Abstract
Efficient regeneration via somatic embryogenesis (SE) would be a valuable system for the micropropagation and genetic transformation of sugar beet. This study evaluated the effects of basic culture media (MS and PGo), plant growth regulators, sugars and the starting plant material on somatic embryogenesis in nine sugar beet breeding lines. Somatic embryos were induced from seedlings of several genotypes via an intervening callus phase on PGo medium containing N6-benzylaminopurine (BAP). Calli were mainly induced from cotyledons. Maltose was more effective for the induction of somatic embryogenesis than was sucrose. There were significant differences between genotypes. HB 526 and SDM 3, which produced embryogenic calli at frequencies of 25–50%, performed better than SDM 2, 8, 9 and 11. The embryogenic calli and embryos produced by this method were multiplied by repeated subculture. Histological analysis of embryogenic callus cultures indicated that somatic embryos were derived from single- or a small number of cells. 2,4-dichlorophenoxyacetic acid (2,4-D) was ineffective for the induction of somatic embryogenesis from seedlings but induced direct somatic embryogenesis from immature zygotic embryos (IEs). Somatic embryos were mainly initiated from hypocotyls derived from the cultured IEs in line HB 526. Rapid and efficient regeneration of plants via somatic embryogenesis may provide a system for studying the molecular mechanism of SE and a route for the genetic transformation of sugar beet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Totipotency, the capacity of a cell to regenerate a whole multicellular organism, is expressed with comparative ease in higher plants. It was first demonstrated some 50 years ago by the phenomenon termed somatic embryogenesis (Steward et al. 1958). Since the first description of somatic embryo formation, many papers have been published addressing this phenomenon (Zimmerman 1993; Thorpe 1995). Somatic embryogenesis offers a valuable opportunity to study various plant developmental processes. Another aspect of somatic embryogenesis is its application to plant breeding and plant production. Micropropagation of somatic embryos and plants can be very advantageous when dealing with desirable genotypes or traits that are difficult to multiply by other means. Large-scale production of somatic embryos can also be applied to the production of artificial seeds (Thorpe 1995).
The development of procedures for the efficient regeneration of plants from cultured cells, tissues and organs are a prerequisite for the application of in vitro culture techniques to plant genetic manipulation and crop germplasm enhancement. Sugar beet is the major sugar-producing crop of temperate zones. Many commercial breeding lines are regarded as recalcitrant with respect to in vitro culture and genetic transformation (Tetu et al. 1987; D’Halluin et al. 1992; Elliott et al. 1996; Snyder et al. 1999; Zhang et al. 2001a; 2001b). The guard cell protoplast transformation system has been developed to produce transgenic sugar beet plants efficiently (Hall et al. 1996). However, this approach often produces multiple foreign gene integration events and leads to gene silencing and instability (Finnegan and McElroy D 1994). A somatic embryogenesis pathway for plant regeneration has been considered desirable for Agrobacterium-mediated transformation because it is assumed that somatic embryos originate from single cells, thus the derived transgenic plants are unlikely to be chimaeric and will originate from low numbers of transgene integration events. In order to produce the transgenic sugar beet plants at a high frequency and without chimaerism it is essential to establish a regeneration protocol via somatic embryogenesis for a range of sugar beet breeding lines.
Sugar beet tissue cultures do not readily undergo somatic embryogenesis in vitro. Tetu et al. (1987) first reported somatic embryogenesis from callus induced on petiole explants. Kubalakova (1990) obtained embryogenic calli from cultured axillary buds of the flower stalk. Tenning et al. (1992) reported somatic embryogenesis from young zygotic embryos using 2,4-D as the sole induction agent. However, these protocols are genotype dependent and less effective for the development of a generic protocol. The work described by D’Halluin et al. (1992) represented a breakthrough in this field by employing mature embryos of sugar beet breeding lines. However, the protocol has not been optimised and the regeneration pathway has not been confirmed by histological analysis. Tsai and Saunders (1995) obtained somatic embryos from suspension culture cells of a highly regenerable clone REL-1 at a frequency of 15 embryos per ml suspension. These approaches are applicable to a very limited number of genotypes and the frequency of regeneration is generally low. The procedures involved are complicated due to the fact that several regimes of induction and subculture are required.
In our previous work, we have shown that TDZ is more effective than BAP for the promotion of adventitious shoot formation from sugar beet (Zhang et al. 2001b, 2004). Our results also demonstrated rapid somatic embryogenesis from seedlings of sugar beet, and that subsequent plant regeneration could be achieved after a period of exposure to TDZ. However, TDZ treatment was not invariably superior to BAP treatment, depending upon genotype. In this study, the effect of the basal medium, BAP and 2,4-D and carbohydrates (sucrose versus maltose) on somatic embryogenesis regeneration from a range of sugar beet breeding lines was investigated with an attempt to further improve the procedures for regeneration of sugar beet.
Materials and methods
Plant materials
Plant materials used in this investigation were sugar beet breeding lines: SDM 2, 3, 5, 8, 9, 10, 11, HB 526 and CMS 22003 (2n = 18). Seeds of breeding lines (SDM 2-11) were provided by Lion Seeds Ltd, Essex, UK. Seeds of HB 526 were provided by Hilleshog AB, Novartis Seeds, Landskrona, Sweden. CMS 22003, a Czech breeding line with a high capacity for somatic embryogenesis (Kubalakova 1990), was maintained as sterile shoot cultures and embryogenic callus cultures.
