Abstract
An efficient protocol for high frequency in vitro regeneration of multiple shoots and somatic embryos from the embryonic axis of common bean (Phaseolus vulgaris) was developed. Ten common bean cultivars representing a wide range of diversity among current commercial market classes were used for in vitro regeneration evaluation in our study. These cultivars were tested on 63 different media formulations consisting of combinations of cytokinins, namely benzyladenine (BA) and thidiazuron (TDZ) at concentration levels of 0.0, 1.0, 2.5, 5.0 and 10.0 mg l−1 and auxin, namely naphthalene acetic acid (NAA) and indole-3-acetic acid (IAA) at concentration levels of 0.0, 0.05, 0.1 and 1.0 mg l−1. P. vulgaris cv. Olathe pinto bean performed the best producing over 20 multiple shoots per explant while cv. Condor black bean was the poorest with nine multiple shoots per explant. The optimum media for regeneration of multiple shoots was 4.4 mg l−1 Murashige and Skoog (MS) containing 2.5 mg l−1 BA and 0.1 mg l−1 IAA supplemented with 30 mg l−1 silver nitrate. Adventitious shoots and somatic embryos were regenerated on 4.4 mg l−1 MS medium containing 1 mg l−1 TDZ and 0.05 mg l−1 NAA supplemented with 30 mg l−1 silver nitrate or activated charcoal. Efficient and effective rooting of plantlets was achieved by dipping the cut end base of in vitro regenerated shoots in 1.0 mg l−1 indole-3-butyric acid (IBA) solution and culturing on media containing 4.4 mg l−1 MS supplemented by 0.1 mg l−1 IAA, NAA or IBA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Common bean (Phaseolus vulgaris) is a very important staple food crop, which provides valuable protein, calories, vitamins and minerals to people in many parts of the world (Blair et al. 2006). Major improvements in agronomic traits of cultivated common bean have been achieved through years of conventional breeding. While breeding has contributed to the improvement of desirable agronomic traits of this major food crop, it is, however, limited and restricted to improvements and traits available in the bean gene pool. As a consequence, novel traits from other species cannot be inserted into common bean. Therefore, genetic transformations are needed to allow breeders to introduce novel traits that could contribute to improved performance and quality, tolerance to abiotic and biotic factors that limit yield and reduce profitability (Aragao et al. 1996, 1998, 2001; Veltcheva et al. 2005).
In vitro shoot regeneration of P. vulgaris has posed the greatest obstacle and challenge limiting potential for an efficient genetic transformation system of common bean. However, in an experiment, when an intact seedling (IS) and cotyledonary node (CN) explants of P. vulgaris were cultured on full MS medium supplemented with 1 mg l−1 benzyladenine (BA) and 0.1 mg l−1 naphthalene acidic acid (NAA), the study resulted into shoot buds and shoots, which were produced more from IS than from CN (Ahmed et al. 2002). In another study (Zambre et al. 1998), protocol was developed for P. vulgaris and P. acutifolius A. Gray using a medium containing thidiazuron (TDZ) and indo-3-acetic acid (IAA), where the cotyledon explants for P. vulgaris XAN-159, regenerated successfully as opposed to embryonic axis explants, which failed. In contrast to P. vulgaris, the P. acutifolius NI574 embryogenic axis regenerated successfully (Zambre et al. 1998). Yet in another experiment, Arellano et al. (2009) developed an in vitro regeneration protocol for P. vulgaris cv. Negro Jamapa using indirect organogenesis, where only half of explants were able to regenerate. In this experiment, apical meristems and cotyledonary node explants were used for callus induction on medium containing 1.5 μM 2,4-dichlorophenoxyacetic acid and shoot development on medium containing 22.2 μM BA. Delgado-Sanchez et al. (2006) used embryonic axes explant of P. vulgaris cv. Flor de Junio Marcela (FJM) and Flor de Mayo Anita (FMA) to regenerate shoots with 83% and 50% regeneration efficiency, respectively, when cultured on MS supplemented with 5.0 or 10.0 mg l−1 BA.
