Abstract
A protocol for in vitro regeneration via indirect organogenesis for Phaseolus vulgaris cv. Negro Jamapa was established. The explants used were apical meristems and cotyledonary nodes dissected from the embryonic axes of germinating seeds. Several auxin/cytokinin combinations were tested for callus induction. The best callus production was obtained with medium containing 1.5 μM 2,4-dichlorophenoxyacetic acid. After 2 weeks of growth calli were transferred to shooting medium containing 22.2 μM 6-benzylaminopurine. Shoots regenerated with a frequency of approximately 0.5 shoots per callus, and upon transfer to rooting medium these shoots produced roots with 100% efficiency. Histological analyses of the regeneration process confirmed the indirect organogenesis pattern. Greenhouse grown regenerated plants showed normal development and were fertile. The protocol was reproducible for other nine P. vulgaris cultivars tested, suggesting a genotype independent procedure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Legumes account for 27% of the world’s major crop production, with grain legumes providing more than one-third of humankind nutritional nitrogen requirement. Legumes are not easily amenable to stable genetic transformation and hence, protocols for high throughput generation of transgenic legume plants are not available. In general, the difficulty for achieving efficient genetic transformation of legumes is related to their low responsiveness for in vitro regeneration. The best approach for obtaining stable transgenic legumes has been through indirect in vitro regeneration, defined as the induction of regenerative somatic embryos or shoots from morphogenetic calli.
Common bean, Phaseolus vulgaris L., is the most important grain legume for human consumption in the world. This and other species from the Phaseolus genus, are recalcitrant for in vitro regeneration and for genetic transformation (Broughton et al. 2003). Although many protocols for in vitro regeneration of P. vulgaris have been reported (reviewed by Nagl et al. 1997), most of these are based on direct organogenesis or shoot development from meristematic cells. More recently, Santalla et al. (1998) reported the regeneration through direct organogenesis of 10 P. vulgaris elite cultivars and seven P. coccineus landraces from cotyledons with a small portion of the split embryonic axes. Cruz de Carvalho et al. (2000) achieved a high frequency direct regeneration protocol, using traverse thin cell layers, derived from 14-days-old plantlets of P. vulgaris cv Carioca. Delgado-Sánchez et al. (2006) established a procedure for efficient regeneration of two commercial cultivars of P. vulgaris through direct organogenesis from embryonic axes derived from mature seeds. Zambre et al. (2001) reported the indirect regeneration, from apical and axillary buds from mature plants and from cotyledons and embryonic axes obtained from in vitro germinated seeds, of several P. polyanthus cultivars, a species phyllogenetically close related to P. vulgaris. To our knowledge there are only two reports on indirect regeneration of Phaseolus vulgaris. Mohamed et al. (1993) tried the in vitro indirect regeneration from morphogenetic calli of 5 different P. vulgaris cultivars but only succeeded with the Tara and Xan-159 cultivars that are hybrids obtained by crossing P. acutifolius x P. vulgaris. Moreover their results could not be reproduced by Zambre et al. (1998). The second report was from Zambre et al. (1998) who obtained fertile P. vulgaris plants through indirect organogenesis and in vitro shoot regeneration of the inbred line Xan159; their results are highly dependent on the cultivar used.
A protocol for obtaining P. vulgaris fertile transgenic plants through the biolistic method, albeit with a very low efficiency, has been reported (Rech et al. 2008). However, the only successful procedure for regeneration of transgenic plants via Agrobacterium mediated transformation for a Phaseolus species was reported by Dillen et al. (1997) this was achieved for P. acutifolius using an indirect regeneration method coupled to A. tumefaciens genetic transformation.
Since the only two reported protocols for indirect regeneration of P. vulgaris are genotype dependent (Mohamed et al. 1993; Zambre et al. 1998), the aim of this work was to establish a method for in vitro regeneration via indirect organogenesis of different P. vulgaris cultivars. We describe a protocol for morphogenetic callus induction from apical meristems and cotyledonary nodes obtained from embryonic axes and their regeneration into fertile plants. This protocol is highly reproducible for ten different P. vulgaris cultivars of both Mesoamerican and Andean origin.
