Abstract
Analysis of cell wall polysaccharide composition of embryogenic and non-embryogenic calli obtained from hypocotyl and petiole explants from Medicago arborea L. revealed significant differences. For calli induced from both hypocotyls and petioles, levels of total sugars, pectins, and hemicelluloses were higher in embryogenic than in non-embryogenic calli. Whereas in the residual cellulose fraction, the highest levels of sugar were detected in non-embryogenic calli. When comparing the two donor sources of callus explants, the highest total sugar levels were detected in embryogenic calli induced from petioles, mainly in the pectin fraction and to a lesser extent in the hemicellulose fraction. Moreover, analysis of uronic acids revealed higher levels in embryogenic calli, primarily in the pectin fraction. Analysis of those sugars associated with cell walls of calli suggested that these polysaccharides consisted of pectic polysaccharides and glucans, and that their levels were higher in embryogenic than non-embryogenic calli.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Medicago arborea L., a member of the legume family,. is an important agricultural crop due to its drought resistance and lack of dormancy requirement during summer and winter seasons (Gonzalez-Andrés and Ceresuela 1998; Gallego et al. 2001). Thus, it is deemed a good forage crop for grazing in dry areas (Corleto et al. 1980; Gonzalez-Andrés and Ceresuela 1998), and particularly in Mediterranean countries.
In vitro culture techniques offer opportunities for rapid propagation of M. arborea (Gallego et al. 2001). When introduced in tissue culture, plant cells undergo cell division. Often, cultured cells proliferate indefinitely in a dis-organized manner, producing a mass of relatively undifferentiated callus (Sakhanokho et al. 2001; Martin 2003). From these, additional calli may be produced or cells may undergo morphogenesis leading to formation of organized tissues, such as shoots and/or roots (Schween and Schwenkel 2002; Singh et al. 2003) and/or embryos; i.e., somatic embryogenesis (Gallego et al. 2001; Pueschel et al. 2003; Linhares et al. 2006). It is suggested that calli have different capacities for somatic embryogenesis as cells are of different forms with different morphologies and capabilities (Moghaddam and Taha 2005). The observed different responses between embryogenic (E) and non-embryogenic (NE) calli are attributed to cellular differences. These cellular differences may be observed at both structural and ultrastructural levels and may be accompanied by metabolic differences in callus tissues (Behrouz et al. 2005; Villalobos et al. 2007). By varying the concentrations and type of growth regulator added to the culture medium, it is possible to induce somatic embryogenesis from explants of cotyledons, petioles, hypocotyls, and leaves of M. arborea (Martín et al. 2000; Gallego et al. 2001).
Cell walls do not have uniform structures, and they vary both in morphology and composition among various plant tissues. In recent years, studies addressing changes occurring in cell wall architecture during differentiation of different cell types have revealed the importance of th cell wall structure in these events (Serpe et al. 2002; Morvan et al. 2003), and in transitions from primary to secondary cell types (Aspeborg et al. 2005; Gorshkova and Morvan 2006).
Changes in tissue and organ morphology that occur during plant development are largely attributed to modification and re-organization of cell wall components and to synthesis and deposition of new material in pre-existing cell walls (Meijer and Murria 2001). As cell walls play important roles during cell differentiation and regeneration, the present study was undertaken to investigate differences in composition and structure of those M. arborea L. cells that undergo embryogenesis versus those that remain recalcitrant (Villalobos et al. 2007). In particular, cell wall polysaccharide components of embryogenic and non-embryogenic calli of M. arborea, as well as analysis of neutral sugars and uronic acids of cell wall polysaccharides were determined.
Materials and methods
Plant material
Seeds of M. arborea L. subsp. arborea (2n = 6x = 48), obtained from the Germplasm Bank (Department of Plant Biology, Higher Technical Institute of Agricultural Engineering, Madrid; stock no. 9504), were germinated aseptically. Three weeks old seedlings were used as sources of explants as described by Gallego et al. (2001).
