Abstract
Despite the widespread use of tissue culture as a means of propagating begonias and concerns regarding the preservation of germplasm, little information is available on the cryopreservation of these commercially important plants. For this reason studies were conducted to develop an encapsulation–dehydration method for the cryopreservation of adventitious shoots of the rhizomatous begonia, Begonia x erythrophylla. Adventitious shoots of B. x erythrophylla were found to be sensitive to dehydration and very sensitive to freezing. While pre-treatment with 0.75 M sucrose significantly increased the percentage of encapsulated shoots surviving dehydration, pre-treatment with sucrose did not afford cryoprotection without prior dehydration. Addition of abscisic acid and proline to the pre-treatment medium significantly improved the percentage of shoots surviving freezing. Pre-treatment of shoots with a medium containing, 0.75 M sucrose, 3.8 μM abscisic acid and 2.15 mM proline resulted in greater than 50% of shoots surviving freezing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Begonias are popular ornamental plants that, despite being readily multiplied by vegetative cuttings, are often propagated using tissue culture techniques as they are susceptible to pathogenic bacteria, fungi and nematodes (Takayama 1990). Begonia x erythrophylla J. Neuman, also known as B. x feistii Hort. ex L.H. Bailey, is a hybrid rhizomatous begonia (B. manicata x B. hydrocotylifolia) and is considered to be among the most important ornamental begonias (Espino et al. 2004). Like most hybrid begonias B. x erythrophylla is not readily propagated from seed and so is commonly micropropagated via organogenesis, using leaf or petiole sections taken from stock plants maintained in vitro (Peck and Cumming 1984; Simmonds and Werry 1987; Burritt and Leung 1996, 2003). The maintenance of stock plants in vitro is a labour-intensive task, requiring periodic subculture with the inherent risk of contamination. In addition, there are significant on going costs associated with the maintenance of stock plants, such as labour, culture media and culture room space. Cryopreservation has proven to be an effective method for the long-term preservation of plant material, that when thawed can be used to rapidly produce in vitro stock plants, with good preservation of genetic and physiological characteristics (Scottez et al. 1992).
A number of different techniques can be used to cryopreserve plant tissues, including slow freezing, vitrification, and encapsulation–dehydration. Although these methods each have their pros and cons, encapsulation–dehydration has a number of advantages. For example, it does not require the use of expensive programmable freezers and generally uses mild cryoprotectants, such as sugars, thus avoiding the potential toxicity issues associated with other cryoprotectants (Scottez et al. 1992).
As little published information is available on the cryopreservation of rhizomatous begonias, this study was conducted to develop an encapsulation–dehydration method for the cryopreservation of adventitious shoots of B. x erythrophylla.
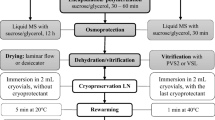
Materials and methods
Plant material
All experiments were carried out using adventitious shoots derived from 5 mm long petiole explants, taken from stock begonia plants grown on half-strength Murashige and Skoog’s (MS) salts (Murashige and Skoog 1962). Explants, 10 per 9 cm plastic tissue culture dish, were cultured on a shoot inducing medium (SIM) consisting of full-strength MS salts plus, 0.55 mM myo-inositol, 40.6 μM nicotinic acid, 2.43 μM pyridoxine HCl, 1.48 μM thiamine HCl, 26.7 μM glycine, 1.13 μM folic acid, 0.2 μM biotin, 0.54 μM naphthalene acetic acid (NAA), 4.44 μM benzyladenine (BA), 0.088 M sucrose and 0.7% w/v agar (Burritt and Leung 1996). The medium was adjusted to pH 5.8 prior to autoclaving. Both cultures and stock plants were maintained at 24°C, under continuous light (50 μmol m−2 s−1), provided by fluorescent lamps (Phillips Aquarella, TLD 36 W/89) (Burritt and Leung 2003). Dishes were regularly randomized in the culture chamber.
Pre-encapsulation treatments
Pre-encapsulation treatments involved transferring shoot-forming explants onto full-strength MS salts, plus vitamins and amino acids (as found in the SIM detailed above), supplemented with glucose, manitol, sorbitol or sucrose, or sucrose plus Abscisic acid (ABA) and/or proline, for 7 days, prior to shoot dissection and encapsulation.
