Abstract
An efficient system was established for a higher frequency of protocorm-like body (PLB) formation from the callus of Dendrobium candidum Wall ex Lindl. The calluses were induced from longitudinally bisected segments of protocorms and subcultured two times every 40d on Murashige and Skoog medium with macronutrients at half strength, micronutrients at full strength, 2% sucrose, and with 8.8μM 6-Benzylaminopurine. PLB formation was achieved when calluses were transferred onto the same basal medium without any plant growth regulators. PLBs developed into intact plantlets about 2cm in height and with four roots when on basal medium with 2.7μM 1-naphthaleneacetic acid. Plantlets were transplanted into vermiculite with a 95% survival rate in a greenhouse. Histological observations proved that globular somatic embryos could be produced from the inside and surface of the embryogenic callus during PLB formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dendrobium candidum Wall ex Lindl., a sympodial epiphytic orchid, is one of the most famous orchids distributed in a few countries in Southeast and South Asia. It is mainly used as a decorative plant and in Chinese traditional medicine. However, its number is steadily declining because of a lower rate of propagation in nature and overexploitation. Therefore, an in vitro propagation technique could be a useful approach for the mass scale propagation of this orchid for commercial purposes. Tissue culture techniques have been widely used for in vitro mass propagation of several commercially important orchids over the past few decades (Chung et al. 1985; Chen and Chang 2000, 2004).
Plant tissues such as shoot tips (Sagawa and Kunisaki 1982; Malabadi et al. 2004; Malabadi et al. 2005), root tips (Kerbauy 1984; Chen and Chang 2000), floral stalks (Lim-Ho and Lee 1987; Young et al. 2000), stem nodes (Van Le et al. 1999; Nayak et al. 2002), apical buds (Bagde and Sharon 1997), protocorm-like bodies (Ishii et al. 1998; Lee and Lee 2003; Huan et al. 2004), leaves (Chen et al. 1999; Chen and Chang 2002; Chung et al. 2005), rhizomes (Chang and Chang 1998; Sheelavantmath et al. 2000), and mature seeds (Shimura and Koda 2004) have been widely used as explants to obtain protocorm-like bodies (PLBs) or shoots and, subsequently, plantlets. In D. candidum, several tissue culture systems using different tissues such as mature seeds (He et al. 1982), immature seeds (Ye et al. 1988), protocorms (Hou and Guo 2005), stem segments (Chen and Cun 2002), shoot tips (Zhang and Fang 2005), or axenic nodal segments (Shiau et al. 2005) as explants have been established since the 1980s.
However, because of very limited growth and a tendency for necrosis upon the subculturing of the callus (Kerbauy, 1984), there are only a few reports on callus culture in orchids such as D. fimbriatium (Roy and Banerjee 2003), Oncidium ‘Gower Ramsey’ (Chen and Chang 2000), Cymbidium ensifolium (Chang and Chang 1998), and Phalaenopsis Richard Shaffer ‘Santa Cruz’ (Ishii et al. 1998). Regenerated plantlets from orchid calluses are usually achieved through PLB formation, a process thought that involve somatic embryogenesis (Begum et al. 1994; Chang and Chang 1998). However, clear histological evidence of somatic embryogenesis in orchids is limited. In this study, the role of exogenous plant growth regulators is examined in plant regeneration through PLB formation from calluses using protocorms as explants to make use of the embryogenic potential of the callus as an effective system of micropropagation. The process of PLB formation was elucidated by histological study.