Explants, consisting of 5-mm sections of both petioles and hypocotyls, were used to establish callus cultures., Explants were incubated in Murashige and Skoog (MS) medium (Murashigue and Skoog 1962) supplemented with 10 μM 2,4-dichlorophenoxyacetic acid (2,4-D) and 9.3 μM kinetin, on sterile Petri dishes sealed with Parafilm under a 16 h photoperiod at a light intensity of 20 μmol m−2 s−1 provided by Osram 36 W white-fluorescent lamps, at 23 ± 2°C. After 2 months, developing calli were transferred to MS medium supplemented with 2.5 μM of 2,4-D, but lacking kinetin. Cultures were transferred to fresh medium on a monthly basis. By the end of the third month, calli of embryogenic (E) and non-embryogenic (NE) calli from each of hypocotyls and petioles were collected, and used for determining cell wall components. Calli were lyophilised, weighed before and after lyophilization, and finally frozen at −10°C until used for analysis. A total of three replications were used for each explant source, and the experiment was repeated three times over time.
Preparation of cells walls and cell wall fractionation
Cell wall preparations were conducted following the protocol of Rupérez et al. (1985), but with some modifications. Lyophilised calli (0.5 g) were incubated in 80% methanol for 10 min and centrifuged at 10,000×g for 20 min, after which the supernatant was discarded. The cell wall fraction was extracted from the precipitate with hot water (100°C) for 15 min, and then centrifuged at 10,000×g. The supernatant was subjected to dialysis against water, and used as the “hot-water-soluble pectin fraction”. The residue was sequentially extracted with 1 and 4 M NaOH using centrifugation at 10,000×g for 10 min. The pH of the supernatant was neutralized with glacial acetic acid in an ice bath, and then subjected to dialysis (Rupérez et al. 1985).
Finally, the sediment was washed with 0.03 M acetic acid, and the precipitate was resuspended in deionized water. Following dialysis of this fraction, corresponding to the cellulose fraction, it was hydrolyzed with sulphuric acid 72% at room temperature for 1 h, followed by dilution with water, and hydrolyzed with sulphuric acid 1 M at 105°C for 1–1/2 h.
Sugar analysis
For each of the fractions collected, the amount of total sugars was determined using the phenol-sulphuric acid method (Dubois et al. 1956), with glucose (25–150 μg/ml) used as a standard. Whereas, the amount of uronic acids was determined following the protocol of Scott (1979), using galacturonic acid (20–140 μg/ml) as a standard. Different cell wall fractions obtained were lyophilised, and used for the analysis of sugars by gas liquid chromatography. Derivatization and analysis by gas liquid chromatography of neutral sugars present in different cell wall fractions were done as reported by Rupérez and Leal (1986). Acetylated monosaccharides were dissolved in dichloromethane and analyzed with an HP 6890 plus 6890A (Germany) gas chromatograph equipped with an SP-2340 capillary column (SUPELCO; 30 m; 0.25 mm id; 0.2 μm film thickness) at 215°C up to 9 min and subjected to a ramp of 215–240°C at 10°C/min. The carrier gas was nitrogen, and detection was performed using flame ionization.
Statistical analysis
All data were subjected to analysis of variance using the SPSS program (Pérez 2005). For significant treatment effects (P < 0.01 and P < 0.05), the least significant difference test, Fisher LSD, was used for mean comparisons at 0.01 and 0.05 levels of significance.
Results and discussion
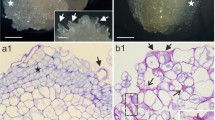
Although embryogenic (E) and non-embryogenic (NE) calli have been obtained previously from different explants of M. arborea L. (Martín et al. 2000; Gallego et al. 2001), a detailed morphological characterization of these calli is herein provided in this study. A high frequency of embryogenic calli (E) was obtained from hypocotyls (28%) and petioles (28%), and similar to those reported by Martín et al. (2000). Embryogenic calli were soft, yellowish, and slow-growing; whereas, NE calli were green, hard, and fast-growing (Gallego et al. 2001). Morphological differences between E and NE calli have also been observed in carrot cell cultures in liquid medium, the former type of calli forming cell clusters, is in this case greater in size than those that had lost embryogenic competence (Kikuchi et al. 1995). The smaller size of the NE calli seems to be associated with a reduction in intercellular union (Kikuchi et al. 1996). In our case, the size of nonembryogenic calli was always bigger (further than 34 mm) than those with embryogenic competence (between 9–10 mm).