Materials and methods
Plant materials
Phaseolus vulgaris cultivars belonging to both Mesoamerican and Andean genotypes used in the present study are listed in Table 1. Plants derived from various bean cultivars were grown in the greenhouse for multiplication.
Callus induction
Bean seeds were surface sterilized in the neutral detergent Hyclin-plus (HYCEL, Mexico DF, Mexico) for 1 min, then in 70% (v/v) ethanol for 1 min and in 10% (v/v) commercial bleach for 10 min. Finally seeds were rinsed four times with sterile distilled water, allowing 5 min for each rinse. Sterile seeds were germinated in 16 h light/8 h dark for 22–24 h over a wet sterile filter paper in a growth chamber maintained at 25°C. Subsequently from these germinating seeds embryo axes were separated from cotyledons and further grown in liquid MS medium (Murashige and Skoog 1962) containing B5 vitamins (Gamborg et al. 1968), 3% (w/v) sucrose, 22.2 μM 6-benzylaminopurine (BAP), 0.5 mM 2-(N-Morpholino) ethanesulfonic acid (MES), and 0.5 g l−1 polyvinylpyrrolidone 360,000 molecular weight (PVP-360; Sigma, St. Louis MO, USA) for another 22–24 h. After the completion of incubation, three different explants were dissected from the embryonic axes under the stereomicroscope, namely the shoot apical meristem (0.5–1.0 mm) region (AM) that includes part of the epicotyl without plumule, the cotyledonary node region (1.0–1.5 mm) (CN) and the hypocotyl region (1.0–1.5 mm) devoid of primary root, and used for callus induction. Cotyledons were also used for callus induction.
Callus induction medium (CIM), contained N6 macronutrient salts (Chu et al. 1975), MS micronutrient salts, B5 vitamins, 0.1 g l−1 myo-inositol, 3% (w/v) sucrose, 0.5 mM MES, 7 g l−1 agar type A (Sigma, St. Louis MO, USA), 0.5 g l−1 PVP-360 (pH 5.7 ± 0.1) together with various combinations of auxins and cytokinins. For different experimental trials CIM was supplemented with: 0, 1, 1.5 or 2 μM indole-3-acetic acid (IAA), 2,4-dichlorophenoxyacetic acid (2,4-D) or α-naphthaleneacetic acid (NAA) in combination with: 0, 1, 10 or 20 μM BAP or thidiazuron. To determine the best auxin cytokinin combination and the best explant type for callus induction, 3 callus induction experiments were carried out for each auxin/cytokinin combination. Each experiment consisted of 16 different treatments with 4 flasks (baby food jars) per treatment, and 5 explants of each type: AM, CN, cotyledons and hypocotyls per flask. All the explants were cultivated 4 weeks on CIM in a growth chamber at 24°C and 16 h light/8 h dark photoperiod provided by cold fluorescent lamps (54 μmol m2 s−1). Effect of different auxin/cytokinin combinations on callus growth was evaluated based on the fresh weight, the appearance and the consistence of calli produced in each treatment.
Shoot regeneration and rooting
After 1, 2, 3 or 4 weeks of callus induction on CIM media, only well developed calli were transferred to shooting medium (SM) based in MS macro and micro elements, B5 vitamins, 3% (w/v) sucrose, 0.5 mM MES, and 0.5 g l−1 PVP-360, and supplemented with 22.2 μM BAP or 10.0 μM thidiazuron. The number of well developed shoots (stem with leaves 1.0–2.0 cm long) regenerated per callus was evaluated after 4 weeks of incubation on SM. Buds or shoot buds like structures were not considered for scoring. The regenerated shoots were transferred for 2–4 weeks to rooting medium (RM) that contained MS macro and micronutrient salts, B5 vitamins and 7 g l−1 agar, 0.444 μM BAP and 0.054 μM NAA, pH 5.7 ± 0.1. Plantlets with well-developed root system were transferred to pots with vermiculite and covered with plastic bags to maintain good humidity conditions. These were cultured in growth chambers with controlled conditions (24°C and 16 h light/8 h dark photoperiod) for 1–2 weeks. After this adaptation period regenerated plants were grown in the greenhouse until seed production.
