantified for the aerial wild plant (0.003 mg g−1 of plant). In cell suspensions cultivated in MS medium with 2.74 mM nitrate, SA production (0.0359 mg g−1) was 12-fold higher, while in cell suspension cultivated in MS medium with 0.274 mM nitrate (0.0672 mg g−1) SA production was 22-fold higher. To our knowledge, this is the first time that the SA compound has been detected in the wild plants.
Modification of nutritional factors, as in the case of nitrate concentrations in culture media, has proven an adequate strategy to enhance the production of several secondary metabolites of commercial value. Examples are capsaicin in suspension cultures of Capsicum frutescens, anthraquinones in Morinda citrifolia, anthocyanins in Vitis species, taxuyunnanine C (8.07 mg g−1, 2 mM NH4 +) in Taxus chinensis, ginseng saponins in Panax quinquefolius, Panax notoginseng, and Panax ginseng (0.55 g g−1, 10 mM total nitrogen), chlorogenic acid (43.98 mg g−1, 8 mM nitrate) in Cecropia obtusifolia, and with anolide A (9.29 g g−1) in Withania somnifera (Liu and Zhong 1997; Smetanska 2008; Zhou and Zhong 2009; Nicasio-Torres et al. 2012; Nagella and Murthy 2011). It will be necessary to explore other nitrate concentrations, as well as other abiotic elicitors, such as metals (copper, zinc, or cobalt), to improve SC, TO, and SA production in cell suspension cultures of S. angustifolia (Mithöfer et al. 2004).
Anti-inflammatory activity
In the present work, we performed a temporal analysis of the effect of dichloromethane extracts deriving from the biomasses and culture media of S. angustifolia cell suspensions after 16 days in culture. The results demonstrated here indicate that the maximal level of carrageenan-induced inflammation was reached between 3 (3.05 ± 0.17 mm) and 5 h (3.02 ± 0.12 mm) and that the model was reproduced (Gepdiremen et al. 2005). Indomethacin, the anti-inflammatory drug employed as test control, inhibited edema formation by 27 % in the first h, with maximal activity of 60 % at 5 h, maintaining this at 55 % until 7 h. The data indicated that i.p. administration (100 mg per kg BW) of the dichloromethane extracts of biomasses (p < 0.01) and culture media (p < 0.001) from S. angustifolia cell suspensions, as well as the dichloromethane extract from the wild plant’s aerial parts, inhibited edema subplantar formation in the mouse after application of the pro-inflammatory agent (Tables 3, 4). Maximal anti-inflammatory effect observed for biomass, culture media, and plant dichloromethane extracts occurred at 3 and 5 h. Statistical analysis indicated that activities from biomass dichloromethane extracts (p = 0.8221 at 3 h and p = 0.7324 at 5 h) were similar (Table 3); however, the anti-inflammatory effect of medium dichloromethane extracts from cell suspensions cultivated under nitrate restriction (Table 4) were higher (p < 0.001) than that obtained from cell suspensions cultivated in whole MS medium. This effect could be explained because the anti-inflammatory compounds (SC, TO, and SA) are mainly produced under this condition. According to the Student t test, the anti-inflammatory effect at 5 h (42 ± 3 %) of i.p. administration (100 mg per kg BW) of dichloromethane extracts from the medium of S. angustifolia cell suspensions, cultivated in MS with 2.74 mM nitrate was similar (p = 0.1479) to that obtained (39 ± 9.3 %) with dichloromethane extract from the biomass of the cell suspension cultivated under the same conditions. The effect of biomass dichloromethane extract with i.p. administration was dose dependent, with a median Effective Dose (ED50) of 137.63 mg per kg BW (Fig. 4).
Relationship between the dose of biomass dichloromethane extract from Sphaeralcea angustifolia cell suspensions developed in Murashige and Skoog medium complemented with 2.74 mM nitrate concentrations administered intraperitoneally (i.p.) and λ-Carrageenan Footpad Edema (CFE), as well as between dose and CFE edema inhibition. The “best-fit” line shown was generated by linear regression of the data (n = 7) and square of correlation coefficient (R 2) regression equations are reported. Vertical and horizontal bars represent the standard error of the means (SEM) (n = 7)
At the same dose (100 mg per kg BW), the effect at 3 h of biomass dichloromethane extracts (39 ± 9.3 %) of S. angustifolia cell suspensions cultivated in MS medium with 2.74 mM nitrate was higher than that obtained with the biomass dichloromethane–methanol (9:1) extract (24 %) of cell suspensions cultivated in whole MS medium (Pérez-Hernández et al. 2014). This effect could be explained because the extract possessed higher concentrations of SC, TO and SA; the anti-inflammatory effect of these compounds has been reported as ≈60–70 % at doses of 45 mg per kg BW (Pérez-Hernández et al. 2014).
