Abstract
A novel protocol for indirect shoot organogenesis of Dieffenbachia cv. Camouflage was established using leaf explants excised from in vitro shoot cultures. The frequency of callus formation reached 96% for explants cultured on Murashige and Skoog (1962) basal medium supplemented with 5 μM thidiazuron and 1 μM 2,4-dichlorophenozyacetic acid. The number of shoots regenerated was high, with up to 7.9 shoots produced per callus cultured on basal medium supplemented with 40 μM N 6-(Δ2-isopentenyl)adenine and 2 μM indole-3-acetic acid. Regenerated shoots rooted well in a soilless substrate, acclimatized ex vitro at 100%, and grew vigorously under shaded greenhouse conditions. Somaclonal variations in leaf variegation, color, and morphology have been observed in regenerated plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Dieffenbachia Schott is a member of the family Araceae and is composed of 30 species native to South and Central America (Mayo et al. 1997). Dieffenbachia has been produced as an ornamental foliage plant for interiorscaping since 1864 (Birdsey 1951) and consistently ranks among the top five most popular foliage plant genera based on annual wholesale value (McConnell et al. 1989; USDA, 1999). In addition to its tolerance to low light levels, another factor contributing to its popularity is the increasing release of new and attractive cultivars that provide consumers with a wide range of selection for novelty.
Interspecific hybridization has been the primary method of developing new cultivars (Henny and Chen 2003). Hybridization of Dieffenbachia, however, has been hampered by naturally occurring dichogamy, a long breeding cycle, and limited seed production (Henny 1988). With the commercial application of in vitro propagation of Dieffenbachia, new cultivars have been released following selection of somaclonal variants (Chen and Henny 2006). The frequency of somaclonal variants is generally high, and the time required for a new cultivar release can be only 2–3 years compared to 7–10 years required using traditional breeding methods (Skirvin et al. 1994; Chen et al. 2003; Henny and Chen 2003). Chen et al. (2004) analyzed genetic relatedness of some cultivated Dieffenbachia using amplified fragment length polymorphism and found that cultivars selected from somaclonal variants differ genetically from their parents.
Successful use of in vitro techniques for producing somaclonal variants depends on the establishment of an efficient method for regenerating a large number of plants indirectly from an intervening callus stage (Maralappanavar et al. 2000; Niwa et al. 2002; Hossain et al. 2003; Anu et al. 2004; Arce-Montoya and Rodriguez-Alvarez 2006; Hammerschlag et al. 2006). Although plant regeneration via indirect shoot organogenesis has been achieved in a vast array of plant species, a protocol for indirect shoot organogenesis has not been developed in Dieffenbachia. Currently, commercial in vitro propagation of Dieffenbachia is through shoot culture (Knauss 1976; Chase et al. 1981; Voyiatzi and Voyiatzis 1989; Henny et al. 2000). The objective of this study was to establish a protocol for inducing indirect shoot organogenesis in Dieffenbachia cv. Camouflage.
Materials and methods
Media and sterilization conditions
A basal medium (BM) consisting of MS (Murashige and Skoog 1962) mineral salts, 0.4 mg l−1 thiamine, 2.0 mg l−1 glycine, 100.0 mg l−1 myo-inositol, 0.5 mg l−1 pyridoxine, 0.5 mg l−1 nicotinic acid, and 25 g l−1 sucrose in combination with plant growth regulators (PGRs) was used. Medium was adjusted to pH 5.8 with 0.1 N KOH prior to the addition of 6 g l−1 TC agar (PhytoTechnology Laboratories, Shawnee Mission, KS) and autoclaved at 1.2 kg cm−2 for 20 min.
Plant materials and establishment of shoot cultures
Stem segments about 10-mm long, containing lateral buds, were cut from stock plants of Dieffenbachia cv. Camouflage. The stock plants were maintained in a shaded greenhouse with a maximum irradiance of 345 μmol m−2 s−1 under natural photoperiod (10–14.5 h light) and a temperature range 20–31°C. Lateral buds were excised, rinsed under running water for about 10 min, and used as explants to initiate in vitro shoot cultures. After surface sterilization in aqueous 1.2% sodium hypochlorite (20%, v/v, Clorox Ultra) containing several drops of Tween-20 for 10 min on a shaker and rinsing three times, 5 min each, with sterile water, lateral buds were further trimmed by removing the outermost one or two bud scales. Lateral buds still attached to a 2-mm2 thick square of stem tissue were cut and placed individually into 25 × 150 mm2 culture tubes containing 15-ml BM supplemented with 80 μM N 6-(Δ2-isopentenyl)adenine (2iP) and 2 μM indole-3-acetic acid (IAA). Cultures were maintained under a 16-h light photoperiod at 40 μmol m−2 s−1 provided by cool white fluorescent lamps (General Electric F20WT12CW) at 22 ± 3°C. Shoot clusters were divided and transferred to the same fresh medium every 8 weeks to increase in vitro stock shoot cultures.