Seed sterilisation and germination
The seed coats were removed by hydration–dehydration treatment of the seed balls followed by manual removal using a dissecting needle and forceps (Zhang et al. 1994). Seeds with the seed coats removed were surface sterilised by dipping in 70% ethanol for 1 min, immersion in 50% Domestos (Lever Industrial Ltd, Cheshire, UK) with a hypochlorite concentration of 2.5% (w/v) for 30–50 min and rinsing 3 times with sterilised deionised water at 10 min intervals. Ten to fifteen sterilised seeds were inoculated on 25 ml of culture medium in a disposable Petri dish (9 cm, Sarstedt, Germany). The Petri dishes were sealed with polyethylene clingfilm, wrapped in aluminium foil and incubated at 24°C in the dark for germination.
Media and culture conditions
The culture media used were MS medium (Murashige and Skoog 1962), PGo medium (De Greef and Jacobs 1979), and the corresponding supplemented media, MSRV and PGoRV, containing additional amino acids and vitamins (Freytag et al. 1988). Culture media were supplemented with various concentrations of BAP, 2,4-D and sugar (sucrose or maltose). MS medium was supplemented with 0.8% agar (Topley House, Bury, England) and 3% sucrose, and the pH was adjusted to 5.8 with 1 M KOH. PGo medium was made up with 0.75% agar, 3–9% sucrose or maltose and the pH was adjusted to 6.0 with 1 M NaOH. All media were autoclaved at 121°C for 20 min. After germination on the solid medium, sterile seedling cultures were kept on the same medium at 24°C in the dark for inducing somatic embryogenesis (Fig. 1a).
Cultures for plant regeneration were incubated in continuous light under 36W white Pluslux 3500 fluorescent tubes at 42 ± 14 μE M−2 S−1 (350–750 nm) at 24°C.
Somatic embryogenesis in seedlings, secondary embryogenesis and regeneration
Seedlings from various sugar beet breeding lines were cultured in the dark on a range of solid media for the induction of somatic embryogenesis (see Figs. 1–6). Three Petri dishes of culture, ten to thirteen seedlings per dish, was regarded as one treatment, and each treatment was repeated three to five times. The efficiency of somatic embryogenesis was scored 8 weeks after germination of the seeds on the same medium.
Somatic embryogenesis in sugar beet. (a) An embryogenic callus produced by culture of HB 526 on PGo B2 medium; (b) secondary somatic embryogenesis from SDM 3 after subculture on PGo B2 medium; (c) newly isolated immature embryo of SDM 11; (d) proembryos on germinated immature embryo of HB 526 on MS 1D medium. Bars represent 0.5 mm (a, b, c, d). Arrows pointed to the specified tissue
The effect of culture medium on somatic embryogenesis in sugar beet. The concentration of BAP used in this experiment was 8.9 μM. The frequency for somatic embryogenesis after 8 weeks of culture is plotted against medium used. Error bars indicate standard errors from mean values. Letters above the plots indicate the significance of differences between mean values (P = 0.05)
The effect of BAP concentration on the frequency of induction of somatic embryogenesis from intact seedlings in sugar beet. The frequency for somatic embryogenesis after 8 weeks of culture is plotted against the concentration of BAP in PGo medium. Seedlings grown on plant growth regulator-free medium produced no somatic embryos. Error bars indicate standard errors from mean values. Letters above the plots indicate the significance of differences between mean values (P = 0.05)
The effect of sugar concentrations on somatic embryogenesis in sugar beet. The concentration of BAP used in this experiment was 8.9 μM. The frequency for somatic embryogenesis after 8 weeks of culture is plotted against the concentration and type of sugars in PGo medium. Error bars indicate standard errors from mean values. Letters above the plots indicate the significance of differences between mean values (P = 0.05)
Genotype difference among sugar beet breeding lines in response to somatic embryogenesis. The concentration of BAP at 8.9 μM and maltose at 30 g l−1 in PGo medium were used in this experiment. The frequency for somatic embryogenesis after 8 weeks of culture is plotted against the breeding lines. Error bars indicate standard errors from mean values. Letters above the plots indicate the significance of differences between mean values (P = 0.05)
PGo medium containing 4.4 μM BAP and 1 μM TIBA (herein designated as 1B + 0.5T), 8.9 μM BAP (B2) or 4.5 μM 2,4-D were used for inducing callus from excised cotyledon explants or germinated seedlings in the dark (Fig. 1b). Callus induced from cotyledons or seedlings was subcultured onto B2 or B2 + 0.6 μM GA3 + 0.6 μM IAA (GA) and grown for 2 weeks in the light. They were then sub-cultured onto 8.9 μM BAP + 0.1 M mannitol (MT) and then onto medium GA and 0.9 μM BAP (0.2B) or directly onto 0.2B for the induction of somatic embryogenesis. About 15 calli per Petri dish was regarded as one treatment, and each treatment was repeated 3–5 times. The frequency of somatic embryogenesis and shoot development was determined four and 8 weeks later, respectively. The frequency was calculated as the number of somatic embryogenic calli/number of calli tested.