The S. angustifolia cell suspension cultivated in MS medium with adequate total nitrate content (2.74 mM) represents an alternative with great potential for upscaling the process into bioreactors of mechanical stirring bioreactors. The excretion of active compounds into the culture medium renders the process of extract obtention and compound purification simpler.
Considering the potential of S. angustifolia as an anti-inflammatory phytomedicine and the urgency of SEMARNAT in Mexico to protect the plant from overexploitation, the cell suspension comprises a controlled culture with high-quality production of SC, TO, and SA useful as an alternative for obtaining extracts and for isolating the compounds for their evaluation in an animal model of polyarthritis induced with kaolin and carrageenan and, in addition, to supply vegetal tissues with a continuous method for obtaining a standardized extract to produce the phytomedicine for clinical evaluation.
Author contribution statement
Dr. María del Pilar Nicasio-Torres wrote and designed the experimental protocol. Besides, Dr. Nicasio together with Dr. Juanita Pérez Hernández worked for the establishment of in vitro cultures (calli and cell suspensions), the growth kinetics, and production kinetics of scopoletin, tomentin and sphaeralcic acid in the cell suspension cultures. Dr. Mariana Meckes-Fischer and Dr. Francisco Cruz made chemical analyses by HPLC of biomass- and medium dichloromethane extracts. Dr. Manasés González contributed to the chemical work for sphaeralcic acid and tomentin purification, and their identification by H RMN. Dr. Jaime Tortoriello contributed to the anti-inflammatory activity evaluation of extracts from biomasses and media of cell suspensions and aerial parts of plants. All authors contributed to the writing of this paper.
Abbreviations
- BW:
-
Body weight
- CFA:
-
Complete Freund’s adjuvant
- CFE:
-
Carrageenan footpad edema
- Dt:
-
Doubling time
- DW:
-
Dry weight
- GI:
-
Growth index
- HPLC:
-
High-performance liquid chromatography
- IL:
-
Interleukins
- i.p.:
-
Intraperitoneal
- Kn:
-
Kinetin
- µ:
-
Growth rate
- MS:
-
Murashige and Skoog
- NAA:
-
α-Naphthalene acetic acid
- SC:
-
SCopoletin
- SA:
-
Sphaeralcic acid
- TO:
-
TOmentin
- TNF-α:
-
Tumor necrosis factor alpha
- TPA:
-
12-O-tetradecanoyl phorbol-13-acetate
References
Aguilar A, Camacho JR, Chino S, Jácquez P, López ME (1994) Herbario Medicinal del Instituto Mexicano del Seguro Social. México: Instituto Mexicano del Seguro Social. p 140–251
Alami I, Mari S, Clerivet A (1998) A glycoprotein from Ceratocystis fimbriata F. spp. platani triggers phytoalexin synthesis in Platanus acerifolia cell-suspension cultures. Phytochemistry 48:771–776. doi:10.1016/S0031-9422(97)00892-3
Armstrong GM, Rohrbaugh LM, Rice EL, Wender SH (1970) The effect of nitrogen deficiency on the concentration of caffeoylquinic acids and scopolin in tobacco. Phytochemistry 9:945–948. doi:10.1016/S0031-9422(00)85211-5
Boeris MA, Toso RE, Skliar MI (2004) Actividad antiinflamatoria de Salpichroa origanifolia. Acta Farm Bonaerense 23:138–141
Calderón-Rzedowski G and Rzedowski J (2001) Flora fanerogámica del Valle de México. México: Instituto Nacional de Ecología; pp 393–395, 406–408
Debergh PC, Zimmerman RH (1993) Micropropagation technology and application, 2nd edn. Kluwer Academic Publishers, México, p 488
Filippini R, Piovan A, Innocent G, Caniato R, Cappelletti EM (1998) Production of coumarin compounds by Haplophyllum patavinum in vivo and in vitro. Phytochemistry 49:2337–2340. doi:10.1016/S0031-9422(98)00356-2
Fliniaux M, Gillet-Manceau F, Marty D, Macek T, Monti JP, Jacquin-Dubreuil A (1997) Evaluation of the relation between the endogenous scopoletin and scopolin level of some solanaceous and Papaver cell suspensions and their ability to bioconvert scopoletin to scopolin. Plant Sci 123:205–210. doi:10.1016/S0168-9452(96)04596-7