Materials and Methods
The mature seeds in three capsules of D. candidum were cultured for protocorm induction in three 500-ml Erlenmeyer flasks containing 200ml MS medium supplemented with 1.08μM 1-naphthaleneacetic acid (NAA). The protocorms, about 3mm in diameter, were longitudinally bisected. These protocorm segments were used as explants for callus induction. The explants were inoculated in 100-ml Erlenmeyer flasks containing 30ml 1/2-MS basal medium, which has macronutrients at half strength, 2% sucrose, supplemented with NAA (0, 0.27, 0.81, 1.08, 2.7, 5.4, 8.1, 10.8μM), BA (0, 2.2, 4.4, 6.6, 8.8, 13.2, 17.6, 22.0μM), 2,4-dichlorophenoxyacetic acid (2,4-D; 0, 2.3, 4.6, 6.9, 9.2, 13.8μM), and kinetin (Kin; 0, 2.3, 4.6, 6.9, 9.2, 13.8μM) alone or in combination. The media were solidified with 0.6% (w/v) agar (In Vitro Diagnosticum Sanland International Inc.–Tokyo, Japan). The pH was adjusted to 5.8 with 1N NaOH or HCl before autoclaving at 121°C for 15min. Cultures were maintained at 25 ± 2°C in darkness for 40d. In each treatment, 50 explants were inoculated in five Erlenmeyer flasks (ten explants per flask). The number of explants with calluses on different media was recorded on the 40th day of culture, and the percentage of the explants with calluses was calculated.
Calluses induced from explants were subcultured on the optimum medium for callus induction for 40d. Then, the calluses were transferred onto the same medium for further subculturing. For PLB regeneration, the subcultured calluses were transferred onto the 1/2-MS basal media with either NAA (0, 1.08, 2.7, 5.4μM), 3-indolyl butyric acid (IBA; 0, 2.5, 5.0, 7.5μM), or a combination of both. Subcultured calluses were cultured in 100-ml Erlenmeyer flasks under illumination (30–34μmol m2 s−1) with a light/dark cycle (12/12h) at 25 ± 2°C; 12 pieces of calluses were inoculated for each flask, and three flasks were considered for each treatment. Cultures were examined and photographed with a stereozoom microscope (SMZ-168-TL, Motic) and a digital camera (Nikon 5700). Calluses used for histological observations were fixed in FAA (formalin/acetic acid/50% ethanol, 1:1:18 v/v/v), dehydrated in an ethanol series, and embedded in paraffin wax. The embedded materials were sectioned at 8μm and stained with 1% safranin-0 (Sigma, St. Loius, MO) and 0.5% fast green (Amresco, Solon, OH). The observation and photography were done using a light microscope (Olympus IX71).
Regenerated PLBs about 4mm in diameter with a cotyledon were transferred onto 1/2-MS basal medium with various concentrations of NAA (0, 1.08, 2.7, 5.4, 8.1, 10.8μM) for shoot development and root induction. Six PLBs were inoculated in each 150-ml Erlenmeyer flask containing 40ml medium, and three flasks were considered for each treatment. The height of the stem and the length of the roots were measured using a ruler, and the diameter of the stem and roots were measured with a vernier caliper (Chengdu–Chengdu, The People’s Republic of China). All data were recorded after 60d of culture under illumination (30–34μmol m2 s−1) with a light/dark cycle (12/12h) at 25 ± 2°C. Plantlets with three or four roots were transplanted into vermiculite in a greenhouse at 20 ± 2°C with a 14-h (50μmol m−2 s−1) photoperiod and 70 ± 5% relative humidity.
Results
Effects of plant growth regulators on callus induction.
Within 8d of culturing, white granules emerged from the surface of explants inoculated on 1/2-MS basal medium with different plant growth regulators and became light yellow and granular calluses within 1mo. As shown in Fig. 1, calluses could be induced from explants in most treatments with different frequencies. Of the four plant growth regulators tested, BA was found to be the most effective in inducing calluses from explants. Callus induction was enhanced as the concentration of BA ranged from 0 to 8.8μM and reduced as the concentration of BA from 8.8 to 22.0μM. The frequency of callus induction from explants was 82% on 1/2-MS basal medium with 8.8μM BA. The effectiveness of NAA on callus induction from explants was comparable to that of BA. The frequency of callus induction increased as NAA concentrations ranged from 0 to 1.08μM; it decreased as NAA concentrations ranged from 1.08 to 10.8μM. The frequency of callus induction from explants was 72% on 1/2-MS basal medium with 1.08μM NAA. The effects of 2,4-D and Kin on callus induction followed a similar pattern, but to a lesser degree than BA and NAA. The highest percentage of explants producing calluses was 32% with 6.9μM 2,4-D and 28% at 6.9μM Kin, respectively. There is a significant difference at p = 0.05 among effects with 8.8μM BA, 1.08μM NAA, 6.9μM 2,4-D or 6.9μM Kin according to Ducan’s multiple range test.