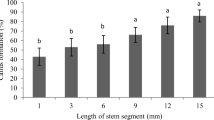
Embryogenic callus cultures were transferred onto PGo media containing 5.4 μM NAA + 1.1 μM BAP (1N + .2B) or 1B + .5T, for multiplication. The size of culture was reduced to about 9 mm2 using a dissecting knife or forceps during this process. Somatic embryos were cultured on the same medium for induction of secondary somatic embryogenesis.
Growth of plant materials in the greenhouse
After the seeds were imbibed in water for 24 h they were planted in pots containing M3 soil compost. The seedlings were watered daily and fed with fertiliser Phostrogen Plant Food (Solaris, Garden division of Monsanto, High Wycome, Buckinghamshire) 2 scoops per 10 l water at 2 weeks interval. Two month-old seedlings (generally growing 5–7 leaves) were transferred to a 4°C cold room under continuous lighting for a three-month period of vernalisation. After this they were moved to the greenhouse for further development. The greenhouse was under natural daylight supplemented by high pressure mercury vapour discharge lamps (400 W) to provide a 16 h day length with maximum day and minimum night temperatures of 25°C and 10°C, respectively.
Immature embryo culture
Flower spikes were obtained from bolted plants grown in the greenhouse 14 days after anthesis. They were sterilised in 30% Domestos for 30 min. Immature embryos (IEs) were isolated from flower stalks and subsequently cultivated for 4 weeks on solid MS media supplemented with various plant growth regulators (see Fig. 1c and Table 2). Immature embryos were cultured in the dark in MS media supplemented with 4.4 μM BAP (B1); 4.9 μM BAP + 2.2 μM NAA (B1 + .4N); 4.5 μM 2,4-D (1D); 1D + 2.2 μM BAP (1D + .5B) or 1D + 2.3 μM kinetin (1D + .5K). Embryos at various developmental stages were tested. About 10 IEs per Petri dish was regarded as one treatment, and each treatment was repeated 2–5 times. The frequency of somatic embryogenesis was calculated as the number of seedlings or immature embryos producing somatic embryos/number tested. MS media supplemented 2.3 μM 2,4-D, 5.4 μM NAA and 0.9 μM BAP or 1D + 0.5B were used for subculturing the somatic embryogenic tissue after size reduction.
Rooting and transplanting
The shoots produced from somatic embryos were subcultured onto MS-hormone free medium in continuous light at 24°C once or twice after every 4 weeks to re-establish apical dominance and for shoot elongation. Apically dominant shoot clumps were separated and transferred. About 60% of shoots were able to develop roots on the same medium. The rest were transferred to 35 ml ½ × MS medium containing 5.4 μM NAA in magenta boxes to induce root formation. Intact plants with 3–4 leaves were then transferred to autoclaved compost and covered with a transparent plastic bag to maintain humidity until new leaves developed. Plants were grown in a glasshouse under the condition described above.
Histological analysis of somatic embryogenesis
For histological studies, embryogenic sugar beet cultures of CMS 22003 and SDM 2, 3 and HB526 at various stages were fixed in Navashin fixation solution for 48 h, dehydrated through a graded ethanol series over 48 h and embedded in paraffin. Sections, 10 μm thick, were stained with basic fuchsin and counterstained with picroindigocarmine following an established protocol (Kubalakova 1990).
Statistical analysis and graphics
The Microsoft Office 2000 Excel program was used for statistical analysis and graph drawing. All the data for regeneration frequency were transformed using the arcsine function of the square root (sin−1 \(\sqrt x\), Steel and Torrie 1980). Analysis of variance (ANOVA) was carried out followed by variable multiple comparison at a probability of 5% using the LSD method.
Results
Somatic embryogenesis in mature embryos
Inoculated mature embryos (seeds) germinated on culture media within 2 weeks. Preliminary experiments with seedlings cultured on PGo medium supplemented with 8.9 μM BAP and 30 g l−1 sucrose indicated that breeding lines HB526 and SDM 3 showed high frequencies of somatic embryogenesis (20–40%). Lines SDM 11, 2, 5, 8 and 9 showed lower frequencies ranging from 0 to 15% of somatic embryogenesis. Seedlings cultured on medium containing BAP showed inhibited apical growth. Calli first appeared on cotyledons or hypcotyls in contact with the medium. The calli multiplied and some of the friable ones formed somatic embryos (Fig. 2a). The initiation of somatic embryos was not synchronous, and embryos could be found ranging from the globular stage through the torpedo stage to a cotyledon stage with well-developed apices. In a number of subsequent experiments, the more embryogenic lines, HB526 and SDM3, were used for further optimisation of the parameters required for efficient somatic embryogenesis.
MS medium, PGo medium and modified media (MSRV, PGoRV) were tested. It was found that PGo medium was superior to MS medium although the differences between the mean values did not reach a significant level (Fig. 3). PGoRV medium (PGo supplemented with additional amino acids and vitamins) further enhanced embryogenic callus production and somatic embryo formation.
Effect of different phytohormones on somatic embryogenesis
A range of concentrations of BAP (1.1–17.8 μM) was tested for the production of embryogenic calli or somatic embryos (Fig. 4), with 8.9 μM being found to be the most effective. Auxin (2,4-D) did not promote somatic embryogenesis from mature embryos (data not shown). Seeds germinated on PGo medium containing 0.5–9 μM 2,4-D showed very limited growth. The seedlings were chlorotic and did not produce any callus or somatic embryos.
