Abstract
Yucca valida is an important potential source of steroidal saponins closely related to Yucca schidigera, the species that is commercially exploited from the wild as a source of steroidal extracts. Neither of the species has been domesticated mainly because of their slow growth and long life span before harvesting. Here, we report a micropropagation method to generate isogenic or clonal lines for plantation purposes. Seventeen clonal lines were propagated and evaluated over a period of 26 months in an experimental plantation and compared with the performance of plants from seeds. The large variability found between the plants derived from seeds is manifested in the differences observed between the different clonal lines; however, these present a much smaller internal coefficient of variation than the one observed in the population of plants derived from seeds. Some clonal lines perform in a superior manner indicating that a process of selection and cloning can generate lines of fast growing individuals for plantations that can satisfy the demand for these materials without putting a natural resource at risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Yucca valida Brandegee is a native species from Baja California Sur (BCS), Mexico, which is a potential source of steroidal saponins used by the agrochemical industry to reduce the toxic levels of ammonia in animal farms, as an activator in oxidation ponds, and to eliminate bad odors in the excreta of pet animals. At present, the main sources of steroidal saponins are the stems of Yucca schidigera, a closely related species whose distribution covers the north of the Baja California peninsula and the south west of the United States of America (Cheeke 2000; García-Mendoza and Galván 1995). The stems of wild plants constitute the raw material for extraction but the slow growth and large variability in biomass in the areas under exploitation make it difficult to collect enough material to meet the international demand. Annually between 3,000 and 5,000 tonnes of stems are extracted to be transformed into different products (Castellón-Olivares et al. 2002). The Mexican official norm NOM 005-RECNAT-1997 only allows the extraction of stems from mature plants where the stem represents 80% of the plant and only 50% of the mature individuals are permitted to be harvested from the natural populations (Diario Oficial 1997).
Therefore, domestication and the establishment of commercial plantations are the best alternatives for a sustainable exploitation. This, however, requires (1) adequate propagation systems, (2) more homogeneous high yielding materials, and (3) appropriate agricultural practices for the management of the plantations.
The use of tissue culture techniques to assist in the selection and propagation of high quality materials is an obvious option. Several Yucca species such as Y. filamentosa (Supniewski 1972); Y. filifera (Quintero et al. 1982) and Y. schidigera have been studied for their capacity to produce steroidal saponins during in vitro culture and some ornamental species like Y. glauca (Bentz et al. 1988), Y. elephantipes (Pierik and Steegmans 1983), and Y. aloifolia (Atta-Alla and Van Staden 1997) have been propagated in vitro. None of the earlier studies, however, report the performance of the micropropagated plants in the field.
In this paper, we present the in vitro propagation of Y. valida, its establishment in an experimental plantation, and the evaluation of the performance of 17 clonal lines compared with plants derived directly from seeds as a first step for the selection and management of elite materials for commercial plantations.
Materials and methods
Biological material and seed germination
Yucca valida seeds collected in February 2000 in the valley of La Paz, BCS, Mexico (23°20′47″ N, 110°16′14″ W) were used to define an in vitro propagation system for the generation of clonal lines and for the establishment of an experimental plantation. The seeds were superficially disinfested by immersing them in 70% ethanol for 60 s and then in 10% sodium hypochlorite (commercial bleach) for 10 min before washing them five times with sterile distilled water.
The seeds were then placed on semisolid MSB, a modified MS medium (Robert et al. 1987) without plant growth regulators and supplemented with 3% sucrose and 0.8% agar (Sigma A-1296), and incubated at 25±2°C under continuous illumination (32.8 μEm−2 s−1).
For in vivo germination, seeds were placed on plastic trays, previously disinfested by washing them with sodium hypochlorite and sterile distilled water, with three layers of absorbent paper damped with sterile distilled water, covered with a transparent top and sealed with plastic wrap and incubated in a growth chamber (Percival MB-60B) at 27±2°C with a photoperiod of 12 h/12 h (32.8 μEm−2 s−1) for 42 days. Water was added whenever it was required to maintain the humidity of the paper.
In vitro multiplication
For clonal propagation, the leaves and roots were removed from plants germinated in vitro and the base of the stems were transferred to MSB medium supplemented with 1 μM IAA and 5 μM BA. After 3 weeks of incubation under the previously described conditions the new shoots were individualized and transferred to fresh medium for a new round of multiplication. For the experiments with plant growth regulators, micropropagated plants from lines P4, Y25, and P14 were previously cultured for 2 months in MSB without plant growth regulators.