Reports by Malik and Saxena (1992) on successful regeneration of P. vulgaris indicate a low regeneration efficiency and frequency of multiple shoots of about 4–8 per explant (Ahmed et al. 2002). The recalcitrant nature of in vitro regeneration of P. vulgaris is unique to this species of Phaseolus (Delgado-Sanchez et al. 2006) as successful efficient in vitro regeneration via somatic embryogenesis, and organogenesis has been reported in other Phaseolus species such as P. acutifolius, P. coccineus and P. polyanthus (Delgado-Sanchez et al. 2006; Dillen et al. 1997; Zambre et al. 1998, 2001).
In vitro rooting of P. vulgaris has also been a major problem. For example, Zambre et al. (1998) had difficulties acclimatizing their in vitro regenerated shoots of P. vulgaris in the greenhouse due to its poor in vitro rooting ability, while P. acutifolius rooted well and established easily in their greenhouse. To establish a better rooting system for P. vulgaris, the same team used an in vitro grafting system of P. vulgaris shoots on P. acutifolius roots to overcome the rooting problem (Zambre et al. 1998).
The objective of our study was to develop highly efficient and reproducible in vitro shoot apical meristem multiplication and somatic embryogenesis protocols, followed by efficient rooting of different cultivars of P. vulgaris, which are commonly grown in the USA for their possible genetic transformation.
Materials and methods
Plant material
Ten cultivars of P. vulgaris used in this experiment are shown in Table 1. The cultivars were selected to include common bean cultivars that represent the current genetic diversity in cultivated P. vulgaris grown in the US. The cultivars belong to eight different commercial classes of common bean and represent the two major gene pools, Andean and Middle American and four contrasting races of P. vulgaris as defined by Singh (2001).
Seed sterilization and explant preparations
Seeds were rinsed twice with sterile distilled water then immersed in 75% ethanol for 3 min, rinsed thrice with sterile distilled water and immersed for 20 min in a solution of 25% commercial Clorox, 5 ml l−1 tween 20 and 10 ml l−1 of 0.02% HgCl2. Following sterilization, the seeds were rinsed five times in sterile distilled water and soaked over night for 20 h. After the soaking period, seeds were dissected, and the embryos were excised. Then, hypocotyls were excised, leaving the epicotyl with plumule intact. The excised embryonic axes were incubated in vitro for 5 days at 25°C with 16 h photoperiod and light intensity of 45–70 μmol m−2 s−1 in the culture media described in the following.
Explant culture media
A total of 63 different media formulations, including different combinations of auxins (NAA and IAA) at concentrations (0.0, 0.05, 0.1 and 1.0 mg l−1) and cytokinins (BA and TDZ) at concentrations (0.0, 1.0, 2.5, 5.0 and 10 mg l−1) were used to determine the optimum concentration and types of growth regulators that favored robust and efficient in vitro regeneration. The growth regulator combinations were filter sterilized and added to 4.43 g l−1 MS (Murashige and Skoog 1962), 3% sucrose, 100 mg l−1 casein hydrorysate and 2.5 g l−1 gelrite. Growth regulators were added after autoclaving the media for 25 min at 120°C and 100 psi. The final media combinations were then poured into 100 mm × 25 mm Petri dishes where they solidified under a laminar flow hood.
Statistical and experimental design for explant culture
The statistical design was a three-way factorial in a completely randomized design (CRD). In this design, ten cultivars were evaluated using nine levels of cytokinins (BA and TDZ) and seven levels of auxin (NAA and IAA). The 10 × 9 × 7 factorial experiment with 630 treatments was replicated three times. Each experimental unit (Petri dish) consisted of five P. vulgaris embryonic axes explants. After regeneration, 3 out of 5 samples were randomly selected for analysis. A total of 1,890 experimental units with 5,670 data points were analyzed using PROC GLM (SAS version 9.1.3). Analysis of variance (ANOVA) was used to test the statistical significance at an alpha level of 0.01.