Calli were produced from hypocotyls and/or cotyledons from seedlings of SDM 5, 9, 10 and 11, which were germinated from seeds and further cultured on MS media without any plant growth regulators. Shoots were regenerated from somatic embryos at a very low frequency (3%).
Effect of carbohydrates on somatic embryogenesis
Somatic embryos or embryogenic calli were produced from sugar beet breeding lines SDM 3 and HB 526 in PGo medium containing 30 g l−1 sucrose. Increasing the sucrose concentration to 60 or 90 g l−1 significantly reduced the embryogenic response (Fig. 5).
The frequency of embryogenic callus production was increased when these breeding lines were germinated on PGo medium containing maltose. The optimal concentration was 30 g l−1 and increasing the maltose concentration to 60 or 90 g l−1 did not give any additional positive response.
Effect of different genotypes on somatic embryogenesis
The full range of breeding lines was screened for their response to the optimised embryogenic culture procedure (Fig. 6). It was found that SDM 3 and HB526 were the most responsive genotypes in terms of frequency of seedlings forming embryogenic calli and somatic embryo production. SDM 2, 5, 8 gave a moderate response. Most of the SDM 9 calli were soft and white, and produced somatic embryos at a low frequency. SDM 11 produced brown calli, which were short-lived and did not produce any somatic embryos.
Multiplication of embryogenic calli and secondary somatic embryogenesis
Embryogenic callus cultures could be maintained and multiplied in PGo media containing BAP and TIBA, or NAA. Trimming coupled with mechanical wounding applied during subculture was useful for efficient multiplication of embryogenic cultures. No reduction in regeneration ability was observed after 6 months subculture in medium containing BAP and NAA. Secondary somatic embryogenesis was also achieved in the medium (Fig. 2b).
The effect of GA3 on the induction of somatic embryos from explant-derived calli
SDM 5, 9 and 11 cotyledons produced friable calli on PGo medium supplemented with BAP and TIBA. The calli were transferred to medium containing BAP or BAP + GA3 to test for the induction of somatic embryogenesis. It was found that GA3 had a stimulatory effect on somatic embryogenesis (Table 1). SDM 5 and SDM 9 cotyledon-derived calli produced somatic embryos at frequencies of 10% and 8% respectively in medium supplemented with BAP + GA3 but not in medium supplemented with BAP alone. SDM 11 calli did not respond to this treatment.
The effect of GA3 and mannitol on the development of somatic embryos in callus
Friable calli of SDM 9 and HB 526 produced from germinated seedlings on PGo medium containing 8.9 μM BAP were subcultured either directly into medium containing GA3 or first into medium containing mannitol for somatic embryo induction and development. Somatic embryos were produced from calli of SDM 9 and HB526 at frequencies of 5.3% and 7.5% respectively following the GA3 treatment (Table 1). Prior culture in medium containing mannitol further enhanced the frequency of embryogenesis to 10% and 13.3% for SDM9 and HB526 respectively. Somatic embryos were germinated in the same medium and routinely developed into shoots.
Somatic embryogenesis from immature embryos cultured in vitro
Several combinations of phytohormones were used for the induction of somatic embryogenesis from immature zygotic embryos (IEs) (Fig. 2c). The IEs either germinated or produced callus on MS media containing 2,4-D and/or BAP, K or NAA. Direct somatic embryogenesis from hypocotyls of germinated IEs was observed in MS medium containing 2,4-D in the dark (Fig. 2d). The frequency of somatic embryogenesis from HB526 in medium 1D (11.8%) was higher that 1D + 0.5B (9.3%) whist media containing high concentrations of cytokinins (BAP or K) alone, or in combination with NAA, did not produce a response. Frequencies of somatic embryogenesis from HB526 (9–12%) were higher than from SDM 10 (3–5%) (Table 2). Old or mature embryos did not produce somatic embryos. SDM 11 did not produce somatic embryos in any of the media tested. MS media supplemented 2,4-D, NAA and BAP were used for subculturing the somatic embryogenic tissue after size reduction. It was found that medium containing a high concentration of NAA and low concentrations of BAP and 2,4-D was effective for the maintenance of regeneration capacity for about 6 months.
Histological analysis of somatic embryogenesis in sugar beet
Embryogenic callus cultures at various stages were collected for histological analysis. Sugar beet embryogenic cells of CMS 22003 undergo asymmetric cell division (Fig. 7a). During the early stages of embryogenesis, chains of cells were seen growing from the surface of the explants (Fig. 7a, b). Figure 7b shows one such chain in the early stages of formation, with a large elongated cell attached to the explant via two smaller cells. Figure 7a shows a chain at a later stage of development showing a long suspensor-like structure of small square-profile cells supporting two heavily staining, elongated cells. After further culture globular embryos attached to the callus tissue via suspensor-like structures (Fig. 7c), and on the surface of the callus (Fig. 7d) appeared. Mature embryos with well-defined shoot meristems and hypocotyls subsequently developed (Fig. 7e). Similar results were obtained with other breeding lines (data not shown).