Fritz C, Palacios N, Fiel R, Stitt M (2006) Regulation of secondary metabolism by the carbon–nitrogen status in tobacco: nitrate inhibits large sectors of phenylpropanoid metabolism. Plant J 46:533–548
García-Rodríguez V, Chamorro G, Siordia G, Jiménez-Arellanes A, Chávez MA, Meckes M (2012) Sphaeralcea angustifolia (Cav.) G. Don extract, a potential phytomedicine to treat chronic inflammation. Bol Latinoam Caribe Plant Med Aromat 11(5):454–465
Gepdiremen A, Mshvildadze V, Süleyman H, Elias R (2005) Acute antiinflammatory activity of four saponins isolated from ivy: alpha-hederin, derasaponin-C, hederacolchiside-E and hederacolchiside-F in carrageenan-induced rat paw edema. Phytomedicine 12:440–444
Gnonlonfin BGJ, Gbaguidi F, Gbenou JD, Sanni A, Brimer L (2011) Changes in scopoletin concentration in cassava chips from four varieties during storage. J Sci Food Agric 91:2344–2347. doi:10.1002/jsfa.4465
Gupta M, Mazumder UK, Kumar RS, Sambath R, Gomathi P, Rajeshwar Y, Kakoti BB, Selven VT (2005) Anti-inflammatory, analgesic and antipyretic effects of methanol extract from Bauhinia racemosa stem bark in animal models. J Ethnopharmacol 98:267–273. doi:10.1016/j.jep.2005.01.018
Hurtado DV, Merino ME (2001) Cultivo de tejidos vegetales. Editorial Trillas, México, pp 122–126
Juárez-Ciriaco M, Román-Ramos R, González-Márquez H, Meckes-Fischer M (2008) Efecto de Sphaeralcea angustifolia sobre la expresión de citocinas pro y antiinflamatorias. LabCiencia Not Tec Lab 2:21–23
Kai K, Shimizu B, Mizutani M, Watanabe K, Sakata K (2006) Accumulation of coumarins in Arabidopsis thaliana. Phytochemistry 67:379–386. doi:10.1016/j.phytochem.2005.11.006
Liu S, Zhong JJ (1997) Simultaneous production of ginseng saponin and polysaccharide by suspension cultures of Panax ginseng: nitrogen effects. Enzyme Microb Tech 21:518–524. doi:10.1016/S0141-0229(97)00023-9
Marja K, Caldentery O, Inzé D (2004) Plant cell factories in the post genomic era: new ways to produce designer secondary metabolites. Trends Plant Sci 9(9):433–440. doi:10.1016/j.tplants.2004.07.006
Martínez M (1979) Catálogo de nombres vulgares y científicos de plantas mexicanas. Fondo de la Cultura Económica; p, México, p 429
Meckes M, David-Rivera AD, Nava-Aguilar V, Jiménez A (2004) Activity of some Mexican medicinal plant extracts on carrageenan-induced rat paw edema. Phytomedicine 11:446–451. doi:10.1016/j.phymed.2003.06.002
Mithöfer A, Schulze B, Boland W (2004) Biotic and heavy metal stress response in plants: evidence for common signals. FEBS Lett 566:1–5
Mora-Izquierdo A, Nicasio-Torres MP, Sepúlveda-Jiménez G, Cruz-Sosa F (2011) Changes in biomass allocation and phenolic compounds accumulation due to the effect of light and nitrate supply in Cecropia peltata plants. Acta Physiol Plant 33:2135–2147. doi:10.1007/s11738-011-0753-5
Morris CJ (2003) Carrageenan-Induced paw edema in the rat and mouse. In: Inflammation Protocols. Springer Protocols, Berlin, p 225, 115–121
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Nagella P, Murthy HN (2011) Effects of macroelements and nitrogen source on biomass accumulation and withanolide—a production from cell suspension cultures of Withania somnifera (L.) Dunal. Plant Cell Tiss Org Cult 104:119–124. doi:10.1007/s11240-010-9799-0
Nicasio-Torres MP, Meckes-Fisher M, Aguilar-Santamaría L, Garduño-Ramírez ML, Chávez-Ávila VM, Cruz-Sosa F (2012) Production of chlorogenic acid and isoorientin hypoglycemic compounds in Cecropia obtusifolia calli and in cell suspension cultures with nitrate deficiency. Acta Physiol Plant 1(34):307–316. doi:10.1007/s11738-011-0830-9