Indexing of established cultures
Following the establishment of in vitro shoot cultures, cultures with visually detectable contamination were immediately discarded. Cultures with no symptom of contamination were routinely indexed for the presence of cultivable bacterial and fungal contamination using the procedure developed by Kane (2000).
Callus induction
Leaves obtained from the in vitro shoot cultures served as explant sources for callus induction. Leaf explants were cut into 5-mm2 sections with mid-vein, and the leaf margins removed with a scalpel. Leaf explants were cultured with abaxial surface in contact with the callus induction medium. A series of eight screening experiments were completed to select the most effective PGR concentrations and combinations for callus induction. Each experiment consisted of factorial PGR combinations with PGR-free medium serving as the control: (1) 6-benzyladenine (BA) at 0, 1, 10, 50 μM and 2,4-dichlorophenozyacetic acid (2,4-D) at 0, 1, 10, 50 μM; (2) [N-(2-chloro-4-pyridyl)-N-phenylurea (CPPU) at 0, 1, 2.5, 5 μM and 2,4-D at 0, 2, 4, 8, 10 μM; (3) CPPU at 0, 1, 2.5, 5 μM and 1-naphthalene acetic acid (NAA) at 0, 2, 4, 8, 10 μM; (4) kinetin at 0, 1, 5, 10 μM and IAA at 0, 1 μM; (5) dicamba at 0, 1, 3, 9 μM and 2,4-D at 0, 1 μM; (6) picloram at 0, 1, 3, 9 μM and 2, 4-D at 0, 1 μM; (7) thidiazuron (TDZ) at 0, 1, 10, 50 μM and NAA at 0, 1, 10, 50 μM; (8) TDZ at 0, 1, 5, 10 μM and 2,4-D at 0, 0.5, 1 μM. Explants were cultured in 100 × 15 mm2 sterile Petri plates containing 20 ml of medium. There were five explants per Petri plate and five replicate plates per treatment. Cultures were initially maintained in dark for 8 weeks and then transferred to the 16-h light photoperiod at 40 μmol m−2 s−1 for another 4 weeks. The number of explants forming calli was scored after 12 weeks of culture. Callus formation frequency was calculated as the percentage of leaf explants forming calli.
Indirect shoot organogenesis
To evaluate medium for inducing shoot formation from callus, calli produced on callus induction medium containing 5 μM TDZ and 1 μM 2,4-D (the medium yielding optimal callus induction) were cultured on BM supplemented with factorial combinations of (1) 2iP at 0, 20, 40, 80 μM and IAA at 0 and 2 μM; (2) kinetin at 0, 1, 2, 4 μM and GA3 at 0, 5, 10 μM. PGR-free medium served as the control. Calli were separated from leaf explants after 12 weeks of callus production. Calli were cut into small pieces with a fresh weight of approximately 150 mg, and then transferred to glass baby food jars (4.5 × 7 cm2) containing 40 ml of medium. There were five callus clumps per jar and five replicates per treatment. The number of shoots formed per callus was determined after 8 weeks culture under the 16-h photoperiod at 40 μmol m−2 s−1.
Histological analysis
To verify the occurrence of indirect shoot organogenesis, callus samples were collected every 3 days after callus clumps were transferred onto shoot induction medium until 42 days culture. For optical light microscopy (OLM), callus specimens approximately 5 mm3 were fixed in Trumps fixative (McDowell and Trump 1976). Fixative infiltration was achieved under vacuum until all samples sank to the bottom of the vials. Calli were rinsed three times in phosphate buffer (pH 7.2) for 10 min each. Calli were postfixed in a 1% buffered osmium tetroxide solution for 1 h, and then rinsed three times in phosphate buffer (pH 7.2) for 10 min each, followed by three rinses in distilled water. Calli were dehydrated in a series of ascending aqueous ethanol solutions at 25, 50, 75% for 30 min each, at 95, 100% for 1 h each, followed by dehydration in 100% acetone for 1 h. Calli were then embedded in Spurr resin (Spurr 1969). Callus sections (10 μm) were cut using a Leica Ultracut rotary ultramicrotome R (Leica Microscopy and Scientific Instruments, Deerfield, IL), and mounted on glass slides. Sections were stained with 0.2% toludine blue and examined under an Olympus BH-2 Epifluorescent Microscope (Olympus America, Inc., Melville, NY). Photographs were taken using a Pixera 120C digital camera.