Overcoming in vitro production of phenolic compounds
An experiment was designed in order to negate the inhibitory effects of phenolic compounds produced in vitro. The experiment was conducted only with the most phenolic producing, cv. Condor. The apical meristem shoot proliferation experiment was repeated in media containing 4.43 mg l−1 MS salts and vitamins, 2.5 mg l−1 BA and 0.1 mg l−1 IAA and four different antioxidants, which included ascorbic acid (2 mg l−1), silver nitrate (30 mg l−1), activated charcoal (15 mg l−1) and glutathione (5 mg l−1) based on modification of published data (Abdelwahd et al. 2008).
Rooting of in vitro regenerated shoots
The cut end of the regenerated shoots (2 cm long) was dipped for 30 s in different concentrations (0.0 1.0, 5.0 or 10 mg l−1) of indole-3-butyric acid (IBA). The treated shoots were then cultured in 4.43 mg l−1 MS medium containing different concentrations (0.0, 0.05, 0.1 or 1.0 mg l−1) of IBA, NAA or IAA to induce rooting.
Statistical and experimental design for rooting
The statistical design for the in vitro rooting was a two factorial experiment in a CRD with the first factor being IBA dipping solution at four levels and the second factor being auxin concentrations at ten levels. The 4 × 10 factorial experiment with 40 treatments was replicated in space three times. From each experimental unit (Petri dish) five explants were cultured, and three plantlets were randomly selected for analysis. A total of 360 experimental units were analyzed using PROC GLM (SAS version 9.1.3). Analysis of variance (ANOVA) was conducted to test for significance at 0.01 level of probability.
Morphogenesis studies via scanning electron microscopy
Samples of in vitro multiplied shoot apices were fixed, dehydrated and dried as described by Klomparens et al. (1986). These samples were then coated with gold particles and microphotographed with JEOL JSM 31 (Tokyo, Japan) scanning electron microscope.
Results
Organogenesis and embryogenesis
Statistically significant differences were observed for in vitro regeneration performance of different P. vulgaris cultivars. The best plant growth regulator (PGR) combination for shoot proliferation of cv. Olathe and Sedona was 2.5 mg l−1 BA and 0.1 mg l−1 IAA, for cv. Matterhorn was 5 mg l−1 BA and 0.1 mg l−1 IAA, for cv. Merlot and Redhawk was 2.5 mg l−1 TDZ and 0.1 mg l−1 NAA, for cv. Montcalm was 5 mg l−1 BA and 0.05 mg l−1 NAA, for cv. Jaguar was 2.5 mg l−1 TDZ and 0.05 mg l−1 IAA, for cv. Beluga was 5 mg l−1 BA and 0.1 mg l−1 NAA for cv. Seahawk and Condor were 1 mg l−1 TDZ and 0.1 mg l−1 IAA (Fig. 1). The results showed that cv. Olathe produced the highest number of multiple shoots followed by cv. Sedona, Merlot, Matterhorn, Seahawk, Jaguar, Redhawk, Beluga, Montcalm and Condor (Fig. 1). The results also showed that the gene pool (race) for each cultivar played an important role in apical shoot meristem multiplication performance (Table 1). No statistically significant differences among the cultivars within their respective races were observed except for race Durango.
There were statistically significant differences among different cytokinins and cytokinin concentration levels. There were no significant differences shown among different auxins. However, there were significant differences between different concentration levels of auxins. Overall the most efficient growth regulator combination for shoot proliferation was a combination of 2.5 mg l−1 BA and 0.1 mg l−1 IAA, which produced a mean number of 12 multiple shoots per explant in all cultivars tested (Fig. 2).
Overcoming in vitro production of phenolic compound
Phenolic compounds exuding from the excised site of the embryonic axis gave a characteristic of browning and black color, which hindered normal growth and development of multiple shoots in vitro. To overcome this problem, the MS media containing growth regulators was supplemented with various antioxidants as described in the materials and methods. When compared to the control treatment with no antioxidants, silver nitrate (30 mg l−1) and activated charcoal (15 mg l−1) gave the best results reducing the degree of browning of the multiple shoot clumps (Fig. 3) and increasing the regeneration frequency by 16 and 18%, respectively, as well as increasing the number of multiple shoots (Table 2).