Histological analysis
For this study, 220 samples of each type of explant (AM and CN) were prepared and incubated on CIM supplemented with 2,4-D 1.5 μM (CIM1.5D), under the conditions described above. For analysis of the de-differentiation process, 10 samples of each type of explant were collected daily from 0 to 7 days and weekly until the 3rd week. For analysis of the morphogenetic process the remaining samples were transferred to SM supplemented with 22.2 μM of BAP (SM5B) at 7, 14 and 21 days. Ten samples of the calli transferred at each time were collected after 1, 2, 3 and 4 weeks.
Tissue samples were fixed in 3% (v/v) glutaraldehyde and 1.5% (v/v) paraformaldehyde in phosphate buffer (pH 7.2) for 24 h. Fixed tissues were gradually dehydrated in ethanol and xylol/Paraplast (Oxford Labware, St. Louis MO USA) series and finally embedded in Paraplast (Trepp et al. 1999). Embedded tissues were sequentially sectioned (8–10 μM) with a microtome, mounted on glass slides and stained with 1% (w/v) safranin in 50% (v/v) ethanol and finally mounted with Permount (Fisher Scientific, Fair Lawn NJ, USA) Observations and photomicroscopy were performed under brightfield optics using Axioskop 2 microscope (Zeiss).
Statistical analysis
The statistical analysis of callus induction experiments was carried out by two way 4 × 3 factorial ANOVA for independent samples. Fresh weight data of each type of explant from 3 independent experiments, each experiment with 3 culture flasks with 5 explants each, were recorded after 4 weeks of incubation in CIM1.5D. The critical values for the Tukey absolute difference between any two means (HSD) was obtained and used to calculate the least significant difference (LSD) of each mean pair. The LSD was obtained separately for each type of explant (AM and CN) (Table 2).
The statistical analysis of morphogenetic calli formation and of shoot regeneration was carried out by two way ANOVA for independent samples. Data coming from 4 independent experiments, each experiment with 20 or 30 AM or CN explants from each of the 10 tested cultivars incubated for 2 weeks in CIM1.5D, were analysed. The critical values for HSD Tukey test were obtained and the LSD of each mean pair between both, the type of explant (AM and CN) and the different cultivars, were calculated (Table 3). The number of shoots formed per callus (Table 3) were considered only from those shoots that developed a root system in RM.
Results and discussion
Shoot regeneration from calli induced from AM and CN explants of Negro Jamapa cultivar
The first objective towards the establishment of an indirect in vitro regeneration protocol for the bean cultivar Negro Jamapa was to develop an optimal medium for the induction of morphogenetic calli. A total of 96 different combinations of auxin and cytokinin were tried in CIM for achieving callus induction from various explants (AM, CN, hypocotyls and cotyledons). The calli formed with different combinations of phytohormones in CIM were transferred, after 1, 2, 3 or 4 weeks, to SM and were evaluated for their shoot induction capacity.
We observed that the auxin supplemented CIM prepared with N6 macronutrient salts facilitated the development of morphogenetic-competent calli. On the other hand, calli formed on MS or B5 macronutrient salts, instead of N6 did not show the morphogenetic capability of N6 macronutrients for regeneration. We also noted that if PVP-360 was omitted from CIM or from SM, calli rapidly turned dark brown, and concomitantly lost the ability to regenerate. The presence of an auxin in CIM was found to be essential for calli induction since no callus was developed on media without auxin. In most of the treatments that included IAA or NAA calli developed only after a long incubation (4 weeks) in CIM, but these were very small, compact and brown in colour. These calli were not morphogenic since no shoots developed when transferred to SM.