Histological analysis of somatic embryogenesis in sugar beet. (a, b) The surface of sugar beet embryogenic callus showing the growth of suspensor-like cell chains connecting large distal cells to the callus; (c) early stage globular embryo attached to the callus by a suspensor-like structure; (d) late stage globular embryo with multicellular attachment to the callus; (e) a mature somatic embryo with well-defined shoot meristem. Bars represent 0.5 mm (a, b, c, d, and e)
Recovery of intact plants
Somatic embryos germinated on MS or PGo media containing BAP, or without added plant growth regulators. The well-defined hypocotyl typically became necrotic without root formation and/or produced secondary somatic embryos. The shoot was dissected from the remaining and subcultured onto MS-hormone free medium for reestablishment of apical dominance and shoot elongation. Apically dominant shoot clumps were separated and transferred onto the same medium. About 60% of shoots were able to form roots and be transferred directly to compost. The rest were transferred to ½ MS medium containing 5.4 μM NAA in magenta boxes to induce root formation. After 4 weeks 85% of shoots were rooted and transferred to soil. Sugar beet lines were obtained with SDM 2 and HB 526 showed better rates of intact plant recovery (90%) than those of SDM 3 (80%) and SDM 8 (70%).
Nearly all the transplanted plants were established in a greenhouse. A low percentage (15%) of the regenerated plants in culture had thin leaves and long petioles with high betacyanin coloration, but when they were transferred to soil, they became phenotypically identical to seed-derived plants.
Discussion
A range of methods has been investigated for inducing somatic embryogenesis in the available sugar beet breeding lines and commercial varieties. The induction of embryogenic calluses and production of somatic embryos succeeded from seedlings derived from mature embryos (seeds) using BAP as a sole induction agent. The advantage of this approach over the use of immature embryos is that the mature embryos can be obtained and stored easily while their quality is assured. It was found that genotypes, culture media, phytohormones and carbohydrates significantly affected this response. There were differences between the genotypes in their response to this treatment. PGo medium was found to give a better response than MS for induction of embryogenic callus. The addition of extra vitamins and amino acids to either medium further increased embryogenic callus production. Freytag et al. (1988) and Catlin (1990) also obtained a high frequency of regeneration using a modified MS medium supplemented with additional vitamins and amino acids (termed RVIM), from petiole and cotyledon callus. Similarly, the frequency of morphogenesis (occasionally somatic embryogenesis) from the clone REL-1 was increased by using the RVIM medium (Owens and Eberts 1992). However, Tenning et al. (1992) did not get positive results when this medium was used for inducing embryogenesis from young zygotes in sugar beet.
BAP was found to be effective in inducing somatic embryogenesis from mature embryos while 2,4-D was inhibitory to this process. On the other end, 2,4-D was effective in somatic embryogenesis in immature embryos from sugar beet breeding lines. In this procedure we found that the use of 2,4-D and younger embryos are two critical factors for success. Somatic embryos are derived more often from hypocotyl tissues via a direct process. Tenning et al. (1992) reported a similar procedure with zygotic embryos of two breeding lines. Further optimisation of this procedure e.g. by inclusion of maltose or ABA and extension to other genotypes should be carried out. The difference between mature embryos and IEs may reflect the difference between the gene regulatory states of two tissues, e.g. genome imprinting has returned with mature embryos (Henderson and Jacobsen 2007).
BAP has been widely employed in the in vitro culture of sugar beet. It plays an important role in both the induction and regeneration of sugar beet via direct shoot organogenesis (Zhong et al. 1993; Grieve et al. 1997; Zhang et al. 2001b). BAP can also induce indirect somatic embryogenesis from germinated seedlings of a few of sugar beet germplasms on PGo medium (D’Halluin et al. 1992). TDZ has been used to induce axillary shoot proliferation, adventitious shoot formation and somatic embryogenesis in numerous plant species (Huetteman and Preece 1993; Murthy et al. 1995). We have shown that TDZ is more effective than BAP for the promotion of adventitious shoot formation from sugar beet (Zhang et al. 2001b). Our results also demonstrated that rapid somatic embryogenesis could be induced from seedlings of sugar beet, and that subsequent plant regeneration could be achieved after a period of exposure to TDZ. However, TDZ treatment was not invariably superior to BAP treatment, depending upon genotype. Interestingly, SDM 11 gave the best response to TDZ pre-treatment, yet was unresponsive to BAP (Zhang et al. 2001b).
GA3 was included in medium for somatic embryogenic culture in sugar beet (D’Halluin et al. 1992). We also found that GA3 increased somatic embryo production in sugar beet callus. GA3 enhanced somatic embryogenesis from Rumex acetosella callus (Culafic et al. 1987), Spinacia oleracea callus (Xiao and Branchard 1993) and Foeniculum vulgare petiole (Hunault and Maatar 1995), and the germination of somatic embryos into plantlets (Culafic et al. 1987). GA3 may act on somatic embryo development or the induction of embryogenic competence. For biotechnological improvement of sugar beet it may still be possible to enhance the SE efficiency of our system by exploiting other phytohormones or growth substances. The application of ethylene inhibitors (silver nitrate or aminoethoxyvinylglycine [AVG]), and polyamines (e.g. spermidine) was found to produce positive effects on somatic embryogenesis in white spruce and carrot (Kong and Yeung 1994; Roustan et al. 1994). ABA stimulated somatic embryogenesis in several plant species e.g. wheat (Brown et al. 1989), rice (Higuchi and Maeda 1990) and white spruce (Attree et al. 1995) by promoting early somatic embryo development and maturation. In suspension-cultured sugar beet cells, ABA was found to enhance somatic embryogenesis while TIBA did not have as much stimulatory effect (Tsai and Saunders 1995). It was reported that leaf explants derived from TIBA-treated plants showed an enhanced embryogenic response in a proline-supplemented medium (Moghaddam et al. 2000). In this study, we found that embryogenic cells can be maintained and multiplied in medium containing BAP and TIBA or NAA. We have previously shown that plant material cultured on this medium also had high transformation efficiencies (Zhang et al. 2001a). It will be interesting to see whether ABA, polyamines and AVG have the same effect in our system and on the transformation efficiencies of the treated tissue.