To further promote callus induction, NAA (0.54, 1.08, 2.7μM) and 2,4-D (4.6, 6.9, 9.2μM) were combined with BA (4.4, 8.8, 13.2μM). The results are shown in Fig. 2. When cultured on 1/2-MS basal medium with 9.2μM 2,4-D and 8.8μM BA, or with 0.54μM NAA and 8.8μM BA, the frequency of callus formation was the highest among all combinations of plant growth regulators tested, and the percentage of explants with calluses was 77.8 or 77.3%. Through Ducan’s multiple range test, these values are not significantly different at p = 0.05 from the percentage of explants with calluses at 8.8μM BA. So, the addition of NAA or 2,4-D to 1/2-MS basal medium with BA was no more effective than BA used alone. Therefore, 1/2-MS basal medium supplemented with 8.8μM BA was considered as the optimal medium for callus induction.
Callus subculturing and plant regeneration from calluses through PLB formation.
After subculturing on the optimal medium for 20d, the initial calluses from explants gradually exhibited three different appearances: light yellow, white, and brown. The light yellow calluses maintained initial growth potential, grew fast, and were comprised of a relatively compact mass of isodiametric granules on the surface (Fig. 3 a). The white calluses gradually turned moist. The brown calluses initially proliferated lighter colored tissue and then gradually turned brown again.
Morphogenesis in callus culture of D. candidum. (a) Compact, yellow callus induced from the PLBs explants (Bar, 1 cm). (b) The subcultured light yellow callus (Bar, 2 mm). (c) In light, the light yellow callus was turning green (Bar, 2 mm). (d) Regeneration of PLBs (Bar, 4 mm). (e) The PLBs elongated and some formed a protrusion (Bar, 4 mm). (f) A regenerated PLBs with the cotyledon (Bar, 3 mm). (g) A PLBs with the cotyledon and the first leaf of the foliage (Bar, 3 mm). (h) A well-developed plantlet with roots (Bar, 1cm). (i) The regenerated plantlets transplanted in a pot (Bar, 2 cm).
Calluses that had been subcultured twice were used for morphogenetic and histological studies. The morphogenetic response was related to appearance of the calluses. They displayed different differentiation potentials on 1/2-MS basal medium with various concentrations and combinations of NAA and IBA. The light yellowish calluses continued to proliferate after being transferred to 1/2-MS basal medium without any plant growth regulators (Fig. 3 b) and gradually turned green (Fig. 3 c). A large number of PLBs were produced from them (Fig. 3 d). There was an average of 47.2 PLBs formation per 16mm2 of callus mass on the 1/2-MS basal medium without any plant growth regulators. Germination of PLBs into well-developed plantlets readily occurred on the same medium or after transferred to a fresh 1/2-MS basal medium without any plant growth regulators. The PLBs first germinated with a protrusion (Fig. 3 e), and then the protrusion developed into a cotyledon (Fig. 3 f). Subsequently, the first leaf of the foliage emerged from cotyledon (Fig. 3 g). Succeeding leaves were differentiated in an alternating sequence (Fig. 3 h).