The best results for morphogenetic calli induction were obtained from AM and CN explants. Though hypocotyl explants produced a good amount of friable calli these were unable to regenerate any shoots, although regeneration of a considerable amount of adventitious roots on SMB5 was observed. The cotyledon explants produced calli in only one of the auxin/cytokinin combinations tested and these were not morphogenetic.
Based on the previous report from Mohamed et al. (1993) about the indirect regeneration of two inbred lines of P. vulgaris we included IAA/thidiazuron combinations added to CIM, however we did not observe any shoot formation from these calli. In agreement, Zambre et al. (1998) had reported that the results from Mohamed et al. (1993) were not reproducible in their conditions. Our best results of morphogenetic calli induction from AM and CN explants (Fig. 1a and i) were using CIM plus a 2,4-D/BAP combination. In these treatments calli were formed very fast during the 1st week of culture. The data of calli fresh weight recorded at the 4th week of culture are shown in Table 2. The statistical analysis showed that there were some significant differences among the fresh weight of calli from different 2,4-D/BAP combinations (Table 2), but there were not significant differences between treatments that gave morphogenetic calli. Interestingly, we noted that although the treatments with higher 2,4-D/BAP concentrations induced heavier calli (Table 2) most of these were not morphogenetic since no shoot formation was observed. The more responsive morphogenetic calli formation was observed in CIM with 1.5 or 2 μM 2,4-D alone or in combination with a maximum of 0.1 μM BAP (Table 2). Figure 1c and j show representative calli induced from AM or NC, respectively, in CIM1.5D. These calli were medium sized, with creamy-light green colour and were friable. The frequency of morphogenetic calli formation in CIM1.5D ranged from 43% to 52% (Table 3).
Indirect organogenesis from AM and CN explants of cv. Negro Jamapa. (a) Dissected shoot apical meristem region (AM) used as explant (time cero) (10×). (b) AM in de-differentiation process after 3 days in CIM1.5D (10×). (c) Friable callus development in CIM1.5D after 2 weeks (2×). (d) Shoots buds regenerated from calli cultured in SM5B after 2 weeks (2×). (e–h) Histological characterization of tissues shown in (a–d), respectively. (e) Longitudinal section of intact AM explant showing the dome (dm) and leaf primordia (lp) (20×). (f) Longitudinal section of shoot apical meristems at initial de-differentiation process (3 days in CIM1.5D) showing axillary shoot buds (asb) and dome (dm (20×). (g) Callus section showing parenchymatic (p) and meristematic cells (m) (5×). (h) Morphogenetic changes in callus after 2 weeks in SM5B, showing typical development of single bud meristemoids (me) close to the surface of the callus (25×). (i) Dissected cotyledonary node (CN) used as explant (time cero) (5×). (j) Compact callus developed on CIM1.5D after 2 weeks (2×). (k) Initial re-differentiation of callus showing shoot buds after 1 week in SM5B. (l) Developed shoot buds derived from organogenic callus in SM5B after 2 weeks (2×). (m–p) Histological characterization of tissues shown in (i–l), respectively. (m) Transversal section of intact CN explant showing epidermis (e), cortex (c) and pith (p) consisting of parenchymatous cells separated by vascular cylinder (v) (15×). (n) Callus section showing parenchymatic (p) and meristematic (m) cells (10×). (o) Shoot buds (sb) regenerated by indirect organogenesis after 1 week in SM5B. (p) Detail of an adventitious shoot bud (sb) showing its attachment to the callus through vascular system (arrows) (25×)
In agreement with the finding of Malik and Saxena (1992), the best response for shoot regeneration from calli was observed in SM supplemented with 22.2 μM BAP (SM5B). Figure 1d, k and l show representative organogenic calli formed in CIM1.5D in which bud development and shoot regeneration was clearly observed after 2 weeks of culture in SM5B. We observed that calli transferred to SM containing 10.0 μM thidiazuron developed only multiple shoot bud-like structures, but none of these structures developed into shoots. The shoot regeneration capability of the Negro Jamapa cultivar averaged around one shoot per every two calli derived from AM or CN explants regarding to the total amount of calli obtained on CIM1.5D (Table 3), even though occasionally up to 3 shoots developed from a single callus.