Rooting and acclimatization of micropropagated plants
Two groups of plants from line P4 of different sizes: short (∼2 cm) and large (∼4.0 cm), were rooted on MSB medium without plant growth regulators. After 3 weeks, the formation of roots and the development (size and number of leaves) of the plants were evaluated.
Rooted plants were treated with a fungicide (Vitisan 50%) and transferred to plastic trays with 5 cm × 5 cm cavities and filled with different substrates: Cosmopeat (Cosmocel S.A.), Sun-shine (Sun Gro Horticulture Canada Ltd.) and a mixture of Sun-shine and local soil (1:2). The trays were placed on a water bed in a shaded acclimatization tunnel for 4 weeks before removing the plastic cover. After 6 weeks the survival rate was evaluated. The plants were maintained without the plastic cover until they were transplanted to the experimental field.
Establishment of micropropagated plants in an experimental plantation
A minimum of 12 micropropagated plants from each of the 17 clonal lines multiplied on MSB with 1 μM IAA and 5 μM BA, and rooted and preadapted as described were planted in November 2002 at El Carrizal, BCS (23°20′47″ N, 110°16′14″ W) at distances of 1.80 × 2.0 m over a surface of 1,782 m2. The plot was cleaned and treated with a herbicide (Ronstar, Bayer AG) 3 months before the planting. The soil was alluvial of secondary origin with a crumb-sandy texture, without superficial rockiness and pH 7.2. Throughout the cultivation period 26.16 l m−2 irrigation was applied per week.
Measurements of plant height, length of leaves, and diameter of rosettes were taken during the cultivation period reported. Annual growth rate was estimated from plant height data. All data were analyzed using the STATISTICA software package (Stat Soft Inc.) and the percentage values were transformed to arcosen values before statistical analysis.
Results and discussion
The objective of this work was to establish a method for the selection and propagation of clonal lines of Yucca valida that can be efficiently cultivated for the extraction of industrial substances. The slow growth rate and inefficient vegetative propagation of this species and the conservation policies that protect them require that, either plants produced from seeds grown in nurseries, or micropropagated materials are used for this purpose.
In vivo and in vitro seed germination
Seeds sown in closed trays with moist paper germinated with an efficiency of 86.2% and only 67% of the total survived the transfer and acclimatization to soil in trays kept in plastic (acclimatization) tunnels. In turn, only 83% of these survived the transfer to the nurseries for an overall survival rate of 55.6%. The emergence of the radicles was observed after 6 days and the first leaf was observed after 10 days.
When the seeds were disinfested and sown in vitro, fungal contamination was observed, after 10 days, in 21.8% of them. Out of the seeds that showed no contamination, 90.8% germinated giving a final efficiency of 70.9%. Many of the contaminants appeared together with the emergence of the radicle suggesting an endogenous presence of microorganisms that cannot be eliminated by the superficial disinfestation procedure and that rapidly develop in the nutrient rich culture media employed to culture plants in vitro.
The germination capacity observed was high in both cases indicating that, contrary to what has been reported for other Agavaceae (Piven et al. 2001), this is not a limiting factor for their reproductive potential. However, during field observations carried out between 2001 and 2003 in the wild populations of Baja California Sur, not a single plantlet was found that would indicate an important role for seeds in the regeneration and maintenance of the Yucca populations. In contrast, wild populations predominantly consist of colonies formed by vegetative propagation through suckers that given the limiting conditions for water availability in this area, would be the main strategy for propagation in the wild in a similar way as it has been proposed for Agave macroacantha (Arizaga and Ezcurra 2002).
Seeds can therefore be used for the production of plantation materials but a great deal of variability resulting from open pollination in the wild might not be the most adequate option for this purpose and therefore micropropagation should be considered.
In vitro multiplication
Methods for the in vitro propagation of agaves that could be used as the basis for this purpose have been previously reported (Robert et al. 1987). They indicated the need for an auxin and a high concentration of a cytokinin to induce the formation of adventitious shoots at the base of the stems of plants cultured in vitro.
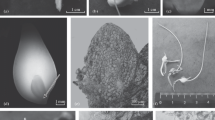
The plantlets germinated in vitro (Fig. 1A) were then propagated to generate clonal lines using 1 μM IAA and 5 μM BA in the culture medium. Figure 2 shows that there was a great deal of variation in the average multiplication efficiency of the 17 lines generated from single seeds after three rounds of propagation. Some lines such as PY, Y24, and PA multiplied in a very inefficient manner producing an average of 1.6 shoots per explant, while S11, Y25, or P14 were propagated with twice the efficiency than the other ones. P4 was the most efficient line producing an average of 3.8 shoots per explant, 2.4 times better than PA and Y24 with the same combination of plant growth regulators.