Encapsulation
For encapsulation, shoots comprising the meristematic dome and 2–4 leaf primordia, were excised, with the aid of a stereo-microscope, from cultured petiole explants and suspended in a liquid encapsulation medium (ECM) consisting of full-strength MS salts, free of calcium, plus vitamins and amino acids (as found in the SIM detailed above), 4.44 μM BA, 2.8 μM gibberellic acid (GA3), 3% w/v sodium alginate and unless otherwise stated 0.75 M sucrose, for 15 h. The shoots were then dispensed, using 3 ml sterile transfer pipettes, into ECM supplemented with 100 mM calcium chloride and free of sodium alginate, and the resultant shoots, encapsulated in alginate beads, were collected.
Dehydration
Dehydration was achieved by placing encapsulated shoots (20) on sterile filter papers (Whatman No. 1) in 9 cm plastic Petri dishes (10, with each dish considered a replicate) and gradually dehydrating them by exposure to a sterile airflow, within a laminar flow hood, for 8 h at room temperature (20–24°C) and 55–60% relative humidity. The water content of the beads during dehydration was determined gravimetrically.
Freezing and thawing
Freezing was carried out by placing dehydrated/hydrated-encapsulated shoots in 2 ml cryovials (Nunc A/S Roskilde, Denmark) and immersing them in liquid nitrogen for 24 h. Encapsulated shoots were thawed by removing the caps from the cryovials and warming them to room temperature (24°C) in a laminar flow hood, taking approximately 20 min. The shoots were then rehydrated by placing them in a sealed plastic chamber at 100% relative humidity for 24 h at 24°C, followed by immersion in liquid SIM, without hormones and the same sugar/sugar-alcohol type/concentration found in the pre-culture medium, for 1 h at 24°C.
Shoot recovery and statistical analysis
Shoot recovery was assessed, by transferring the rehydrated shoots to agar solidified SIM, without hormones, and culturing them for 2 weeks, under the same conditions used for shoot induction. Only shoots exhibiting growth and producing leaves were scored as recovered. Statistical tests were performed using SPSS 10 for the Macintosh. The data were subjected to an analysis of variance (ANOVA), and the Tukey’s multiple range test was used to determine significant differences between the treatment means. P values less than 0.05 were considered significant. Experiments were repeated at least three times using explants derived from different donor plants.
Results and discussion
Preliminary experiments were conducted where shoots were dissected from explants grown on SIM then incubated with different concentrations of sucrose for various times, encapsulated, dehydrated and then frozen, or encapsulated and then treated with different concentrations of sucrose for various times prior to dehydration and freezing. Irrespective of the sucrose concentration, time of incubation or if incubated with increased sucrose concentrations before or after encapsulation, less than 10% of shoots survived freezing, with no one treatment being clearly beneficial with respect to shoot survival (Fig. 1a, b).
The influence of sucrose concentration and time of incubation in liquid ECM before or after encapsulation of the percentage of B. x erythrophylla shoots surviving dehydration, freezing in LN, thawing, and then rehydration. (a) The mean percentage of shoots surviving incubation in liquid ECM before encapsulation (±SE); (b) the mean percentage of shoots surviving incubation in liquid ECM after encapsulation (±SE)
In an attempt to improve the percentage survival, whole explants were cultured on solid media containing different sugars/sugar alcohols at various concentrations for 7 days prior to encapsulation, dehydration and freezing. Pre-treatment of shoots with concentrations of sucrose, glucose, or sorbitol up to 1 M followed by encapsulation, but without dehydration or freezing, did not significantly influence shoot re-growth (Fig. 2a), suggesting that none of these sugars/sugar-alcohol were toxic to shoots at the concentrations tested, and that short-term exposure to increased osmotic potentials had no adverse effects. In contrast, mannitol at concentrations of 0.5 M and above significantly reduced shoot re-growth (Fig. 2a). Treatment with mannitol has been shown to reduce re-growth of shoots in other studies (Paul et al. 2000) and this also appears true for B. x erythrophylla. It has been suggested that mannitol can modify the physical and chemical properties of alginate beads, resulting in mechanical compression damage to shoot apices (Paul et al. 2000). In addition, mannitol has been shown to inhibit adventitous shoot formation in B. Rex petiole explants cultured on a SIM (Mangat et al. 1990). This inhibition was thought to be due to reduced starch synthesis during the early stages of organogenesis. Coffin et al. (1976) suggested that exposure to a non-metabolizable substrate could lead to the depletion of cells energetic reserves and that this could lead to damage affecting survival.