Shoots formed on SM5B were transferred to RM for root development. Our results indicated that the best root promotion was obtained when 1.0–2.0 cm long induced shoots were cut with a very small part (0.5–1.0 mm) of the parental callus, before transferring to RM. Our interpretation is that in this way the damage of the basal part of the regenerated shoot stem was minimized. We observed that all the shoots transferred to RM without growth regulators (Fig. 2a) or with 0.444 μM BAP and 0.054 μM NAA (Fig. 2b) developed roots. The addition of phytohormones (BAP and NAA) to RM was more effective for root induction, since roots developed earlier and were prominent than in the RM alone (Fig. 2b). In agreement, similar RM with phytohormones was successfully used by Pigeaire et al. (1997) as a rooting and propagation medium for Lupinus angustifolius. The high rooting efficiency obtained with Negro Jamapa contrasts with the poor in vitro rooting observed in plantlets of P. vulgaris Xan-159 cultivar reported by Zambre et al. (1998).
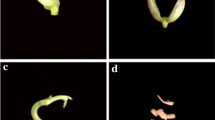
Rooting and greenhouse adaptation of regenerated plants of different bean cultivars. (a) Rooted shoot from AM of Negro Jamapa cultivar on RM without growth regulators. (b) Rooted shoot from AM of Negro Jamapa cultivar on RM with 0.444 μM BAP and 0.054 μM NAA. (c–d) Fertile plants of two different cultivars showing well developed pods after 8 weeks in greenhouse. (c): Negro Jamapa. (d): Carioca
Plantlets with a well developed root system (Fig. 2a and b) were transferred, after 2–4 weeks, from RM to plastic pots with sterile vermiculite. The planted pots were covered with plastic bags and incubated in growth chambers under controlled environmental conditions for 1–2 weeks prior to transferring them to greenhouse. Regenerated plants grown in greenhouses showed normal development and were fertile (Fig. 2c and d), producing from 7 to 12 pods with 6 to 7 seeds each one.
Indirect organogenesis capacity of 10 different P. vulgaris cultivars
It is known that the in vitro regeneration and the genetic transformation of Phaseolus is highly genotype dependent (Zambre et al. 2005). Another objective of our work was to establish a protocol for indirect regeneration of P. vulgaris that could be genotype independent, therefore allowing its application to agronomically important cultivars. Based in the described protocol for indirect regeneration of Negro Jamapa cultivar, we tested other 9 P. vulgaris cultivars that included 7 cultivars of Mesoamerican and 2 cultivars of Andean origin with diverse growth habit and seed colours (Table 1). For the experiments with these cultivars the four 2,4-D/BAP combinations added to CIM that are highlighted in Table 2 were tested. Similar as for Negro Jamapa cultivar, best results for morphogenetic calli induction for the other cultivars were obtained with CIM1.5D. Calli from AM and CN explants from all the cultivars tested showed shoot regeneration (Table 3). The frequency of morphogenetic calli formation varied among the tested cultivars, showing higher percentage frequency when AM explants were used (Table 3). Statistical analysis indicated significant differences in number of shoots formed per calli among the explants used (AM vs. CN) and the different cultivars (Table 3). The best regeneration capability was observed for the Carioca cultivar followed by Olathe and Cardinal using the AM explant (Table 3). The shoots regenerated from explants of the different cultivars were transferred to RM for root development followed by transference to plastic pots and greenhouse adaptation. Plants regenerated from every cultivar produced normal pods and seeds (Fig. 2c and d).