A pool of three of these lines (P4, P14, and Y25) was used to determine the optimal balance of plant growth regulators for in vitro micropropagation (Fig. 1B). The effect of the combination of IAA and BA on lateral shoot induction is presented in Table 1. In the absence of a cytokinin, with the sole addition of IAA, a single shoot developed from the apical meristem without hypertrophy or callus formation. The addition of BA always increased the development of lateral shoots in a significant manner but no statistically significant effect was observed by the interaction with IAA. The highest numbers of shoots were obtained at concentrations of 10 or 20 μM BA combined with 5 μM IAA. The use of other auxins such as 2,4-D and NAA induced callus formation (data not shown); therefore, the combination of 10 or 20 μM BA and 5 μM IAA appears to be the best choice for general purpose micropropagation. In some lines, a slight hyperhydricity and abnormal morphological development was observed at these concentrations. Nevertheless, it disappeared when the plants were taken back to 5 μM BA and 1 μM IAA without any effect on their subsequent development when they were transferred to soil.
Shoots started appearing after 10 days in culture irrespectively of the treatment and reached the maximum number per explant after 30 days, the use of 30 μM BA produced hypertrophy and induced callus formation. The induction of shoot formation by BA has been reported by Bentz et al. (1988) on Y. glauca, and by Pierik and Steegmans (1983) and Atta-Alla and Van Staden (1997) for Y. elephantipes and Y. aloifolia, respectively. Roots were always present in the absence of plant growth regulators and IAA did not stimulate their development in a significant manner. On the other hand, the presence of BA almost completely inhibited root formation.
Acclimatization of micropropagated plants
Shoots from clonal line P4 were rooted in vitro (Fig. 1C) by sub-culturing them to a medium without plant growth regulators. Table 2 shows that the initial size of the shoots does have a great effect on the percentage of plants that developed roots. Only 27.8% of the smaller (∼2 cm) plants produced roots compared to 82.9% of the larger ones (∼4 cm). Roots start forming at day 10 and after 21 days they were fully developed; most shoots produced at least one new leaf although the increase in size was not significantly different from their initial size. Significant differences between the substrates used on the survival rate or plantlet growth were not observed (p>0.05).
Rooted plants were transferred to soil and acclimatized for 5 weeks after which they did not increase in size or in number of leaves (Table 3). The average survival rate was 99%. When micropropagated plants from all the 17 clonal lines were rooted and acclimatized under the same conditions, an 87% survival rate was observed.
Field performance of micropropagated plants in an experimental plantation
The development of micropropagated plants from the 17 clonal lines was evaluated in an experimental plantation (Fig. 3) for a period of 26 months (Table 4). The micropropagated plants, on an average, showed a lower survival rate than did the plants derived from seeds with most of the losses taking place during the first 6 months. This, however, is unlikely to be attributable to their micropropagation origin and is probably due to variability present in the seed population from which they originated, as it has been reported for Betula pendula, another perennial species (Vihera-Aarnio and Velling 2001).
Table 4 shows that there is also a considerable variability among the different lines with respect to plant height, number of leaves, length of the leaves, and diameter of the rosette. It also shows that clonal lines were considerably less variable in all parameters than were the population of plants derived directly from seeds.
Table 4 shows that, on an average, the clonal lines Y18, S11, Y24, Y26, Y25, and Y10 developed faster, with annual growth rates of 27.49, 24.67, 24.66, 24.05, 22.66, and 22.39 cm per year, respectively, than did the average plants derived from seed with an AGR of 20.6 cm per year. The ACV for the 17 clonal lines was, however, nearly half of that one from plants derived from seeds for all the parameters measured; the height of plants derived from seeds showed a CV of 35.1% versus only an ACV of 17.7% for the clonal lines. In a similar manner, the CV for the length of the leaves was 20.3% for the seeds and 10.9% for the clones, and the CV for the diameter of the rosette was 22.1% versus 11.9%, respectively. Figure 4 illustrates the comparative growth rate in the experimental plantation of two micropropagated clonal lines: Y18 with the highest growth rate of 27.5 cm per year and P14 with the lowest growth rate of 17.4 cm per year, with plants derived directly from seeds that showed a growth rate of 20.6 cm per year.