Maltose was found to give a better response than sucrose in the tissue culture of cereal species e.g. rice and wheat (Asano et al. 1994; Karsai et al. 1994). Maltose also enhances somatic embryogenesis from shoot apices in pea (Loiseau et al. 1995) and immature embryos in sunflower (Jeannin et al. 1995). In this work it was proved that maltose supported a better response than sucrose at a concentration of 30 g l−1. Sugars function as both a carbon source and as an osmotic regulator in culture media. The superiority of maltose over sucrose for the initiation of somatic embryos from cereal anthers or microspores has been explained in terms of the metabolism of sucrose leading to the accumulation of potential toxic products (Scott and Lyne 1994), or that maltose is degraded at a much slower rate than sucrose (Navarro-Alvarez et al. 1994).
Increasing osmolarity by increasing sugar concentration or by the addition of mannitol or polyethylene glycol 4,000 has enhanced somatic embryogenesis in several species e.g. pea (Loiseau et al. 1995), sunflower (Jeannin et al. 1995) and white spruce (Attree et al. 1995). High osmoticum treatment leads to osmotic stress, reduces water content and makes cells more compact and embryogenic. High osmoticum treatment may also lead to higher ABA content in the treated tissue. We found that 30 g l−1 of maltose was optimal for somatic embryogenesis in sugar beet. A further increase in the sugar concentration reduced the percentage of explants undergoing somatic embryogenesis. However, the inclusion of mannitol in the callus culture medium enhanced the embryogenic response. Mannitol is an osmotic regulator and is generally considered not to be metabolised in most of the higher plants (Kishor and Reddy 1986). Mannitol treatment may deliver an osmotic stress which leads to cellular alterations and an enhanced capacity for ABA synthesis in treated tissues. It also acts as an active oxygen scavenger to protect cells or organs from active oxygen toxity (Zhang et al. 1993; Shen et al. 1997). This work indicates that the use of mannitol promotes somatic embryogenesis in sugar beet.
The good responsiveness of sugar beet lines HB526 and SDM3 and promoting effect of GA3 and mannitol on SE reported here may be related to transcription of characterised somatic embryogenic genes. PEG treatment of pine somatic embryos increased the expression of several genes including the apparent homologs to the Arabidopsis genes ZWILLE/PINHEAD, FIDDLEHEAD, FUSCA, and SCARECROW, which are known to be involved in the formation of the embryo body plan and in the control of the shoot and root apical meristems (Stasolla et al. 2003). Changes in the transcript levels of many pine genes involved in sucrose catabolism and nitrogen assimilation and utilization were also observed. Auxin treatment of soybean cotyledons induced changes in the mRNA abundance of genes characteristic of oxidative stress, the synthesis of gibberellic acid and storage proteins and genes indicative of cell division in the adaxial side of the cotyledons (Thibaud-Nissen et al. 2003). This suggests that the arrangement of the new cells into organized structures might depend on a genetically controlled balance between cell proliferation and cell death. Homologous or orthologous sugar beet ESTs to characterised somatic embryogenic genes have been identified by BLAST searches, e.g. BQ583288 and BQ587788 to MtSERK1 (M. truncatula somatic embryogenesis receptor kinase 1), BQ594754 to AtBBM (Arabidopsis AP2-like ethylene-responsive transcription factor BABY BOOM), BQ590430 to translation initiation factor ZWILLE, CV301527 to homeobox transcription factor WUS and CK136457 to DcAGP1 (D. carota secreted basic proline-rich arabinogalactan protein). It is of interest to check the differential expression of these genes as BAP is the sole PGR in indirect somatic embryogenesis in sugar beet.
Procedures for multiplying the somatic embryogenic tissues that involved mechanical wounding during subculture were developed, and a large number of secondary embryos could normally be produced from the trimmed embryogenic tissues. The maintenance of regeneration capacity for mature embryo-derived embryogenic callus was achieved in medium supplemented with NAA and BAP rather than TIBA. The advantages of including vitamins or calcium salt in this application were recognised for several species (Freytag et al. 1988; Martin et al. 2007a; 2007b). The procedures for indirect somatic embryogenesis described in this paper are efficient and rapidly produce normal intact plants. Histological observation with embryogenic callus cultures indicated that somatic embryos were derived from single cells or a few cells. This may have some impact on investigations into molecular mechanisms of somatic embryogenesis or the development of routine transformation procedures for sugar beet.