Ex vitro transfer and acclimatization of shoots
Shoots, some with roots, were removed from the baby food jars and excised individually from the callus clumps. Medium was carefully rinsed off shoots. Shoots longer than 20 mm with 2–3 leaves were planted individually in 60-cell plug trays (4.5 × 4 × 5 cm3 each cell, REB Plastics, Inc, Apopka, FL) containing a 2:1:1 (v/v/v) soilless mixture of Canadian peat:vermiculite:perlite. All plantlets were maintained in a greenhouse under shade cloth with a maximum irradiance of 345 μmol m−2 s−1, natural photoperiod (10–14.5 h light), and a temperature range 20–31°C. Plugs were hand-watered twice a week. Peters 20N–10P–20K liquid fertilizer (200 mg l−1 N; The Scotts Company, Marysville, OH) was applied weekly following 2 weeks acclimatization.
Statistical analysis
All experiments were established in a completely randomized design. The experiments showing response were repeated once. Data were subject to analysis of variance and regression analysis using SAS (SAS Institute, Inc., 1999). Mean separation was achieved by least significant difference test at 95% level.
Results
Callus induction
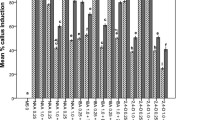
The PGR combinations and concentrations screened were totally ineffective for inducing callus formation in seven out of the eight experiments. Callus formed only on BM supplemented with the factorial combinations of TDZ at 1, 5, 10 μM and 2,4-D at 0, 0.5, 1 μM. Other PGR combinations failed to induce callus formation. Additionally, the frequency of callus occurrence from medium containing TDZ and 2,4-D differed significantly based on their concentrations. No callus formation was observed on medium without PGR (the control) or with 2,4-D alone at 0.5 and 1.0 μM. However, TDZ alone at concentrations of 1, 5, and 10 μM induced 4, 10, and 26% of explants to produce calli, respectively. The higher frequency of callus formation occurred in medium supplemented with both TDZ and 2,4-D. Induction medium containing 5 μM TDZ and 1 μM 2,4-D resulted in the maximum of 96% of explants to produce calli (Table 1).
Indirect shoot organogenesis
A low number of shoots developed from calli cultured on BM alone (1.6 shoots/callus) or BM supplemented with 2 μM IAA (1.2 shoots/callus) (Table 2). BM supplemented with 2iP alone at concentrations of 20, 40, and 80 μM elevated shoot numbers to 3.6, 6.3, and 4.0 per callus, respectively. Combining 2iP with IAA further increased shoot numbers compared to 2iP at respective concentrations alone, but the increase was not statistically significant. Highest shoot number (7.9 shoots/callus) occurred on BM supplemented with 40 μM 2iP and 2 μM IAA (Table 2). No shoot formation was observed on BM supplemented with combinations of kinetin and GA3.
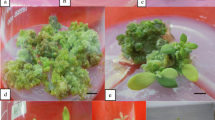
Green nodular calli were observed on leaf explants after 12 weeks of culture on BM supplemented with 5 μM TDZ and 1 μM 2,4-D (Fig. 1a). When calli were separated from primary leaf explants and transferred onto BM supplemented with 40 μM 2iP and 2 μM IAA, small green meristems were visible on the surface of calli within 4 weeks (Fig. 1b) and later developed into shoot buds (Fig. 1c). Leaf formation and shoot elongation occurred in the following 2 weeks (Fig. 1d). Single shoot or shoot clusters with leaves and roots were developed by the end of 8 weeks of culture (Fig. 1e).