Osti-Castillo MR, Torres-Valencia JM, Villagómez-Ibarra JR, Castelán-Pelcastre I (2010) Estudio químico de cinco plantas mexicanas de uso común en la medicina tradicional. Bol Latinoam Caribe Plant Med Aromat 9:359–367
Osuna L, Tapia N, Zamilpa A, Jiménez-Ferrer E, Tortoriello J (2014) Biosynthesis stimulation of nor-secotriterpene anxiolytics in cell suspension cultures of Galphimia glauca Cav. Eng Life Sci 14:68–75. doi:10.1002/elsc.201200209
Pérez-Hernández J, González-Cortazar M, Marquina S, Herrera-Ruiz M, Meckes-Fischer M, Tortoriello J, Cruz-Sosa F, Nicasio-Torres MP (2014) Sphaeralcic acid and tomentin, anti-inflammatory compounds produced in cell suspension cultures of Sphaeralcea angustifolia. Planta Med 80:1–6. doi:10.1055/s-0033-1360302
Quintero R (1981) Ingeniería Bioquímica: teoría y aplicaciones. Alhambra Mexicana, México, p 332
Romero-Cerecero O, Meckes-Fischer M, Zamilpa A, Jiménez-Ferrer JE, Nicasio-Torres P, Pérez-García D, Tortoriello J (2013) Clinical trial for evaluating the effectiveness and tolerability of topical Sphaeralcea angustifolia treatment in hand osteoarthritis. J Ethnopharmacol 147:467–473
Ryabushkina NA (2005) Synergism of metabolite action in plant responses to stress. Russ J Plant Physiol 52(4):614–621. doi:10.1007/s11183-005-0081-y
Sharan M, Taguchi G, Gonda K, Jouke T, Shimosaka M, Hayashida N, Okazaki M (1998) Effects of methyl jasmonate an elicitor in the activation of phenylalanine ammonia-lyase and the accumulation of scopoletin and scopolin in tobacco cell cultures. Plant Sci 132:13–19. doi:10.1016/S0168-9452(97)00260-4
Smetanska I (2008) Production of secondary metabolites using plant cell cultures. Adv Biochem Eng Biotechnol 111:187–228. doi:10.1007/10-2008-103
Stafford AM (2002) Plant cell cultures as a source of bioactive small molecules. Curr Opin Drug Discov Dev 5:296–303
Taguchi G, Yoshizawa K, Kodaira R, Hayashida N, Okazaki M (2001) Plant hormone regulation on scopoletin metabolism from culture medium into tobacco cells. Plant Sci 160:905–911. doi:10.1016/S0168-9452(00)00464-7
Tapia N, Zamilpa A, Bonfil M, Ventura E, Cruz-Vega D, Del Villar A, Cruz-Sosa F, Osuna L (2013) Effect of the culture medium and biotic stimulation on taxane production in Taxus globosa Schltdl in vitro cultures. Acta Physiol Plant 35(12):3447–3455
Urbanczyk-Wochniak E, Fernie AR (2005) Metabolic profiling reveals altered nitrogen nutrient regimes have diverse effects on the metabolism of hydroponically-grown tomato (Solanum lycopersicum) plants. J Exp Bot 56:309–321
Vanisree M, Lee CY, Lo SF, Nalawade SM, Lin CY, Tsay HS (2004) Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Bot Bull Acad Sin 45:1–22
Zhong JJ (2001) Biochemical engineering of the production of production of plant-specific secondary metabolites by cell suspension cultures. Adv Biochem Eng Biotechnol 72:1–26. doi:10.1007/3-540-45302-4_1
Zhou X, Zhong JJ (2009) Effect of initial ammonium concentration on taxoid production and biosynthesis genes expression profile in suspension cultures of Taxus chinensis cells. Eng Life Sci 9(3):261–266. doi:10.1002/elsc.200800109
Acknowledgments
This research received financial support provided by FIS-IMSS-PROT/G13/1225 from the Instituto Mexicano del Seguro Social (IMSS, México).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J van Staden.
Rights and permissions
About this article
Cite this article
del Pilar Nicasio-Torres, M., Pérez-Hernández, J., González-Cortazar, M. et al. Production of potential anti-inflammatory compounds in cell suspension cultures of Sphaeralcea angustifolia (Cav.) G. Don. Acta Physiol Plant 38, 209 (2016). https://doi.org/10.1007/s11738-016-2211-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2211-x