Abbreviations
- BAP :
-
N6-Benzylaminopurine
- 2,4-D:
-
2,4 Dichlorophenoxyacetic acid
- GA3 :
-
Gibberellic acid
- IEs:
-
Immature zygotic embryos
- IAA:
-
3-Indole acetic acid
- K:
-
Kinetin
- MS:
-
Murashige and Skoog
- NAA:
-
1-Naphthalene acetic acid
- PGR:
-
Plant growth regulator
- SE:
-
Somatic embryogenesis
- TDZ:
-
Thidiazuron or N-phenyl-N′-1,2,3-thiadiazol-5-ylurea
- TIBA:
-
2,3,5-Triiodobenzoic acid
References
Asano Y, Ito Y, Ohara M, Sugiura K, Fujiie A (1994) Improved regeneration response of creeping bentgrass and japonica rice by maltose and lactose. Plant Cell Tiss Org Cult 39:101–103
Attree SM, Pomeroy MK, Fowke LC (1995) Development of white spruce. Picea glauca (Moench.) Voss) somatic embryos during culture with abscisic acid and osmoticum, and their tolerance to drying and frozen storage. J Exp Bot 46:433–439
Brown C, Brooks FJ, Pearson D, Mathias RJ (1989) Control of embryogenesis and organogenesis in immature wheat embryo callus using increased osmolarity and abscisic acid. J Plant Physiol 133:727–733
Catlin DW (1990) The effect of antibiotics on the inhibition of callus induction and regeneration from cotyledons of sugarbeet. Plant Cell Rep 9:285–288
Culafic L, Budimir S, Vujicic R, Neskovic (1987) Induction of somatic embryogenesis and embryo development in Rumex acetosella L. Plant Cell Tiss Org Cult 11:133–139
De Greef W, Jacobs M (1979) In vitro culture of the sugarbeet: description of a cell line with high regeneration capacity. Plant Sci Lett 17:55–61
D’Halluin K, Bossut M, Bonne E, Mazur B, Leemans J, Botterman J (1992) Transformation of sugarbeet (Beta vulgaris L.) and evaluation of herbicide resistance in transgenic plants. Biotechnology 10:309–313
Elliott MC, Chen DF, Fowler MR, Kirby MJ, Kubalakova M, Scott NW, Zhang CL, Slater A (1996) Towards the perfect sugar beet via gene manipulation. Sugar Crops China (1):23–30
Finnegan J, McElroy D (1994) Transgene inactivation: plants fight back. Bio/Technol 12:883–888
Freytag AH, Anand SC, Rao-Arelli AP, Owens LD (1988) An improved medium for adventitious shoot formation and callus induction in Beta vulgaris L. in vitro Plant Cell Rep 7:30–34
Grieve TM, Gartland KMA, Elliott MC (1997) Micropropagation of commercially important sugar beet cultivars. Plant Growth Reg 21:15–18
Hall RD, Riksen-Bruinsma T, Weyens GJ, Rosquin IJ, Denys PN, Evans IJ, Lathouwers JE, Lefbvre MP, Dunwell JM, Van Tunen A, Krens FA (1996) A high efficiency technique for the generation of transgenic sugar beets from stomatal guard cells. Nat Biotechnol 14:1133–1138
Henderson IR, Jacobsen SE (2007) Epigenetic inheritance in plants. Nature 447:418–424
Higuchi N, Maeda E (1990) Enhanced plant regeneration in rice callus cultures following abscisic acid accumulation and water stress in leaves of rice (Oryza sativa L.). Ann Bot 54:569–582
Huetteman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tiss Org Cult 34:71–76
Hunault G, Maatar A (1995) Enhancement of somatic embryogenesis frequency by gibberellic acid in fennel. Plant Cell Tiss Org Cult 41:171–176
Jeannin G, Bronner R, Hahne G (1995) Somatic embryogenesis and organogenesis induced on the immature zygotic embryo of sunflower (Helianthus annuus L.) cultivated in vitro: role of the sugar. Plant Cell Rep 15:200–204
Karsai I, Bedo Z, Hayes PM (1994) Effect of induction medium pH and maltose concentration on in vitro androgenesis of hexaploid winter triticale and wheat. Plant Cell Tiss Org Cult 39:49–53
Kishor PBK, Reddy GM (1986) Retention and revival of regenerating ability by osmotic adjustment in long-term cultures of four varieties of rice. J Plant Physiol 126:49–54
Kong L, Yeung EC (1994) Effects of ethylene and ethylene inhibitors on white spruce somatic embryo maturation. Plant Sci 104:71–80
Kubalakova M (1990) Somatic embryogenesis and cytoplasmic sterility in Beta vulgaris L. var Saccharifera. Biol Plant 32:414–419
Loiseau J, Marche C, Deunff YL (1995) Effects of auxins, cytokinins, carbohydrates and amino acids on somatic embryogenesis induction from shoot apices of pea. Plant Cell Tiss Org Cult 41:267–275
Martin KP, Zhang CL, Slater A, Madassery J (2007a) Control of shoot necrosis and plant death during micro-propagation of banana and plantains (Musa spp.). Plant Cell Tiss Org Cult 88:51–59
Martin KP, Shahanaz Beegum A, Zhang CL, Slater A, Madhusoodanan PV (2007b) In vitro propagation of Ophiorrhiza prostrata through somatic embryogenesis. Biol Planta 51:769–772