Indirect shoot organogenesis in Dieffenbachia cv. Camouflage. (a) Induction of calli on leaf explants on BM supplemented with 5 μM TDZ and 1 μM 2,4-D after 8 weeks culture in dark and 4 weeks culture in 16 h photoperiod. Bar = 1 mm. (b) Small green meristems formed on the surface of calli on BM containing 40 μM 2iP and 2 μM IAA within 4 weeks. Bar = 1 mm. (c) Shoot buds formed from these meristems 2 weeks later. Bar = 1 cm. (d) Shoots and leaf development. Bar = 1 cm. (e) Well-developed shoots with roots within 8 weeks of culture. Bar = 1 cm. (f) Acclimatized plants in the greenhouse exhibiting variation in leaf variegation and color. Bar = 5 cm
The regeneration of shoots via indirect shoot organogenesis was confirmed using histological techniques. Although the appearance of nodular calli resembled somatic embryos, only unipolar structures (shoot or root meristems) were observed under OLM (Fig. 2a and b). No bipolar structures (unique feature of somatic embryos) could be identified. Vascular connections were also detected between developing shoots and callus tissue which was another distinct feature of shoot organogenesis (Fig. 2c).
Histological verification of shoot organogenesis from calli cultured on BM supplemented with 40 μM 2iP and 2 μM IAA in Dieffenbachia cv. Camouflage. (a) Development of apical meristem (AM) and leaf primordia (LP) by day 18. Bar = 500 μm. (b) Root formation by day 39. Bar = 250 μm. (c) Vascular connection between developing shoot and callus tissue. Bar = 500 μm
Acclimatization
Shoot and root formation were observed in most Dieffenbachia cultures (Fig. 1e). Of 2,248 shoots (both rooted and unrooted), 100% survival rate was observed. Acclimatized plants exhibited variation in leaf variegation and color with a total somaclonal variation rate of 40.4% (Fig. 1f).
Discussion
A plant regeneration system via indirect shoot organogenesis was established in this study. To our knowledge, this is the first report of indirect shoot organogenesis in Dieffenbachia. Our observations indicated that Dieffenbachia, in general, was recalcitrant in regard to shoot organogenesis. This may, in part, explain why in vitro regeneration via organogenesis in Dieffenbachia has not been previously reported.
The type, concentration, and combination of PGRs are the key factors influencing indirect shoot organogenesis in Dieffenbachia. Similar PGR effects on callus formation have been reported in other species (Khanam et al. 2000; Reddy et al. 2001; Ma and Xu 2002; Giridhar et al. 2004; Azad et al. 2005; Datta and Majumder 2005; Zhou and Brown 2005). 2,4-D has been shown to be most effective for callus induction in many species. In contrast, our study showed that 2,4-D was not a prerequisite for callus initiation as calli were induced on BM without 2,4-D. However, TDZ ranging from 1 to 10 μM was essential to induce callus formation in Dieffenbachia cv. Camouflage. There have been several reports of significant TDZ effects on callus formation and shoot organogenesis in other species (Gurriaran et al. 1999; Mithila et al. 2003; Datta and Majumder 2005; Landi and Mezzetti 2005). TDZ, a cotton defoliant, has both auxin- and cytokinin-like activity and can be substituted for auxins or the combination of auxins and cytokinins (Singh et al. 2003). Hutchinson et al. (1996) reported that TDZ could result in an increase in endogenous levels of auxins. It could be possible that TDZ might have fulfilled both auxin- and cytokinin-like roles in callus induction of this Dieffenbachia.
Cytokinin type and concentration have significant effects on subsequent shoot regeneration from calli. Results from this study indicated that 2iP was more effective than kinetin because no shoot regeneration was observed on BM supplemented with kinetin alone or combination with GA3. Voyiatzi and Voyiatzis (1989) also found that 2iP was more effective in inducing lateral shoot multiplication in Dieffenbachia than kinetin, and 80 μM 2iP with 2 μM IAA was optimal for shoot formation of Dieffenbachia exotica cv. Marianna. The present results suggested that 40 μM 2iP with 2 μM IAA was optimal for shoot organogenesis of cv. Camouflage. This difference might be due to the fact that different cultivars and explants were used. This study used calli derived from leaves, while axillary buds and shoot tips were used for shoot multiplication of cultivar Marianna (Voyiatzi and Voyiatzis 1989). Differential responses of genotypes and explant sources to PGR requirements have been well documented (Magioli et al. 1998; Choi et al. 2003).
Dieffenbachia shoots regenerated in vitro proved to be easily adaptable to ex vitro conditions. No separate in vitro rooting stage was required as shoot survival rate was 100% in a soilless substrate. This is different from many other species where rooting is an obstacle for plant establishment (Reed 1995; Gavidia et al. 1996; Pruski et al. 2000). Dieffenbachia cv. Camouflage belongs to an easy-to-root type, which is in contrast to its very limited shoot formation ability. It was conceivable that endogenous level of auxin in Dieffenbachia might be sufficient for root formation. A promotive carry over effect of the IAA in the shoot induction medium on rooting was also possible.