The brownish calluses had no response on 1/2-MS basal medium without any plant growth regulators, but when transferred to 1/2-MS basal medium supplemented with NAA (1.08, 2.7, 5.4μM) and IBA (2.5, 5.0μM), some new light green granules emerged from the surface of brownish callus. The newly produced granules gradually turned green and then developed into PLBs on this medium or on MS basal medium without any plant growth regulators. The highest frequency of PLB regeneration from the brownish calluses was about 10% on 1/2-MS basal medium with NAA (1.08μM) and IBA (2.5, 5.0μM). The whitish calluses had a similar process of PLB regeneration, but the highest frequency of PLB regeneration was only about 3%, and this process was also dependent on exogenous NAA and IBA.
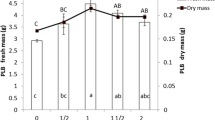
Meanwhile, exogenous auxins had different effects on PLB regeneration from light yellowish to brownish calluses. For whitish and brownish calluses, exogenous auxins (NAA and IBA) are indispensable to the regeneration of PLBs. For light yellowish calluses, exogenous auxins are not essential. The results were shown in Table 1. 1/2-MS basal medium without any plant growth regulators or 1/2-MS basal medium with NAA (1.08, 2.7μM) or 1/2-MS basal medium with NAA (0.54μM) and IBA (2.5μM) was more suitable for plant regeneration from callus. The percentage of differentiation of light yellowish calluses was 100% on these media. PLB regeneration was promoted at low concentrations of NAA (1.08, 2.7μM), but retarded at high concentrations during the same culture period. The calluses cultured on 1/2-MS basal medium with high concentrations of NAA gradually deteriorated to a necrotic brown color. On the medium with IBA, PLB regeneration was first promoted and then inhibited after 40d of culture at lower concentration (2.5, 5.0μM). For the combination of NAA (0.54μM) and IBA (2.5μM), PLB regeneration was significantly improved. Although PLBs could be regenerated on 1/2-MS basal medium with or without plant growth regulators, PLBs regenerated on 1 basal medium with NAA alone or combined with IBA were smaller than those on 1/2-MS basal medium without any plant growth regulators.
Effects of NAA on shoot development and root induction.
As newly formed PLBs with shoots were transferred to 1/2-MS basal medium with various concentrations of NAA, almost all of the PLBs developed into individual plantlets under lower NAA (up to 2.7μM) concentrations (Fig. 3 h). The effects of NAA on shoot development and root induction are shown in Table 2. Lower concentrations of NAA (up to 5.4μM) promoted shoot development, and up to 2.7μM promoted root induction. Higher concentrations of NAA (5.4μM and higher) suppressed root induction. As cultured on 1/2-MS basal medium with 2.7μM NAA, PLBs developed into plantlets about 2.0cm in height with about four roots after 60d. All these regenerated plantlets were acclimated for a week and then transplanted into vermiculite in the greenhouse. After a month, these plantlets grew well with a 95% survival rate in greenhouse (Fig. 3 i).
Morphogenesis of PLBs.
As callus differentiation proceeded, somatic embryos were found to initiate from the inside and the surface of the callus. They sequentially expanded to form a globular structure comprised of cells with densely stained cytoplasm and turned into granular somatic embryos (Fig. 4 a), which then developed into the PLBs.
Histological study on the process of PLB formation. (a) A somatic embryo (arrow) derived from inner cells of callus (Bar, 200 μm). (b) An ellipsoid-shaped embryo (arrow) derived from outer cells of callus (Bar, 400 μm). (c) A regenerated PLB (arrow) with a shoot apex (Bar, 400 μm). (d) Secondary embryos (arrow) formed from the surface of the somatic embryo (Bar, 400 μm).
The sections of calluses cultured on 1/2-MS basal medium without any plant growth regulators showed globular somatic embryos derived from the inside or the surface of the callus (Fig. 4 a,b). From histological observations, the globular somatic embryos were composed of cells with dense cytoplasm; no vascular connections were observed between the globular somatic embryos and the peripheral tissues. As the differentiation of callus progressed, these somatic embryos developed into PLBs, and then the PLBs developed with a shoot apex (Fig. 4 c). Most of these PLBs could further proliferate to produce secondary PLBs (Fig. 4 d) which eventually developed into plantlets.