Effect of four antioxidant treatments on the quality of multiple shoots of cv. Condor; a an extreme case of an untreated explant failing to regenerate due to phenolics; b a different untreated explant; c explant treated with ascorbic acid; d Explant treated with glutathione; e Explant treated with activated charcoal; f Explant treated with silver nitrate
Morphogenesis studies via scanning electron microscopy
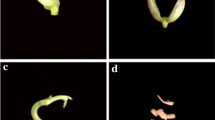
Direct adventitious shoot primordia were formed (Fig. 4a) after 3 weeks of culturing the embryonic axes in culture media A (Fig. 5). Direct somatic embryogenesis (Fig. 4b) occurred after the same duration of culturing the embryonic axis in culture medium B (Fig. 5). Shoots developed from clumps of adventitious shoot primordia after 4 weeks on culture medium D (Fig. 4c, d) and from direct somatic embryos after 7 weeks on culture medium D (Fig. 4c). Rooted P. vulgaris plantlets were obtained after 5 weeks by culturing of 2–3 cm long shoots on rooting medium E (Fig. 4e). The type and concentration of growth regulators were the key elements in determining the morphological pathway of in vitro regeneration of P. vulgaris. Overall BA promoted organogenesis, whereas, TDZ promoted embryogenesis (Fig. 5).
Differentiation of somatic embryos, multiple shoots and regenerated mature greenhouse grown rooted cv. Olathe common bean. a Scanning electron micrograph of a section of a multiple shoot clump 3 weeks after in vitro culture of an embryonic axis. AvS, adventitious shoot; LP, leaf primordia; ST, shoot tip; LH, leaf hair (×20); bar = 200 μm; b Scanning electron micrograph of a mixture of embryogenesis and organogenesis resulting 6 weeks after in vitro culture of an embryogenic axis explant. IDSE, indirect somatic embryo; DSE, direct somatic embryo; LP, leaf primordia; ST, shoot tip resulting from organogenesis (×40); bar = 200 μm; c A regenerating organogenic and embryogenic tissue 6 weeks after in vitro culture of an embryonic axis (×8); d An advanced apical multiple shoot clump regenerated through organogenesis 10 weeks after in vitro culture of an embryonic axis; e Efficient rooting after 30 s dipping of the cut end of a single in vitro regenerated shoot in 1.0 mg l−1 IBA followed by 5 weeks of in vitro culture in 0.1 mg l−1 NAA; f Greenhouse grown mature plants produced from rooted shoots
Rooting
The higher levels of auxin and lower levels of cytokinin had the greatest effect on root establishment. Auxin level of 0.1 mg l−1 gave the best results while lesser amount (0.05 mg l−1) led to poor or no root development (Fig. 6). High amounts of auxin (1 mg l−1) in the presence of low amount of cytokinin (1 mg l−1) gave many roots with little or no shoots, while the same high amount of auxin in the presence of high concentration of cytokinin (5–10 mg l−1) gave no roots and a few short shoots with many large leaves. There were no statistically significant differences among different auxin types based on the number and length of roots produced. However, there were significant differences among different concentration levels used on the root length and number of roots produced. The effect of dipping the base of each shoot in IBA was most significant. Overall the best treatment that produced strong multiple root establishment was dipping in 1.0 mg l−1 IBA solution and then culturing of the IBA treated shoots in media containing 0.1 mg l−1 of NAA, IAA or IBA, which resulted in a mean of nine roots per explant, each with mean length of 26.6 cm (Fig. 6).
Acclimation of rooted plantlets and transfer to greenhouse
Rooted plantlets were removed from Petri dishes, the agar media was removed from the roots by direct rinsing under running tap water, and the washed rooted plantlets were transferred into small pots containing BACTO potting soil. The pots were covered with plastic bags to eliminate evaporation resulting in high humidity around the potted plantlets to mimic the high humidity in Petri dishes. Potted covered plantlets were maintained under fluorescence light for 3 weeks or until new leaves emerged on the plantlets. Approximate 3-mm diameter holes were punched in the plastic bag covers every other day to gradually reduce humidity and to eventually acclimate plantlets to the low humidity of the greenhouse. Acclimated plants were transferred into larger pots and kept in a greenhouse where they grew to maturity and produced seeds (Fig. 4f).