In the present study, significant differences (P < 0.05) in polysaccharide composition of cell walls were observed between E and NE calli obtained from explants of M.arborea L. hypocotyls and petioles. Moreover, significant differences in total sugars (Fig. 1) and uronic acids (Fig. 2), except for the 4 M NaOH fraction, were observed between E and NE calli in the different fractions obtained following chemical fractionation of cell walls. The recovery was higher than 80% (Table 1), thus indicating that only part of the cell wall samples was lost during extraction. Similar recovery values were reported by Thomas and Thibault (2002) using Chaenomeles japonica fruit. The amount of material released may vary as a function of the extraction process employed (Shiga and Lajolo 2006).
Total sugar content in the different cell wall fractions of embryogenic (E) and non-embryogenic (NE) calli obtained from petiole and hypocotyl explants of M. arborea L. Comparisons of E versus NE: blank indicates non-significance, “b” corresponds to significance at P < 0.05), and “a” corresponds to significance at P < 0.01
Uronic acid content of the different cell wall fractions in embryogenic (E) and embryogenic (NE) calli obtained from petiole and hypocotyl explants of M. arborea L. Comparisons of E versus NE: blank indicates non-significance, “b” corresponds to significance at P < 0.05), and “a” corresponds to significance at P < 0.01
For calli obtained from hypocotyls (Fig. 1), total sugars extracted in the hot-water fraction were significantly higher in E than in NE calli, and this finding was detected in both 1 and 4 M NaOH fractions. In contrast, sugar levels in the residual fraction were higher in NE than in E calli.
For calli induced from both petioles and hypocotyls (Fig. 1), total sugars were higher in E than in NE calli in those fractions corresponding to pectins and hemicelluloses. However, total sugars, primarily in the pectin fraction, and to a lesser extent in the hemicellulose fraction, were higher in calli induced from petioles than those induced from hypocyotyls. Whereas in the cellulose fraction, the lowest amounts of total sugars were detected in E calli derived from petioles. Overall fractions, higher levels of total sugars of both types of calli (E and NE) from hypocotyls and petioles were detected in the hot water fraction, 1 M NaOH, and cellulose fraction (Fig. 1); whereas, these sugar levels in the 4 M NaOH fraction reached an optimum of 11 mg/g callus dry weight.
Analysis of uronic acids content in polysaccharides from each of the cell wall fractions of E and NE calli revealed higher levels in E calli (derived from both petiole and hypocotyl explants), mainly in the hot-water fraction, but also in 1 M and 4 M Na fractions, latter fractions were between 11- and 17-fold lower than those detected in the pectin fraction (Fig. 2). Uronic acids levels in the hot-water and NaOH fractions of NE calli were 12 to 22-fold lower than those of E calli. Overall, calli induced from petioles had the highest levels of uronic acids. These high amounts of uronic acids indicated presence of pectic polysaccharides in this fraction (Femenia et al. 1999). Most of the uronic acids were extracted in the hot-water fraction. Polysaccharides obtained in the hot-water fraction were likely to be derived from the middle lamella of cell walls, as reported previously in other plant species (Ryden and Selvendran 1990). These pectic polysaccharides were readily degraded, thus affording fragments rich in neutral sugars and derived from pectin side chains (Femenia et al. 1998).
In the 1 and 4 M NaOH fractions, uronic acid contents were low, with highest levels reaching only 1 mg/g dry weight. In the cellulose fraction, containing similar uronic acid levels for both E and NE calli; however, the highest uronic acid levels were detected in calli induced from petioles. These high observed values of pectin components detected in this fraction suggested a possible inter-or intramolecular interaction among pectic polysaccharides resistant to chemical reagents during the sequential extraction process, resulting in physical adhesion of pectic polysaccharides to cellulose microfibrils (Femenia et al. 1998). Briefly, those carbohydrate polymers forming cell walls of these calli were mainly pectic polysaccharides and glucans. These were notable observations as strong intercellular adhesion was likely to be critical for both morphogenesis and embryogenesis. Strength of intercellular contacts must depend on the structure of the cell wall and, in particular, on pectins of the middle lamella of plant cells (Kikuchi et al. 1995). Neutral sugars of the side chains of pectins could play an important role in this process (Iwai et al. 2001). To date, there are few supportive data primarily from cultures grown in liquid media.