Histological analysis
Histological analyses of the tissue from different stages of the regeneration process was performed in order to delineate the pattern of indirect organogenesis in Negro Jamapa (Fig. 1e–h and m–p). At time cero, longitudinal sections of AM explant showed a typical arrangement of dicotyledonous shoot apical meristem with dome and leaf primordia (Fig. 1e). The transversal section of CN at time cero showed an unstratified epidermis and a large parenchymatous pith in the centre surrounded by a ring of discrete vascular bundles with a narrow external cortex (Fig. 1m), typical structures for dicotyledonous plants. Upon cultivation in CIM1.5D the morphology of the AM and NC explants changed, starting with swelling of the explants and subsequent calli formation, that started during the 1st week and continued until the 3rd week of culture (Fig. 1b, c and j). Within the 1st week it was possible to observe preexisting meristematic cells confined to the axillary shoot bud region (Fig. 1f), which clearly differ from meristemoids originated after the de-differentiation process (Fig. 1g, h and n–p). Micrographs of calli at the 2nd week revealed clearly cell de-differentiation with parenchymatic and meristematic cell types not connected to preexisting organized structures (Fig. 1g and n). After 14 days on SM5B, de novo meristemoid structures and unipolar adventitious buds connected to the parental tissue through vascular bundles were observed (Fig. 1h, o and p). These results clearly demonstrate the regeneration via indirect organogenesis. Malik and Saxena (1992) also reported a similar developmental pattern during in vitro indirect regeneration in four Phaseolus species.
The efficient protocol reported here, is summarized in the Fig. 3. With this protocol we have achieved regeneration of plants from ten different P. vulgaris cultivars via indirect organogenesis. We consider that the use of N6 macronutrient salts and PVP-360, that have not been used as part of media for common bean regeneration, were important factors for the successful formation of morphogenetic calli. Although the regeneration frequency that we report is low it could be improved in the future, and the values obtained are in the range of the results obtained previously by Mohamed et al. (1993) and Zambre et al. (1998). Our protocol shows the advantage of being applicable to ten different cultivars and therefore it may be genotype independent. It should be noted that Dillen et al. (1997) achieved Agrobacterium-mediated genetic transformation of P. acutifolius coupled to an indirect organogenesis protocol with low regeneration efficiency, similar to what we report. In addition, the reports dealing with direct organogenesis and high regeneration frequency had not been successful for genetic transformation of common bean via Agrobacterium. Therefore the procedure that we report opens the possibility for future improvement of regeneration frequency, and may be the basis for establishing a procedure for P. vulgaris Agrobacterium-mediated genetic transformation.
Protocol for indirect regeneration of common bean P. vulgaris L. This protocol is effective for the 10 P. vulgaris cultivars shown in Table 1
Abbreviations
- AM:
-
Apical meristem
- BAP:
-
6-Benzylaminopurine
- CIM:
-
Callus induction medium
- CN:
-
Cotyledonary node
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- IAA:
-
Indole-3-acetic acid
- MES:
-
2-(N-Morpholino) ethanesulfonic acid
- MS:
-
Murashige and Skoog medium
- NAA:
-
α-naphthaleneacetic acid
- PVP-360:
-
Polyvinylpyrrolidone 360,000 molecular weight
- SM:
-
Shooting medium
- RM:
-
Rooting medium
References
Broughton WJ, Hernández G, Blair M, Beebe S, Gepts P, Vanderleyden J (2003) Beans (Phaseolus spp.)—model food legumes. Plant Soil 252:55–128. doi:10.1023/A:1024146710611
Chu CC, Wang CC, Sun SC, Hsu C, Yin KC, Chu CY, Bi FY (1975) Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Sci Sin 18:659–688