Discussion
We demonstrated that it was possible to regenerate efficient apical shoot meristem multiplication from embryonic axes of P. vulgaris by optimizing appropriate combinations and concentrations of cytokinin and auxin. Cultivar played a significant role in apical shoot proliferation of P. vulgaris. Similar results were shown in shoot proliferations of cereal crops (Sticklen and Oraby 2005). Our results clearly show that closely related cultivars have similar shoot proliferation efficiency as opposed to distantly related cultivars. The cultivars that performed better were those that were able to heal faster from the wounding caused by excising the hypocotyl and the cotyledonary nodes and those that produced less secondary callus tissue at the excision site. We conclude that it is primarily the inability to heal fast from excision wounds and the excessive production of secondary callus tissue at the excision site that causes P. vulgaris to be recalcitrant toward in vitro regeneration.
We showed that cytokinin more than auxin was the key in accelerating wound healing of explants in vitro and reducing the amount of callus produced on the wounded explant. This is because auxins induce callus tissue in vitro. Moderate levels of cytokinin ranging from 2.5 to 5 mg l−1 favored the acceleration of wound healing and reduction in callus tissue, which resulted in an increase in the number of multiple shoots. On the other hand, high levels of cytokinin 10 mg l−1 delayed wound healing and inhibited the production of multiple shoots.
We observed that in vitro plantlet development from somatic embryos in P. vulgaris is more difficult with low regeneration efficiency and takes a longer time when compared to shoot development from adventitious shoots followed by rooting. However, somatic embryogenesis has an advantage over adventitious shoots because more plantlets per explant can be regenerated from somatic embryogenesis in vitro.
In our studies, rooting of in vitro grown shoots was a challenge mainly because the base of the shoots developed phenolic compounds in vitro causing blackening and death of cells, which prevented rooting. Similar problems were encountered by other researchers (Mohamed et al. 1991; Santalla et al. 1998; Zambre et al. 1998). In order to overcome this problem, we reduced the effect of phenolics with a solution composed of 15 mg l−1 activated charcoal and 30 mg l−1 silver nitrate and dipped the base of each shoot in IBA solution for 30 s. The rooting media also needed to be void of cytokinin as cytokinin delays root establishment.
As had been observed by other researchers (Ozyigit 2008), a combination of callus and phenolic compounds is naturally produced as a defense mechanism following wounding to aid in healing of plant tissues and to prevent entry of micro-organisms. Although the presence of callus and phenolics assists in wound healing, the greatest limiting factor in rooting of in vitro regenerated shoots of P. vulgaris was its propensity to produce high amounts of callus tissue that blocked root formation and phenolics compounds that caused death of tissues due to tissue oxidation (Arnaldos et al. 2001). Other researchers also discovered that oxidized phenolics prevent multiple shoot development, rooting or regeneration of explants (Ozyigit 2008). In our studies, the anti-oxidants including activated charcoal and silver nitrate were able to effectively reduce the oxidative effect of the phenolic compounds resulting into more and better quality multiple shoots with increased in vitro regeneration efficiency.
Conclusion
A major obstacle in in vitro regeneration of P. vulgaris includes the relatively inefficient in vitro shoot regeneration system mostly due to the in vitro production of phenolic compounds. In the research presented here, we were able to resolve the phenolic compound problem and therefore successfully regenerated in vitro multiple shoots up to 20 per explant of P. vulgaris. The excretion of phenolic compounds from wounds is normally associated with high production of callus tissue, which in our studies resulted in blocking of the in vitro regeneration of P. vulgaris. Supplementation of anti-oxidants to the culture media significantly improved the quality and increased the regeneration efficiency (i.e. the percent of explant regenerating) as well as the numbers of multiple shoots per explant of P. vulgaris.