Gas chromatography analysis of neutral sugars solubilized in the hot-water fraction (Table 2) revealed that galactose was the major sugar, at levels ranging between 58 and 49 mg/g dry weight, followed by arabinose, ranging between 11.66 and 18.83 mg/g dry weight, and glucose, ranging between 9 and 16 mg/g dry weight. The presence of high levels of galactose and arabinose indicated that pectins were the main polysaccharides present in these extracts. These findings were consistent with those reported in calli of Linum usitatissimum L. (David et al. 1994). In our study, sugar levels were significantly higher in E calli. Among the sugars, low amounts of xylose and mannose were found; however, the lowest sugars were fuctose and rhamnose. For xylose, the highest levels of this sugar detected in E calli may be derived from pectins, probably xylogalacturonans, the main component of cell walls in legumes as reported by Shiga and Lajolo (2006).
In this study, polysaccharides released by saponification with 1 M NaOH solution (N1) (Table 2) were mainly formed by xylose, at levels ranging between 7 and 11 mg/g dry weight. Among the polysaccharides, high amounts of galactose and arabinose were also detected, followed by glucose, ranging between 5 and 10 mg/g dry weight, with levels of the remaining sugars as low as 2 mg/g dry weight in any explant or callus type.
Xylose and glucose are known as the main components of hemicelluloses (Thomas and Thibault 2002; Femenia et al. 1999). In this study, the highest levels of xylose, glucose, and arabinose have been detected in NE calli. Previously, it has been reported that xylose levels in carrot cells grown in liquid medium increased directly with size of cell clusters and with increased embryogenic capacity (Kikuchi et al. 1996). This is contrast to observations in this study whereby the smallest cell clusters of M. arborea were found to be the most amenable to embryogenisis and contained the lowest levels of xylose.
In general, an increase in NaOH concentration in the extraction medium (N4) (Table 2) elicited a dramatic decrease in levels of neutral sugars released, primarily in calli derived from petioles, with low mannose, fructose, or rhamnose contents detected in this fraction. Significant differences were observed between E and NE calli induced from hypocotyls for all other sugars analyzed, with higher levels detected in NE calli. The major sugar in the cellulose residue (C) was glucose, and mainly detected in NE calli (55.55 mg/g dry weight) (Table 2). Significant differences were observed for all other sugars in this cellulose fraction between E and NE calli. The highest levels of galactose and arabinose were also detected in NE calli; whereas, levels of xylose and rhamnose did not surpass 3 mg/g dry weight, and mannose was not detected in this fraction (Table 2).
The detection of pectic polysaccharides in all fractions analyzed in this study may be due to the presence of uronic acids (Femenia et al. 1998). Moreover, detection of high amounts of xylose indicate presence of non-cellulosic polysaccharides such as xyloglucans and xylans in most aloe fractions as reported by Femenia et al. (1998). The presence of high amounts of glucose and xylose in NE calli in both hemicellulosic and cellulosic fractions also suggested a likely relationship between compactness of NE callus and abundance of xyloglucan and cellulose, as they are linked through hydrogen bridges (Femenia et al. 1999).
Overall in this study, there is a likely relationship between total sugars and uronic acids contents in pectin and hemicellulose fractions and embryogenic capacity of M. arborea calli. This may contribute to greater development of the middle lamella in cell walls. In coconut the acquisition of embryogenic competence was linked to the appearance of an outer layer of fibrillar material containing pectin epitope, fully coating the embryogenic cells (Verdeil et al. 2001). Branched arabinan was actively synthesized in asparagus embryogenic calli (Up-Dong et al. 1998). The soft texture of embryogenic calli could be attributed to increased cell division, resulting in incomplete cell wall formation with lower cellulose and hemicellulose contents. In contrast, the compact feature of NE calli could be due to the higher cellulose content in these cell walls, contrary to what is described at Kikuchi et al. 1996 in carrots. Among the major polysaccharides in the pectin fraction analyzed in this study are galactose and arabinose, while levels of xylose are low, thus indicating presence of xylogalacturonans. Whereas, the observed high xylose content in the hemicellulose fraction may be attributed to presence of xylans or xyloglucans. As for the cellulose fraction, the major form of sugar detected is glucose, although other polysaccharides, such as galactose, arabinose, xylose, and rhamnose have also been detected, thus indicating a close relationship between cellulose and other cell wall polysaccharides.