Cruz de Carvalho MH, Van Le B, Zuily-Fodil Y, Pham Thi AT, Tran Thanh Van K (2000) Efficient whole plant regeneration of common bean (Phaseolus vulgaris L.) using thin-cell-layer culture and silver nitrate. Plant Sci 159:223–232. doi:10.1016/S0168-9452(00)00346-0
Delgado-Sánchez P, Saucedo-Ruiz M, Guzmán-Maldonado SH, Villordo-Pineda E, González Chavira M, Fraire-Velázquez S, Acosta-Gallegos JA, Mora-Avilés A (2006) An organogenic plant regeneration system for common bean (Phaseolus vulgaris L). Plant Sci 170:822–827. doi:10.1016/j.plantsci.2005.11.015
Dillen W, De Clercq J, Goossens A, Van Montagu M, Angenon G (1997) Agrobacterium mediated transformation of Phaseolus acutifolius A. gray. Theor Appl Genet 94:151–158. doi:10.1007/s001220050394
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158. doi:10.1016/0014-4827(68)90403-5
Malik KA, Saxena PK (1992) Somatic embryogenesis and shoot regeneration from intact seedlings of Phaseolus acutifolius A., P. aureus (L.) Wilczek, P.coccineus L., and P. wrightii L. Plant Cell Rep 11:163–168. doi:10.1007/BF00232172
Mohamed MF, Coyne DP, Read PE (1993) Shoot organogenesis in callus induced from pedicel explants of common bean (Phaseolus vulgaris L.). J Am Soc Hortic Sci 118:158–162
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Nagl W, Ignacimuthu S, Becker J (1997) Genetic engineering and regeneration of Phaseolus and Vigna. State of the art and new attempts. J Plant Physiol 150:625–644
Pigeaire A, Abernethy D, Smith PM, Simpson K, Fletcher N, Lu CY, Atkins CA, Cornish E (1997) Transformation of a grain legume (Lupinus angustifolius L) via Agrobacterium tumefaciens-mediated gene transfer to shoot apices. Mol Breed 3:341–349. doi:10.1023/A:1009642620907
Rech EL, Giovanni R, Vianna GR, Aragao FJL (2008) High-efficiency transformation by biolistics of soybean, common bean and cotton transgenic plants. Nat Protoc 3:410–418. doi:10.1038/nprot.2008.9
Santalla M, Power JB, Davey MR (1998) Efficient in vitro shoot regeneration responses of Phaseolus vulgaris and P coccineus. Euphytica 102:195–202. doi:10.1023/A:1018317327302
Trepp GB, Van de Mortel M, Yoshioka H, Miller SS, Samac DA, Gant JS, Vance CP (1999) NADH-glutamate synthase in alfalfa root nodules. Genetic regulation and cellular expression. Plant Physiol 119:817–828. doi:10.1104/pp.119.3.817
Zambre M, Goossens A, Cardona C, Van Montagu M, Terryn N, Angenon G (2005) A reproducible genetic transformation system for cultivated Phaseolus acutifolius (tepary bean) and its use to assess the role of arcelins in resistance to the Mexican bean weevil. Theor Appl Genet 110:914–924. doi:10.1007/s00122-004-1910-7
Zambre MA, De Clercq J, Vranová E, Van Montagu M, Angenon G, Dillen W (1998) Plant regeneration from embryo-derived callus in Phaseolus vulgaris L (common bean) and P acutifolius A. Gray (tepary bean). Plant Cell Rep 17:626–630. doi:10.1007/s002990050455
Zambre MA, Geerts P, Maquet A, Van Montagu M, Dillen W, Angenon G (2001) Regeneration of fertile plants from callus in Phaseolus polyanthus Greenman (Year bean). Ann Bot (Lond) 88:371–377. doi:10.1006/anbo.2001.1468
Acknowledgments
We thank Drs. M. Blair, S. Beebe and D. Debouck from CIAT for kindly supplying seeds, PhD Pallavolu Maheswara Reddy from CCG-UNAM for critical review, Victor Bustos from CCG-UNAM, Elizabeth González from CEIB-UAEM and to Ana Isabel Bieler Antolín from Microcine FC-UNAM, for technical assistance on plant maintenance, histological analysis and microphotography, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arellano, J., Fuentes, S.I., Castillo-España, P. et al. Regeneration of different cultivars of common bean (Phaseolus vulgaris L.) via indirect organogenesis. Plant Cell Tiss Organ Cult 96, 11–18 (2009). https://doi.org/10.1007/s11240-008-9454-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9454-1