The second obstacle associated with in vitro culture of P. vulgaris is its low number and poor rooting resulting in unsuccessful establishment of plants in greenhouses. To establish a good greenhouse rooting system for P. vulgaris, Zambre et al. (1998) grafted their in vitro regenerated P. vulgaris shoots on the P. acutifolius roots. In our research, we were able to resolve the rooting problem via the dipping of the in vitro-produced shoots in IBA followed by their cultures in media containing IBA, IAA or NAA.
Experiments have shown that in vitro regeneration of Phaseolus is species-specific. Our results indicate that, P. vulgaris regeneration is also cultivar-specific, and media formulation has to be made specifically for a particular cultivar in order to obtain maximum in vitro regeneration and rooting.
We have developed an efficient system for in vitro apical shoot proliferation of different cultivars of P. vulgaris. Apical shoot proliferation is important for mass propagation of virus free and true-to-type plants, which can be distributed to farmers in regions of the world where there is high prevalence of seed borne viral diseases (Bonfim et al. 2007; Delgado-Sanchez et al. 2006).
Furthermore, despite a few reports (Dillen et al. 2000; Bonfim et al. 2007; Aragao et al. 1996, 1998, 2001; Brasileiro et al. 1996; Liu et al. 2005), reproducible genetic engineering of P. vulgaris still remains a challenge mostly due to its recalcitrance toward in vitro regeneration and rooting. Efficient and reproducible in vitro plant regeneration and rooting protocols are the most important requirements for successful genetic transformation of different cultivars of P. vulgaris. The research presented here may assist in system development for efficient genetic transformation of P. vulgaris.
References
Abdelwahd R, Hakam N, Labhilili M, Udupa SM (2008) Use of an absorbent and antioxidants to reduce the effect of leached phenolics in in vitro plantlet regeneration of faba bean. Afr J Biotechnol 7(8):997–1002
Ahmed E, Ahmed EE, Bisztray GYD, Velich I (2002) Plant regeneration from seedling explants of common bean (Phaseolus vulgaris L.). Acta Biologica Szegediensis 46(3–4):27–28
Aragao FJL, Barros LMG, Brasileiro ACM, Ribeiro SG, Smith FD, Faria JC, Rech EL (1996) Inheritance of foreign genes in transgenic bean (Phaseolus vulgaris L.) co transformed via particle bombardment. Theor Appl Genet 93:142–150
Aragao FJL, Ribeiro SG, Barros LMG, Brasileiro ACM, Maxwell DP, Rech EL, Faria JC (1998) Transgenic beans (Phaseolus vulgaris L.) engineered to express viral antisense RNAs showed delayed and attenuated symptoms to bean golden mosaic geminivirus. Mol Breeding 4:491–499
Aragao FJL, Viana GR, Albino MMC, Rech EL (2001) Transgenic dry bean tolerant to the herbicide glufosinate ammonium. Crop Sci 42:1298–1302
Arellano J, Fuentes SI, Castillo-Espanã P, Hernández G (2009) Regeneration of different cultivars of common bean (Phaseolus vulgaris L.) via indirect organogenesis. Plant Cell Tiss Organ Cult 96:1–11
Arnaldos TL, Munoz R, Ferrer MA, Calderon AA (2001) Changes in phenol content during strawberry (Fragaria × ananasa, cv. Chandler) callus culture. Physiol Plant 113:315–322
Blair MW, Caldas GV, Avila P, Lascano C (2006) Tannin content of commercial classes of common bean. Annu Rep Bean Improv Coop 49:151–152
Bonfim K, Faria JC, Nogueira EOPL, Mendes EA, Aragao FJL (2007) RNAi-mediated resistance to Bean golden mosaic virus in genetically engineered common bean (Phaseolus vulgaris). Mol Plant-Microbe Interact 20:717–726