Discussion
As the first shoot tip culture in orchids for mass propagation was reported by Morel in 1960, plant regeneration syste1 from various types of explants have been achieved in a number of orchid species. Since then, about four protocols for mass propagation have been established in orchids. The first is adventitious buds induced from node segments, leaf segments through direct organogenesis (Chen and Cun 2002; Chen and Chang 2004; Shiau et al. 2005). The second is protocorm induction from seed embryos (He et al. 1982; Ye et al. 1988), which eventually developed into plantlets. The third is PLB regeneration from shoot tips, root tips, stem segments through direct embryogenesis (Chen and Chang 2000; Zhang and Fang, 2005). The fourth is the tTCL culture system established using transverse thin cell layers of stem nodes (Zhao et al. 2007). The four protocols could be classified into two types: bud formation via organogenesis and direct PLBs formation through embryogenesis from explants. Either bud formation or direct PLBs formation is related to the cultural conditions and types of explants. It is reported that plantlets could be induced in about 1~2mo. through direct PLB formation (Sheelavanthmath and Murthy 2005) or 3~5mo. through indirect PLBs formation from callus (Lu 2004). Less variant regenerated plants were observed in regenerated plantlets through direct PLB formation. As for the number of regenerated plantlets, up to 5.3 shoots could be directly induced from leaf explants of Paphiopedilum in optimal conditions (Chen and Chang 2004), whereas an average of 90.7 PLBs developed from callus of Cymbidium (Huan et al. 2004).
Although callus exhibited great importance for mass propagation in many species and have been induced in a number of orchids previously, tissue cultures in orchids are not focused on callus because of the lower growth rate and necrosis in culture. In recent years, callus lines can be successfully established in some orchid species (Lee and Lee 2003; Lu 2004), and these calluses give rise to plantlets through indirect PLBs formation for mass propagation. It is reported that calluses could be formed from seed-derived protocor1 at 50% frequency (Lu 2004), PLB segments at 53% (Huan et al. 2004), shoot tips at 66.7% (Roy and Banerjee 2003), and root tips at 25% (Chen and Chang 2000). After several subcultures, all these calluses proliferated by three to fivefold within a month, and the average number of PLBs was 90.7 per cultured callus (Lu, 2004), 134 per 0.01 g fresh weight callus (Huan et al. 2004), 32.5 per callus mass (Roy and Banerjee 2003), and 29.1 per 9 mm2 of callus mass (Chen and Chang 2000) in a month. Thus, the mass propagation through indirect PLB formation may be more effective than through the direct formation in orchids. Because of the high frequency of somatic embryo formation and efficiency of embryo conversion into plants, somatic embryogenesis could be best accomplished through indirect PLB formation from callus. Until now, there was no report about plantlet regeneration from callus culture via PLB formation in D. candidum. In the present study, a novel regeneration system of D. candidum was established. Calluses were induced from protocorm segments under various conditions. Of four plant growth regulators tested in callus induction, the explants exhibited a higher frequency of callus formation with 8.8 μM BA. The superiority of BA in promoting callus induction was also observed in D. fimbriatum (Roy and Banerjee 2003).
Although the presence of plant growth regulators was essential for callus induction, callus differentiation was achieved on 1/2-MS basal medium without any plant growth regulators. The process of PLB regeneration from the callus and their subsequent germination is independent of exogenous plant growth regulators, which is similar to the previous reports on the other orchid species such as Phalaenopsis Richard Shaffer ‘Santa Cruz’ (Ishii et al. 1998) and D. fimbriatum (Roy and Banerjee 2003). In contrast, the embryogenic calluses of many other species require continuous exposure to specific plant growth regulators for somatic embryogenesis (Huang et al. 1999; Luo et al. 1999; Chengalrayan et al. 2001). The dedifferentiation, differentiation, and PLB regeneration in orchids may be an interesting system suitable to study somatic embryogenesis. It is plausible that at the time of callus induction, the endogenous hormonal synthesis system is triggered on and the increased level of hormones allowed the proliferation and differentiation of cells in the absence of exogenous plant growth regulators (Smith and Krikorian 1990).