Abbreviations
- C:
-
Cellulose fraction
- E:
-
Embryogenic calli
- NE:
-
Non-embryogenic calli
- N1:
-
1 M NaOH fraction
- N4:
-
4 M NaOH fraction
References
Aspeborg H, Schrader J, Coutinho PM, Stam M, Kallas A, Djerbi S, Nilsson P, Denman S, Amini B, Sterky F et al (2005) Carbohydrate active enzymes involved in the secondary cell wall biogenesis in hybrid aspen. Plant Physiol 137:983–997. doi:10.1104/pp.104.055087
Behrouz E, Mat R (2005) Cellular behaviour in embryogenic and nonembryogenic sugar beet calluses. In Vitro Cell Dev Biol Plant 41:465–469
Corleto A, Venezian ME, Magín L, Eroli A, Cordella S (1980) Provedi addattamento e produzione di arbusti da pascolo in deverse localita della Puglia e della Basilicata. Riv Agron 14:42–49
David H, David A, Bade P, Millet J, Morvan O, Morvan C (1994) Cell wall composition and morphogenic response in callus derived from protoplasts of two fibre flax (Linum usitatissimum L.) genotypes. J Plant Physiol 143:379–384
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method of determination of sugars and related substances. Anal Chem 28:350–356. doi:10.1021/ac60111a017
Femenia A, Garosi P, Roberts K, Waldron KW, Selvendran RR, Robertson JA (1998). Planta 205:438–444. doi:10.1007/s004250050341
Femenia A, Waldron KW, Robertson JA, Selvendran RR (1999) Compositional and structural modification of the cell wall of cauliflower (Brassica oleracea L. var botrytis) during tissue development and plant maturation. Carbohydr Polym 39:101–108. doi:10.1016/S0144-8617(99)00004-1
Gallego P, Hita O, Villalobos N, Dorado A, Martin L, Guerra H (2001) Somatic embryogenesis and plant regeneration with Medicago arborea L. plantlet. In Vitro Cell Dev Biol Plant 37:199–203. doi:10.1007/s11627-001-0034-x
Gonzalez-Andrés F, Ceresuela JL (1998) Chemical composition of some Iberian Mediterranean leguminous shrubs potentially useful for forage in seasonally dry areas New Zeland. J Agric Res 41:139–147
Gorshkova T, Morvan C (2006) Secondary cell-wall assembly in flax phloem fibres: role of galactans. Planta 223:149–158. doi:10.1007/s00425-005-0118-7
Iwai H, Ishii T, Satoh S (2001) Absence of arabinan in the side chains of the pectic polysaccharides strongly associated with cell walls of Nicotiana plumbaginifolia non-organogenic callus with loosely attached constituent cells. Planta 213:907–915
Kikuchi A, Satoh S, Nakamura N, Fujii T (1995) Differences in pectic polysaccharides between carrot embryogenic and nonembryogenic calli. Plant Cell Rep 14:279–284. doi:10.1007/BF00232028
Kikuchi A, Edashige Y, shii T, Fujii T, Satoh S (1996) Variations in the structure of neutral sugar chains in the pectic polysaccharides of morphologically different carrot calli and correlations with the sizes of the cell clusters. Planta 198:634–639. doi:10.1007/BF00262652
Linhares F, Ferreira Gomes A, Fernandes Heredia F, Barbeta e Silva CP, Facó O, Assis de Paiva Campos F (2006) Somatic embryogenesis and plant regeneration in Opuntia picus-indica (L.) Mill. (Cactaceae). Sci Hortic (Amsterdam) 108:15–21. doi:10.1016/j.scienta.2005.12.007
Martin KP (2003) Plant regeneration through somatic embryogenesis on Holostemma ada-kodien, a rare medicinal plant. Plant Cell Tissue Organ Cult 72:79–82. doi:10.1023/A:1021229422172
Martín JP, Pintos B, Rebordinos I, Villalobos N, Guerra H, Martín L (2000) Embryogenic response in different Medicago arborea L. explants depending on cytokinin/auxin balances. J Plant Physiol 156:801–804
Meijer M, Murria J (2001) Cell cycle controls and the development of plant form. Curr Opin Plant Biol 4:44–49. doi:10.1016/S1369-5266(00)00134-5