The ability to regenerate shoots from calli has several advantages. A great number of shoots can be produced from an explant through callus induction and shoot formation. Indirect shoot organogenesis has a greater potential for regenerating somaclonal variants. In Dieffenbachia cv. Camouflage, phenotypical variations in leaf variegation and color of acclimatized plants were observed. The feasibility of inducing indirect shoot organogenesis in other Dieffenbachia cultivars, and then evaluating this regeneration protocol for potential isolation of somaclonal variants will be described in a subsequent study.
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenozyacetic acid
- 2iP:
-
N 6(Δ2-isopentenyl)adenine
- BA:
-
6-benzyladenine
- CPPU:
-
N(2-chloro-4-pyridyl)-N-phenylurea
- IAA:
-
indole-3-acetic acid
- NAA:
-
1-naphthalene acetic acid
- TDZ:
-
thidiazuron
References
Anu A, Babu KN, Peter KV (2004) Variations among somaclones and its seedling progeny in Capsicum annuum. Plant Cell Tiss Org Cult 76:261–267
Arce-Montoya M, Rodriguez-Alvarez M (2006) Micropropagation and field performance of Yucca valida. Plant Cell Rep 25:777–783
Azad MAK, Yokota S, Ohkubo T, Andoh Y, Yahara S, Yoshizawa N (2005) In vitro regeneration of the medicinal woody plant Phellodendron amurense Rupr. through excised leaves. Plant Cell Tiss Org Cult 80:43–50
Birdsey MR (1951) The cultivated aroids. The Gillick Press, Berkeley, CA
Chase AR, Zettler FW, Knauss JF (1981) Perfection-137B: a pathogen-free selection of Dieffenbachia maculata derived through tissue culture. Circular S-280, Florida Agricultural Experiment Stations, Institute of Food and Agricultural Sciences, University of Florida, pp 1–7
Chen J, Devanand PS, Norman DJ, Henny RJ, Chao CT (2004) Analysis of genetic relatedness of Dieffenbachia cultivars using AFLP markers. J Am Soc Hort Sci 129:81–87
Chen J, Henny RJ (2006) Somaclonal variation: an important source for cultivar development of floriculture crops. In: Teixeira da Silva JA (eds) Floriculture, ornamental and plant biotechnology II. Global Science Books, London, pp 244–253
Chen J, Henny RJ, Chao CT (2003) Somaclonal variation as a source for cultivar development of ornamental aroids. Recent Res Dev Plant Sci 1:31–43
Choi PS, Cho DY, Soh WY (2003) Plant regeneration from immature embryo cultures of Vigna unguiculata. Biol Plant 47:305–308
Datta MM, Majumder A (2005) Organogenesis and plant regeneration in Taxus wallichiana (Zucc.). Plant Cell Rep 25:11–18
Gavidia I, Perez-Bermudez P, Segara J (1996) Micropropagation of bay laurel (Daphne gnidium). J Hort Sci 71:977–983
Giridhar P, Kumar V, Ravishankar GA (2004) Somatic embryogenesis, organogenesis, and regeneration from leaf callus culture of Decalepis hamiltonii Wight & Arn., an endangered shrub. In Vitro Cell Dev Biol Plant 40:567–571
Gurriaran MJ, Revilla MA, Tames RS (1999) Adventitious shoot regeneration in cultures of Humulus lupulus L. (hop) cvs. Brewers Gold and Nugget. Plant Cell Rep 18:1007–1011
Hammerschlag F, Garces S, Koch-Dean M, Stephanie R, Lewers K, Maas J, Smith BJ (2006) In vitro response of strawberry cultivars and regenerants to Colletotrichum acutatum. Plant Cell Tiss Org Cult 84:255–261
Henny RJ (1988) Ornamental aroids: culture and breeding. Hort Rev 10:1–33
Henny RJ, Chen J (2003) Cultivar development of ornamental foliage plants. Plant Breed Rev 23:245–289
Henny RJ, Goode L, Ellis W (2000) Micropropagation of Dieffenbachia. In: Trigiano RN, Gray DJ (eds) Plant tissue culture concepts and laboratory exercises, 2nd ed. CRC, Boca Raton, pp 97–102
Hossain MdA, Konisho K, Minami M, Nemoto K (2003) Somaclonal variation of regenerated plants in chili pepper (Capsicum annuum L.). Euphytica 130:233–239