Plant regeneration from callus culture in orchids is usually through an intermediary PLB phase. This type of morphogenetic development has been reported in a number of species (Colli and Kerbauy 1993; Chen and Chang 2000; Lee and Lee 2003; Roy and Banerjee 2003; Huan et al. 2004), and various investigators have suggested that the process of somatic embryogenesis is a part of the early steps of PLB regeneration in orchids (Steward and Mapes 1971; Kerbauy 1984; Begum et al. 1994; Chen and Chang 2000; Huan et al. 2004). However, well-defined routines of somatic embryogenesis in orchids have not been reported. In our result, as the differentiation of callus progressed, globular granules emerged from the inner or outer parts of the callus, which were composed of cells with dense cytoplasm and small vacuoles. This is characteristic of embryogenic cells (Eady et al. 1998; Li et al. 2001; Nikam et al. 2003). The granules gradually could develop into PLBs, and these PLBs could develop into plantlets under suitable conditions. Also, most of these PLBs could further proliferate to produce secondary PLBs, which is a common characteristic of many orchids (Morel 1960; Wimber 1963; Arditti and Ernst 1993). In this study, the histological observations suggest that plantlet regeneration of D. candidum through PLBs could be considered as a form of somatic embryogenesis and that globular somatic embryos originated from the inner or outer cells of the callus.
References
Arditti, J.; Ernst, R. Micropropagation of orchids. New York: Wiley; 1993.
Bagde, P.; Sharon, M. In vitro regeneration of Oncidium ‘Gower Ramsey’ by high frequency protocorm like bodies proliferation. J Plant Physiol 2: 10–14; 1997.
Begum, A.A.; Tamaki, M.; Tahara, M.; Kato, S. Somatic embryogenesis in Cymbidium through in vitro culture of inner tissue of protocorm-like bodies. J Jpn Soc Hortic Sci 63: 419–427; 1994.
Chang, C.; Chang, W.C. Plant regeneration from callus culture of Cymbidium ensifolium var. misericors. Plant Cell Rep 17: 251–255; 1998.
Chen, J.T.; Chang, C.; Chang, W.C. Direct somatic embryogenesis on leaf explants of Oncidium ‘Gower Ramsey’ and subsequent plant regeneration. Plant Cell Rep 19: 143–149; 1999.
Chen, J.T.; Chang, W.C. Efficient plant regeneration through somatic embryogenesis from callus of Oncidium (Orchidaceae). Plant Sci 160: 87–93; 2000.
Chen, J.T.; Chang, W.C. Effects of tissue culture conditions and explant characteristics on direct somatic embryogenesis in Oncidium ‘Gower Ramsey’. Plant Cell Tissue Organ Cult 69: 41–44; 2002.
Chen, T.Y.; Chang, W.C. Plant regeneration through direct shoot bud formation from leaf cultures of Paphiopedilum orchids. Plant Cell Tissue Organ Cult 76: 11–15; 2004.
Chen, W.; Cun, S.X. In vitro rapid propagation of stems of Dendrobium candidum Plant Physiol Commun 38: 145; 2002 (In Chinese).
Chengalrayan, K.; Hazra. S.; Gallo-Meagher, M. Histological analysis of somatic embryogenesis and organogenesis induced from mature zygotic embryo-derived leaflets of peanut (Arachis hypogaea. L.). Plant Sci 161: 415–421; 2001.
Chung, H.H.; Chen, J.T.; Chang, W.C. Cytokinins induce direct somatic embryogenesis of Dendrobium Chiendmai Pink and subsequent plant regeneration. In Vitro Cell Dev Biol Plant 41: 765–769; 2005.