Moghaddam BE, Taha RM (2005) Cellular behavior in embryogenic and non-embryogenic sugar beet calluses. In Vitro Cel Dev Biol—Plant 41:465–469
Morvan C, Ande` me-Onzighi C, Girault R, Himmelsbach DS, Driouich A (2003) Building flax fibres: more than brick in the walls. Plant Physiol Biochem 41:935–944. doi:10.1016/j.plaphy.2003.07.001
Murashigue T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Pérez C (2005) Técnicas estadísticas con SPSS 12: aplicaciones al análisis de datos. Pearson Educación. Madrid (España)
Pueschel AK, Schwenkel HG, Winkelman T (2003) Inheritance of the ability for regeneration via somatic embryogenesis in Cyclamen persicum L. Plant Cell Tissue Organ Cult 72:43–51. doi:10.1023/A:1021231806838
Rupérez P, Leal JA (1986) Age-related changes in Penicillium erythromellis cell wall. Trans Br Mycol Soc 86:279–285
Rupérez P, Selvendran RR, Stevens BJH (1985) Investigation of the heterogeneity of xyloglucans from the cell walls of apple. Carbohydr Res 142:107–113. doi:10.1016/S0008-6215(00)90737-7
Ryden P, Selvendran R (1990) Cell-wall polysaccharides and glycoproteins of parenchymatous tissues of runner bean (Phaseolus coccineus). Biochem J 269:393–402
Sakhanokho HF, Zipf A, Rajasekaran K, Saha S, Sharma GC (2001) Induction of highly embryogenic calli and plant regeneration in upland (Gossypium hirsutioum L.) and pima (Gossypium barbadense L.) cottons. Crop Sci 41:1235–1240
Schween G, Schwenkel H-G (2002) In vitro regeneration in Primula ssp. via organogenesis. Plant Cell Rep 20:1006–1010. doi:10.1007/s00299-002-0443-2
Scott RV (1979) Colorimetric determination of hexuronic acid in plant materials. Anal Chem 51:936–941. doi:10.1021/ac50043a036
Serpe MD, Muir AJ, Driouich A (2002) Immunolocalization of β-D-glucans, pectins and arabinogalactan-proteins during intrusive growth and elongation of nonarticulated laticifers in Asclepias speciosa. Torr Planta 215:357–370. doi:10.1007/s00425-002-0756-y
Shiga TM, Lajolo FM (2006) Cell wall polysaccharides of common beans (Phaseolus vulgaris L.)- composition and structure. Carbohydr Polym 63:1–12. doi:10.1016/j.carbpol.2005.06.025
Singh ND, Sahoo L, Sarin NB, Jaiwal PK (2003) The effect of TDZ on organogenesis and somatic embryogenesis in pigeonpea (Cajanus cajan L.Millsp). Plant Sci 164:341–347. doi:10.1016/S0168-9452(02)00418-1
Thomas M, Thibault J-F (2002) Cell-wall polysacharides in the fruits of Japanese quince (Chaenomeles japonica): extraction and preliminary characterization. Carbohydr Polym 49:345–355. doi:10.1016/S0144-8617(01)00375-7
Up-Dong Y, Hiroyuki K, Naoki N, Naoki S (1998) Quantitative and qualitative changes of cell wall polysaccharides during somatic embryogenesis and plantlet development of Asparagus (Asparagus officinalis L.). Plant Physiol 39:607–614
Verdeil JL, Hocher V, Huet C, Grosdemange F, Escoute J, Ferriere N, Nicole M (2001) Ultrastructural changes in coconut calli associated with the acquisition of embryogenic competence. Ann Bot (Lond) 88:9–18. doi:10.1006/anbo.2001.1408
Villalobos N, Martín L, Blázquez A, Martín JP, Gallego P, Pintos B, Guerra H (2007) Morphogenic differentiation in Medicago. Funct Plant Sci Biotechnol 1:171–194
Acknowledgments
This work was supported by a grant from the Dirección General de Investigación del Ministerio de Educación y Ciencia, Spain, No BFU2004-00771 and by Castilla y León, Spain, No. SA076A07.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Endress, V., Barriuso, J., Ruperez, P. et al. Differences in cell wall polysaccharide composition between embryogenic and non-embryogenic calli of Medicago arborea L.. Plant Cell Tiss Organ Cult 97, 323–329 (2009). https://doi.org/10.1007/s11240-009-9531-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-009-9531-0