The influence of sugar/sugar alcohol pre-treatments on the re-growth of shoots excised from B. x erythrophylla petiole explants. (a) The mean percentage of shoots surviving pre-treatment and encapsulation (±SE); (b) the mean percentage of shoots surviving pre-treatment and encapsulation followed by dehydration then rehydration (±SE); (c) the mean percentage of shoots surviving pre-treatment and encapsulation, followed by freezing in LN and then thawing (±SE); (d) the mean percentage of shoots surviving pre-treatment and encapsulation followed by dehydration, freezing in LN, thawing, and then rehydration (±SE). The different letters indicate statistically different values at P < 0.05; n = 10 replicates with 10 shoots per replicate. The most effective pre-treatment is indicated by *
Dehydration of encapsulated shoots using the air-drying method detailed above resulted in a water content of between 10 and 20% (Fig. 3). Pre-treatment with different concentrations of sucrose did not significantly influence the rate of water lost or the final water content, although the initial water content of shoots pre-cultured on 0.75 or 1.0 M sucrose were significantly (P = 0.05) lower than those cultured on 0.088 M sucrose (Fig. 3), nor did addition of ABA or proline to the culture medium (data not presented). Dehydration of shoots without a pre-treatment with increased sugar/sugar alcohol concentrations resulted in less than 5% of shoots exhibiting re-growth (Fig. 2b), suggesting a high sensitivity to dehydration. Pre-treatment of shoots with increasing concentrations of sucrose, glucose, sorbitol or manitol, significantly increased the percentage of shoot re-growth, with 0.75 M sucrose proving most effective, with nearly 30% re-growth (Fig. 2b). Rapid dehydration of encapsulated shoots, in sealed containers, over silica gel, or slow dehydration using stepped decreases in relative humidity (100 to 20%) failed to significantly improved shoot re-growth compared to the air-drying protocol (data not presented).
Like the shoots of many plants grown in vitro, those of B. x erythrophylla are extremely sensitive to freezing damage and are unable to withstand freezing in LN without the use of a cryprotectant, and a reduction in cellular water content. Rapid freezing of shoots in LN, without dehydration, resulted in almost complete inhibition of shoot re-growth, irrespective of pre-treatment, with most pre-treatments resulting in less than 5% survival (Fig. 2c). These results suggest that B. x erythrophylla shoots are extremely sensitive to freezing damage when fully hydrated, and that dehydration reduces freezing damage.
However, when shoots were pre-treated with increasing concentrations of sucrose, glucose, or sorbitol, dehydrated and then rapidly frozen in LN a significant increase in shoot re-growth was observed (Fig. 2d). Sugars are commonly used as cryoprotectants and the types and concentrations of sugars used are parameters that must be determined when developing cryopresevation methods (Paul et al. 2000). The resistance of B. x erythrophylla shoots to dehydration and freezing damage was dependant upon the sugar employed and it’s concentration. Although all four of the sugar/sugar alcohols tested were able to protect a proportion of the shoot-tips from terminal dehydration damage, only sucrose, glucose and sorbitol were effective cryoprotectants, with few dehydrated shoot-tips pre-treated with mannitol surviving freezing in LN (Fig. 2d). Other studies developing cryopreservation protocols using encapsulation–dehydration have found sucrose to be the most effective cryoprotectant (Martinez et al. 1999; Paul et al. 2000) and this was also true for B. x erythrophylla. Sucrose at a concentration of 0.75 M was the most effective pre-treatment with 24% of shoots exhibiting re-growth. In addition to inducing osmotic dehydration and increasing the dry matter content of plant tissues, a readily metabolized carbohydrate cryoprotectant such as sucrose is also thought to contribute to the maintenance of membrane integrity during the dehydration and freezing processes. This is thought to be due to the formation of hydrogen bonds between sugars and the hydrophilic components of cellular membranes, thus membrane stability is increased (Taylor 1987).
Experiments were also conducted, on shoots pre-cultured on 0.75 M sucrose, to ascertain if the concentration of sucrose in the ECM and rehydration solutions influenced re-growth. No significant improvement in percentage re-growth was observed at sucrose concentrations higher or lower than the 0.75 M optimum for pre-culture (data not presented).