Chung, J.D.; Chun, C.K.; Choi, S.O. Asymbiotic germination of Cymbidium ensifolium. J Korean Soc Hort Sci 26: 182–196; 1985.
Colli, S.; Kerbauy, G.B. Direct root tip conversion of Catasetun into protocorm-like bodies: effect of auxin and cytokinin. Plant Cell Tissue Organ Cult 33: 39–44; 1993.
Eady, C.C.; Butler, R.C.; Suo, Y. Somatic embryogenesis and plant regeneration from immature embryo cultures of onion (Allium cepa L.). Plant Cell Rep 18: 111–116; 1998.
He, J.B.; Zheng, C.Z.; Wang, S.L. Multiplication of protocorm of Dendrobium candidum. Acta Botanica Yunnanica 4: 211–212; 1982 (In Chinese).
Hou, P.Y.; Guo, S.X. Studies on transplanting suspension-cultured protocorms of Dendrobium candidum onto solid culture medium. China J Chinese Materia Medica 30: 729–732; 2005.
Huan, L.V.T.; Takamura. T.; Tanaka, M. Callus formation and plant regeneration from callus through somatic embryo structures in Cymbidium orchid. Plant Sci 166: 1443–1449; 2004.
Huang, L.T.; Baiocco, M.; Huy, B.P.; Mezzetti, B.; Santilocchi, R.; Rosati, P. Somatic embryogenesis in Canary Island date palm. Plant Cell Tissue Organ Cult 56: 1–7; 1999.
Ishii, Y.; Takamura, T.; Goi, M.; Tanaka, M. Callus induction and somatic embryogenesis of Phalaenopsis. Plant Cell Rep 17: 446–450; 1998.
Kerbauy, G.B. Plant regeneration of Oncidium varicosum (Orchidaceae) by means of root tip culture. Plant Cell Rep 3: 27–29; 1984.
Lee, Y.I.; Lee, N. Plant regeneration from protocorm-derived callus of Cypripedium formosanum. In Vitro Cell Dev Biol Plant 39: 475–479; 2003.
Li, S.; Shen, Z.H.; Qin, Z.; Wang, Y.F. Uptake rate of tracer elements by Lycium barbarum L. in somatic embryogenesis. J Radioanal Nucl Chem 250: 593–597; 2001.
Lim-Ho, C.L.; Lee, G.C. Clonal propagation of Oncidium from dormant buds on flower stalk. Malay Orchid Rev 22: 48–52; 1987.
Lu, M.C. High frequency plant regeneration from callus culture of Pleione formosana Hayata. Plant Cell Tissue Organ Cult 78: 93–96; 2004.
Luo, J.P.; Jia, J.F.; Gu, Y.H.; Liu, J. High frequency somatic embryogenesis and plant regeneration in callus cultures of Astragalus adsurgens Pall. Plant Sci 143: 93–99; 1999.
Malabadi, R.B.; Mulgund, G.S.; Kallappa, N. Micropropagation of Dendrobium nobile from shoot tip sections. J Plant Physiol 162: 473–478; 2005.
Malabadi, R.B.; Mulgund, G.S.; Nataraja, K. Efficient regeneration of Vanda coerulea, an endangered orchid using thidiazuron. Plant Cell Tissue Organ Cult 76: 289–293; 2004.
Morel, G.M. Proding virus-free Cymbidium. Am Orchid Soc Bull 29: 495–497; 1960.
Nayak, N.R.; Sahoo, S.; Patnaik, S.; Rath, S.P. Establishment of thin cross section (TCS) culture method for rapid micropropagation of Cymbidium aloifolium (L.) Sw. and Dendrobium nobile Lindl. (Orchidaceae). Sci Hortic 94: 107–116; 2002.
Nikam, T.D.; Bansude. G.M.; Aneesh Kumar, K.C. Somatic embryogenesis in sisal (Agave sisalana Perr. ex. Engelm). Plant Cell Rep 22: 188–194; 2003.