Hutchinson MJ, Krishnaraj S, Saxena PK (1996) Morphological and physiological changes during thidiazuron-induced somatic embryogenesis in geranium (Pelargonium × hortorum Bailey) hypocotyl cultures. Int J Plant Sci 157:440–446
Kane ME (2000) Culture indexing for bacterial and fungal contaminant. In: Trigiano RN, Gray DJ (eds) Plant tissue culture concepts and laboratory exercise, 2nd ed. CRC, Boca Raton, pp 427–431
Khanam N, Khoo C, Khan AG (2000) Effects of cytokinin/auxin combination on organogenesis, shoot regeneration and tropane alkaloid production in Duboisia myoporoides. Plant Cell Tiss Org Cult 62:125–133
Knauss JF (1976) A tissue culture method for producing Dieffenbachia picta cv. ‘Perfection’ free of fungi and bacteria. Proc Fla State Hort Soc 89:293–296
Landi L, Mezzetti B (2005) TDZ, anxin and genotype effects on leaf organogenesis in Fragaria. Plant Cell Rep 25:281–288
Ma G, Xu W (2002) Induction of somatic embryogenesis and adventitious shoots from immature leaves of cassava. Plant Cell Tiss Org Cult 70:281–288
Magioli C, Rocha APM, de Oliveira DE (1998) Efficient shoot organogenesis of eggplant (Solanum melongena L.) induced by thidiazuron. Plant Cell Rep 17:661–663
Maralappanavar MS, Kuruvinashetti MS, Harti CC (2000) Regeneration, establishment and evaluation of somaclones in Sorghum bicolor (L.) Moench. Euphytica 115:173–180
Mayo SJ, Bogner J, Boyce PC (1997) The genera of Araceae. Royal Bot. Gardens, Kew
McConnell DB, Henley RW, Kelly CB (1989) Commercial foliage plants: twenty years of changes. Proc Fla State Hort Soc 102:297–303
McDowell EM, Trump BF (1976) Histological fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med 100:405
Mithila J, Hall JC, Victor JMR, Saxena PK (2003) Thidiazuron induces shoot organogenesis at low concentrations and somatic embryogenesis at high concentrations on leaf and petiole explants of African violet (Saintpaulia ionantha Wendl.) Plant Cell Rep 21:408–414
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–495
Niwa M, Arai T, Fujita K, Marubashi M, Inoue E, Tsukihashi T (2002) Plant regeneration through leaf culture of yacon. J Jpn Soc Hort Sci 71:561–567
Pruski KW, Lewis T, Astatkie T, Nowak J (2000) Micropropagation of chokecherry and pincherry cultivars. Plant Cell Tiss Org Cult 63:93–100
Reed BM (1995) Screening Pyrus germplasm for in vitro rooting response. HortScience 30:1292–1294
Reddy PS, Rodrigues R, Rajasekharan R (2001) Shoot organogenesis and mass propagation of Coleus forskohlii from leaf derived callus. Plant Cell Tiss Org Cult 66:183–188
SAS Institute, Inc. (1999) Version 8.02. SAS Institute, Cary, NC
Singh ND, Sahoo L, Sarin NB, Jaiwal PK (2003) The effect of TDZ on organogenesis and somatic embryogenesis in pigeonpea (Cajanus cajan L. Mill.). Plant Sci 164:341–347
Skirvin RM, McPheeters KD, Norton M (1994) Sources and frequency of somaclonal variation. HortScience 29:1232–1236
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastr Res 26:31
USDA (1999) 1998 census of horticultural specialties. United States Department of Agriculture, Washington, DC
Voyiatzi C, Voyiatzis (1989) In vitro shoot proliferation rate of Dieffenbachia exotica cultivar ‘Marianna’ as affected by cytokinins, the number of recultures and the temperature. Sci Hort 40:163–169
Zhou S, Brown DCW (2005) High efficiency plant production of North American ginseng via somatic embryogenesis from cotyledon explants. Plant Cell Rep 25:166–173
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, X., Chen, J. & Kane, M.E. Indirect shoot organogenesis from leaves of Dieffenbachia cv. Camouflage. Plant Cell Tiss Organ Cult 89, 83–90 (2007). https://doi.org/10.1007/s11240-007-9214-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-007-9214-7