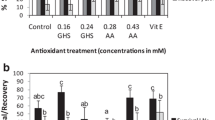
Despite pre-treatment with 0.75 M sucrose enabling a proportion of B. x erythrophylla shoots to survive, a percentage re-growth of less than 25% following freezing is still relatively low, when compared to the cryopreservation protocols developed for other plant species (Martinez et al. 1999; Paul et al. 2000). In an attempt to improve the percentage re-growth, shoots were pre-treated with ABA and/or proline at various concentrations. Pre-treatment with 3.8 μM ABA and 0.75 M sucrose or 2.15 mM proline and 0.75 M sucrose, significantly increased the percentage re-growth from 24%, with 0.75 M sucrose alone, to 42 and 31%, respectively (Fig. 4a). When shoots were pre-treated with 3.8 μM ABA, 2.15 mM proline and 0.75 M sucrose the percentage re-growth increased to 51% (Fig. 3b). Even pre-treatment of shoots with ABA or proline and 0.088 M sucrose, significantly increased shoot re-growth when compare to 0.088 M sucrose alone (Fig. 4b).
The influence of ABA/proline pre-treatments on the re-growth of B. x erythrophylla shoots excised from begonia petiole explants. (a) The mean percentage of shoots surviving pre-treatment with ABA or proline followed by dehydration, freezing in LN, thawing, and then rehydration (±SE); (b) the mean percentage of shoots surviving pre-treatment with ABA (3.8 μM) and/or proline (2.15 mM), at two sugar concentrations, followed by dehydration, freezing in LN, thawing, and then rehydration (±SE). The different letters indicate statistically different values at P < 0.05; n = 10 replicates with 10 shoots per replicate. The most effective pre-treatment is indicated by *
Comparison of a standard protocol where isolated shoots, grown on 0.088 M sucrose, were incubated in liquid medium containing 0.75 M sucrose for 15 h prior to encapsulation, dehydration and freezing, clearly shows the benefit for shoot survival of pre-culturing explants for 7 days on 0.75 M sucrose plus 3.8 μM ABA and 2.15 mM proline (Fig. 5). Pretreatment with ABA has also been shown to increase the survival of desiccated encapsulated somatic embryos of sugarcane (Nieves et al. 2001) and in a recent study Suzuki et al. (2006) demonstrated that ABA and proline are important for both desiccation and cryopreservation tolerance of Gentian axillary buds. Although the importance of ABA in protecting plants against desiccation and low temperatures is well established (Nieves et al. 2001) little is known about the role of proline. Recent research suggests that as well as functioning as a cellular osmoticant and membrane protectant, proline can react with some reactive oxygen species (ROS) potentially reducing oxidative stress. For example, proline has been shown to scavenge singlet oxygen (Alia and Matysik 2001) and hydroxyl radicals (Smirnoff and Cumbes 1989) in vitro and both of these ROS can be produced in plant cells under stress.
The influence of genotype on the mean percentage of shoots surviving cryopreservation using three protocols (± SE). Protocol 1, growth of explants on solid SIM containing 0.088 M sucrose and 15 h incubation in liquid ECM, containing 0.75 M sucrose, prior to encapsulation, dehydration and freezing. Protocol 2, growth of explants on solid SIM containing 0.088 M sucrose, pre-treatment for 7 days on solid SIM containing 0.75 M sucrose and 15 h incubation in liquid ECM, containing 0.75 M sucrose, prior to encapsulation, dehydration and freezing. Protocol 3, growth of explants on solid SIM containing 0.088 M sucrose, pre-treatment for 7 days on solid SIM containing 0.75 M sucrose, 3.8 μM ABA and 2.15 mM proline, and 15 h incubation in liquid ECM, containing 0.75 M sucrose, prior to encapsulation, dehydration and freezing. Be02a, Be03F and Be02h represent three lines of B. x erythrophylla plants established in culture from three independently sourced donor plants. Br04b represents a line of B. rex plants established in culture from a single donor plant. The different letters indicate statistically different values at P < 0.05; n = 10 replicates with 10 shoots per replicate
To ensure the protocol detailed in the present study was not unduly influenced by genotype, shoots from three separately sourced B. x erythrophylla lines and a single line of Begonia rex plants were tested, with no statistically significant differences in the percentage survival observed between any of the lines (Fig. 5). Although no tests of genetic stability were conducted, re-growth of cryopreserved shoots appeared normal. In addition, over 200 plants grown in vitro from cryopreserved B. x erythrophylla shoots showed no morphological abnormalities and over 50 plants acclimated and grown under glasshouse conditions also appeared normal.