Selection of elite materials from wild populations is not possible because we do not know their age or the conditions under which they have developed. The results described previously indicate that clonal micropropagation of plants derived from seeds can produce fast growing clonal lines from the variation that exists in seed populations of Yucca valida. However, these lines were produced randomly and, as can be seen in Table 5, some individuals derived from seed that were not micropropagated, grew even faster and showed higher values for all the parameters measured than did the averages of the best clonal lines, reaching growth rates of 41.9 and 36.25 cm per year compared with 27.49 of Y18. We are now cloning these selected individuals to generate faster growing clonal lines with reduced variability for the establishment of commercial plantations.
Abbreviations
- ACV:
-
Average coefficient of variation
- AGR:
-
Annual growth rate
- BA:
-
6-Benzylaminopurine
- CV:
-
Coefficient of variation
- IAA:
-
Indole-3-acetic acid
- MS:
-
Murashige–Skoog medium
References
Arizaga S, Ezcurra E (2002) Propagation mechanisms in Agave macroacantha (Agavaceae), a tropical arid-land succulent rosette. Am J Bot 89:632–641
Atta-Alla H, Van Staden J (1997) Micropropagation and establishment of Yucca aloifolia. Plant Cell Tissue Organ Cult 48:209–212
Bentz SE, Parliman BJ, Talbott H-J, Ackerman WL (1988) Factors affecting in vitro propagation of Yucca glauca. Plant Cell Tissue Organ Cult 14:111–120
Castellón-Olivares JJ, Rublúo-Islas A, Sepúlveda-Betancourt J, Ruiz-Campos G (2002) Environmental effects on biomass productivity of wild populations of Yucca schidigera in Baja California, Mexico, Southwest. Nature 47:576–584
Cheeke PR (2000) Actual and potential applications of Yucca schidigera and Quillaja saponaria saponins in human and animal nutrition. In: Oleszek W, Marston A (eds) Saponins in food, feedstuffs and medicinal plants. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 241–254
Diario Oficial (1997) Norma Oficial Mexicana NOM-005-RECNAT-1997. Mayo 20, pp 47–56
García-Mendoza A, Galván VR (1995) Riqueza de las familias Agavaceae y Nolinaceae en México. Bol Soc Bot Mex 56:7–24
Kaneda N, Nakanishi H, Staba EJ (1987) Steroidal constituents of Yucca schidigera plants and tissue cultures. Phytochemistry 26:1425–1429
Pierik RLM, Steegmans HHM (1983) Vegetative propagation of a chimerical Yucca elephantipes Regel in vitro. Sci Hort 21:261–272
Piven NM, Barredo-Pool FA, Borges-Argáez I, Herrera-Alamillo MA, Mayo-Mosqueda A, Herrera-Herrera JL, Robert ML (2001) Reproductive biology of Henequen (Agave fourcroydes) and its wild ancestor Agave angustifolia (Agavaceae). I. Gametophyte development. Am J Bot 88:1966–1976
Quintero A, Rosas V, Zamudio F, Capella S, Romo A (1982) Tissue culture of Yucca filifera cells. Identification of steroidal precursors. In: Proceedings of the 5th international plant tissue and cell culture, pp 295–296
Robert ML, Herrera JL, Contreras F, Scorer KN (1987) In vitro propagation of Agave fourcroydes Lem (Henequen). Plant Cell Tissue Organ Cult 8:37–48
Supniewski HJ (1972) Tissue culture of medicinal plants. In: Progress in the field of plant drugs. A supplement to Herba Pol. 13
Vihera-Aarnio A, Velling P (2001) Micropropagated silver birches (Betula pendula) in the field: performance and clonal differences. Silva Fenn 35:385–401
Acknowledgments
The authors express their gratitude to the Government of Baja California Sur and to the technical personnel of Centro de Propagación Vegetal for their support to establish and maintain the experimental plantation of Yucca valida. The work was carried out with the financial support of CIBNOR and Fundación PRODUCE Baja California Sur (Project 2440/2002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. C. Phillips
Rights and permissions
About this article
Cite this article
Arce-Montoya, M., Rodríguez-Álvarez, M., Hernández-González, J.A. et al. Micropropagation and field performance of Yucca valida . Plant Cell Rep 25, 777–783 (2006). https://doi.org/10.1007/s00299-006-0144-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-006-0144-3