Roy, J.; Banerjee, N. Induction of callus and plant regeneration from shoot-tip explants of Dendrobium firmbriatum Lindl. var. oculatum Hk.f. Sci Hortic 97: 333–340; 2003.
Sagawa, Y.; Kunisaki, J. T. Clonal propagation of orchids by tissue culture. Proceedings of 5th congress, Plant tissue and cell culture, pp 683–684; 1982.
Sheelavanthmath, S.S., Murthy, H.N., Hema, B.P., Hahn, E.J., Paek, K.Y. High frequency of protocorm like bodies (PLBs) induction and plant regeneration from protocorm and leaf sections of Aerides crispum. Sci Hortic 106: 395–401; 2005.
Sheelavantmath, S.S.; Murthy, H.N.; Pyati, A.N.; Kumar, H.G..A.; Ravishankar, B.V. In vitro propagation of the endangered orchid, Geodorum densiflorum (Lam.) Schltr. through rhizome section culture. Plant Cell Tissue Organ Cult 60: 151–154; 2000.
Shiau, Y.J.; Nalawade, S.M.; Hsia, C.N.; Mulabacal, V.; Tsay, H.S. In vitro propagation of the Chinese medicinal plant, Dendrobium candidum Wall. Ex Lindl., from axenic nodal segments. In Vitro Cell Dev Biol Plant 41: 666–670; 2005.
Shimura, H.; Koda, Y. Micropropagation of Cypripedium macranthos var. rebunense through protocorm-like bodies derived from mature seeds. Plant Cell Tissue Organ Cult 78: 273–276; 2004.
Smith, D.L.; Krikorian, A.D. Low external pH replaces 2,4-D in maintaining and multiplying 2,4-D initiated embryogenic cells of carrot. Plant Physiol 72: 329–336; 1990.
Steward, F.C.; Mapes, M.O. Morphogenesis in aseptic cell culture of Cymbidium. Bot Gaz 132: 65–70; 1971.
Van Le, B.; Hang Phuong, N.T.; Anh Hong, L.T.; Tran Thanh Van, K. High frequency shoot regeneration from Rhynchostylis gigantean (orchidaceae) using thin cell layers. Plant Growth Regul 28: 179–185, 1999.
Wimber, D.E. Clonal multiplication of Cymbidium through tissue culture of the shoot meristem. Am Orchid Soc Bull 32: 105–107; 1963.
Ye, X.L.; Cheng, S.J.; Wang, F.X.; Qian, N.F. Morphology of immature seeds and development in vitro of Dendrobium candidum. Acta Bot Yunnanica 10: 285–290; 1988 (In Chinese).
Young, P.S.; Murthy, H.N.; Yoeup, P.K. Mass multiplication of protocorm-like bodies using bioreactor system and subsequent plant regeneration in Phalaenopsis. Plant Cell Tissue Organ Cult 63: 67–72; 2000.
Zhang, Q.X .; Fang, Y.M. Tissue culture and in vitro seedling and protocorm-like body examination of Dendrobium candidum. Acta Bot Boreali-Occidentalia Sinica 25: 1761–1765; 2005 (In Chinese).
Zhao, P.; Wang, W.; Feng, F.S.; Wu, F.; Yang, Z.Q.; High frequency shoot regeneration through transverse thin cell layer culture in Dendrobium candidum Wall ex Lindl. Plant Cell Tissue Organ Cult 90: 131–139; 2007.
Acknowledgments
We thank Emily King for critical reading of the manuscript. This work was supported from the science foundation of Southwest Jiaotong University (X1200511130101).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: E. Bunn
Rights and permissions
About this article
Cite this article
Zhao, P., Wu, F., Feng, FS. et al. Protocorm-like body (PLB) formation and plant regeneration from the callus culture of Dendrobium candidum Wall ex Lindl.. In Vitro Cell.Dev.Biol.-Plant 44, 178–185 (2008). https://doi.org/10.1007/s11627-007-9101-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-007-9101-2