Conclusion
In conclusion, this paper describes a method that, for the first time, enables the cryopreservation of B. x erythrophylla shoots, using an encapsulation/dehydration procedure. This procedure involves pre-treatment of shoots with sucrose, proline and ABA, followed by encapsulation of the shoot-tips in alginate beads and then air-drying, prior to freezing in LN.
References
Alia MP, Matysik J (2001) Effect of proline on the production of singlet oxygen. Amino Acids 21:195–200. doi:10.1007/s007260170026
Burritt DJ, Leung DWM (1996) Organogenesis in cultured petiole explants of Begonia x erythrophylla: the timing and specificity of the inductive stimuli. J Exp Bot 47:557–567. doi:10.1093/jxb/47.4.557
Burritt DJ, Leung DWM (2003) Adventitious shoot regeneration from Begonia x erythrophylla petiole sections is developmentally sensitive to light quality. Physiol Plant 118:289–296. doi:10.1034/j.1399-3054.2003.00083.x
Coffin R, Taper CD, Chong C (1976) Sorbitol and sucrose as carbon source for callus-culture of some species of rosaceae. Can J Bot 54:547–551
Espino FJ, Linacero R, Rueda J, Vazquez AM (2004) Shoot regeneration in four Begonia genotypes. Biol Plant 48:101–104. doi:10.1023/B:BIOP.0000024282.01087.a3
Mangat BS, Pelekis MK, Cassells AC (1990) Changes in the starch content during organogenesis in in vitro cultured Begonia rex stem explants. Physiol Plant 79:267–274. doi:10.1111/j.1399-3054.1990.tb06741.x
Martinez D, Tames RS, Revilla MA (1999) Cryopreservation of in vitro-grown shoot-tips of hop (Humulus lupulus L.) using encapsulation/dehydration. Plant Cell Rep 19:59–63. doi:10.1007/s002990050710
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Nieves N, Martinez ME, Castillo R, Blanco MA, Gonzalez-Olmedo JL (2001) Effect of abscisic acid and jasmonic acid on partial desiccation of encapsulated somatic embryos of sugarcane. Plant Cell Tissue Organ Cult 65:15–21. doi:10.1023/A:1010699532641
Paul H, Daigny G, Sangwan-Norreel BS (2000) Cryopreservation of apple (Malus x domestica Borkh.) shoot tips following encapsulation–dehydration or encapsulation–vitrification. Plant Cell Rep 19:768–774. doi:10.1007/s002990000195
Peck DE, Cumming BG (1984) In vitro-propagation of Begonia x tuberhybrida from leaf sections. HortScience 19:395–397
Scottez C, Chevreau E, Godard N, Arnaud Y, Duron M, Dereuddre J (1992) Cryopreservation of cold-acclimated shoot tips of pear in vitro cultures after encapsulation dehydration. Cryobiology 29:691–700. doi:10.1016/0011-2240(92)90073-B
Simmonds JA, Werry T (1987) Liquid-shake culture for improved micropropagation of Begonia x hiemalis. HortScience 22:122–124
Smirnoff N, Cumbes QJ (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28:1057. doi:10.1016/0031-9422(89)80182-7
Suzuki M, Ishikawa M, Okuda H, Noda K, Kishimoto T, Nakamura T et al (2006) Physiological changes in Gentian axillary buds during two-step preculturing with sucrose that conferred high levels of tolerance to desiccation and cryopreservation. Anal Bot 97:1073–1081. doi:10.1093/aob/mcl054
Takayama S (1990) Begonia. In: Ammirato PV, Evans DA, Sharp WR, Bajaj YPS (eds) Handbook of plant tissue culture. Macmillan, New York, pp 253–283
Taylor MJ (1987) Physico-chemical principles in low temperature biology. In: Grout BWW, Morris GJ (eds) The effects of low temperature on biological systems. Edward Arnold Publisher, London, pp 3–70
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burritt, D.J. Efficient cryopreservation of adventitious shoots of Begonia x erythrophylla using encapsulation–dehydration requires pretreatment with both ABA and proline. Plant Cell Tiss Organ Cult 95, 209–215 (2008). https://doi.org/10.1007/s11240-008-9434-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9434-5