Moghaddam BE, Mesbah M, Yavari N (2000) The effects of in planta TIBA and proline treatment on somatic embryogenesi of sugar beet (Beta vulgaris L.). Euphytica 112:151–156
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Murthy BNS, Murch SJ, Saxena PK (1995) Thidiazuron induced somatic embryogenesis in intact seedlings of peanut (Arachis hypogaea): endogenous growth regulator levels and significance of cotyledons. Physiol Plant 94:268–276
Navarro-Alvarez W, Baenziger PS, Eskridge KM, Shelton DR, Gustafson VD, Hugo M (1994) Effect of sugars in wheat anther culture media. Plant Breed 112:53–62
Owens LD, Eberts DR (1992) Sugarbeet leaf disc culture: an improved procedure for inducing morphogenesis. Plant Cell Tiss Org Cult 31:195–201
Roustan J-P, Latche A, Fallot J (1994) Role of ethylene on induction and expression of carrot somatic embryogenesis: relationship with polyamine metabolism. Plant Sci 103:223–229
Scott P, Lyne RL (1994) Initiation of embryogenesis from cultured barley microspores: a further investigation into the toxic effects of sucrose and glucose. Plant Cell Tiss Org Cult 37:61–65
Shen B, Jensen RG, Bohnert HJ (1997) Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts. Plant Physiol 113:1177–1183
Snyder GW, Ingersoll JC, Smigocki AC, Owens LD (1999) Introduction of pathogen defense genes and a cytokinin biosynthesis gene into sugar beet (Beta vulgaris L.) by Agrobacterium or particle bombardment. Plant Cell Rep 18:829–834
Stasolla C, van Zyl L, Egertsdotter U, Craig D, Liu W, Sederoff RR (2003) The effects of polyethylene glycol on gene expression of developing white spruce somatic embryos. Plant Physiol 131:49–60
Steel RGD, Torrie JH (1980) Principles and procedures of statistics, a biometrical approach, 2nd edn. McGraw Hill, New York
Steward FC, Mapes MO, Mears K (1958) Growth and organized development of cultured cells. II organization in cultures grown from freely suspended cells. Am J Bot 45:705–708
Tenning P, Wremerth Weich E, Kjarsgaard U-B, Lelu M-A, Nihlgard M (1992) Somatic embryogenesis from zygotic embryos of sugarbeet (Beta vulgaris L.). Plant Sci 81:103–109
Tetu T, Sangwan RS, Sangwan-Norreel BS (1987) Hormonal control of organogenesis and somatic embryogenesis in Beta vulgaris callus. J Exp Bot 38:506–517
Thibaud-Nissen F, Shealy RT, Khanna A, Vodkin LO (2003) Clustering of microarray data reveals transcript patterns associated with somatic embryogenesis in soybean. Plant Physiol 132:118–136
Thorpe TA (1995) In vitro embryogenesis in plants. Current plant science and biotechnology in agriculture. Kluwer Academic Publishers, Dordrecht
Tsai C-J, Saunders JW (1995) Somatic embryos from callus of sugar beet biotechnology clone REL-1. J Sugar Beet Res 32:215–227
Xiao XG, Branchard M (1993) Embryogenesis and plant regeneration of spinach (Spinacia oleracea L.) from hypocotyls segments. Plant Cell Rep 13:69–71
Zhang CL, Chen DF, Elliott MC, Slater A (2004) Efficient procedures for callus induction and adventitious shoot organogenesis in sugar beet (Beta vulgaris L.) breeding lines. In Vitro Cell Dev Biol-Plant 40:475–481
Zhang CL, Chen DF, McCormac AC, Scott NW, Elliott MC, Slater A (2001a) Use of the GFP reporter as a vital marker for Agrobacterium-mediated transformation of sugar beet (Beta vulgaris L.). Mol Biotechnol 17:109–117
Zhang CL, Chen DF, Elliott MC, Slater A (2001b) Thidiazuron-induced organogenesis and somatic embryogenesis in sugar beet (Beta vulgaris L.). In Vitro Cell Dev Biol-Plant 37:305–310
Zhang CL, Jiao D, Tong H (1993) Varietal difference of photooxidation in rice and effects of active oxygen scavengers. Chinese J Rice Sci 7:175–178
Zhang CL, Peng SF, Guo JN (1994) The regulation and improvement of seed quality in sugar beet. I. effects of PEG osmoconditioning and hydration-dehydration on germination of seeds with different vigor. China Sugarbeet (1):13–18
Zhong Z, Smith HG, Thomas TH (1993) In vitro culture of petioles and intact leaves of sugar beet (Beta vulgaris). Plant Growth Reg 12:59–66
Zimmerman JL (1993) Somatic embryogenesis: a model for early development in higher plants. Plant Cell 5:1411–1423
Acknowledgements
The authors are grateful to MAFF and De Montfort University for supporting the project. We thank Dr. E. S. Mutasa-Göttgens for her comments on the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, CL., Chen, DF., Kubalakova, M. et al. Efficient somatic embryogenesis in sugar beet (Beta vulgaris L.) breeding lines. Plant Cell Tiss Organ Cult 93, 209–221 (2008). https://doi.org/10.1007/s11240-008-9364-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9364-2