Brasileiro ACM, Aragao FJL, Rossi S, Dusi DMA, Barros LMG, Rech EL (1996) Susceptibility of common and tepary beans to Agrobacterium spp. strains and improvement of Agrobacterium-mediated transformation using microprojectile bombardment. J Am Soc Hort Sci 121:810–815
Delgado-Sanchez P, Saucedo-Ruiz M, Guzman-Maldonado SH, Villordo-Pineda E, Gonzalez-Chavira M, Faire-VelAzquez S, Acosta-Gallegos JA, Mora-Aviles A (2006) An organogenic plant regeneration system for common bean (Phaseolus vulgaris L.). Plant Sci 170:822–827
Dillen W, De Clercq J, Goosens A, Van Montagu M, Angenon G (1997) Agrobacterium mediated transformation of Phaseolus acutifolius A. Gray. Theor Appl Genet 94:15–158
Dillen W, Zambre M, De Clercq J, Goossens A, Kapila J, Vranova E, Van Montagu M, Angenon G (2000) In vitro regeneration and Agrobacterium-mediated transformation of Phaseolus vulgaris L. (common bean) and P. acutifolius A. Gray (Tepary Bean). Acta Hort 521:ISHS
Klomparens KS, Flegler S, Hooper GR (1986) Procedures for transmission and scanning electron microscopy for biological and medical sciences—a laboratory manual. Ladd Research Industries, Burlington
Liu Z, Park B-J, Kanno A, Kameya T (2005) The novel use of combination of sonication and vacuum infiltration in Agrobacterium-mediated transformation of kidney beans (Phaseolus vulgaris) with lea gene. Mol Breeding 16:189–197
Malik KA, Saxena PK (1992) Regeneration in Phaseolus vulgaris L.: high-frequency induction of direct shoot formation in intact seedlings by benzyladenine and thidiazuron. Planta 186:384–389 84-389; 1.nta 2
Mohamed MF, Read PE, Coyne DP (1991) Plant regeneration in vitro from the embryonic axes of common and tepary beans. Annu Rep Bean Improv Coop 34:150–151
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant 15:473–497
Ozyigit II (2008) Phenolic changes during in vitro organogenesis of cotton (Gossypium hirsutum L.) shoot tips. Afr J Biotechnol 7(8):1145–1150
Santalla M, Power JB, Davey MR (1998) Efficient in vitro shoot regeneration responses of Phaseolus vulgaris and P. coccineus. Euphytica 102:195–202
Singh SP (2001) Broadening the genetic base of common bean cultivars: a review. Crop Sci 41:1659–1675
Sticklen MB, Oraby HF (2005) Shoot apical meristem: a sustainable explant for genetic transformation of cereal crops. In Vitro Cell Dev Biol Plant 41:187–200
Veltcheva M, Svetleva D, Petkova SP, Pearl A (2005) In vitro regeneration and genetic transformation of common bean (Phaseolus vulgaris L.)—problems and progress. Sci Hortic 107:2–10
Zambre MA, De Clercq J, Vranova E, Van Montagu M, Angenon G, Dillen W (1998) Plant regeneration from embryo-derived callus in Phaseolus vulgaris L. (common bean) and P. acutifolius A. Gray (tepary bean). Plant Cell Rep 17:626–630
Zambre MA, Geerts P, Maqeut A, Van Montagu M, Dillen W, Angenon G (2001) Regeneration of fertile plants from callus in Phaseolus polyanthus Greenman (Year Bean). Ann Bot 88:371–377
Acknowledgments
Kingdom Kwapata’s Ph.D. research has been supported by funding from the W. Fulbright fellowship, the W. Leo and Rae Phelps Mericle Memorial Scholarship and the MSU College of Agriculture and Natural Sciences. The authors would like to thank Dr. Sasha Kravchenko for providing advice on the statistical design, and Halima Awale for her assistance on maintaining plants in greenhouses. Scanning microscopy presented in this article was performed through services of the MSU Center for Advanced Microscopy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kwapata, K., Sabzikar, R., Sticklen, M.B. et al. In vitro regeneration and morphogenesis studies in common bean. Plant Cell Tiss Organ Cult 100, 97–105 (2010). https://doi.org/10.1007/s11240-009-9624-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-009-9624-9