Abstract
Two albino mutants (ab1 and ab2) have been derived from long-term shoot proliferation of Bambusa edulis. Based on transmission electronic microscopy data, the chloroplasts of these mutants were abnormal. To study the mutation of gene regulation in the aberrant chloroplasts, we designed 19 pairs of chloroplast-encoded gene primers for genomic and RT-PCR. Only putative NAD(P)H-quinone oxidoreductase chain 4L (ndhE; DQ908943) and ribosomal protein S7 (rps7; DQ908931) were conserved in both the mutant and wild-type plants. The deletions in the chloroplast genome of these two mutants were different: nine genes were deleted in the chloroplast genomic aberration in ab1 and 11 genes in ab2. The chloroplast genes, NAD(P)H-quinone oxidoreductase chain 4 (ndhD; DQ908944), chloroplast 50S ribosomal protein L14 (rpl14; DQ908934), and ATP synthase beta chain (atpB; DQ908948) were abnormal in both mutants. The gene expressions of 18 of these 20 genes were correlated with their DNA copy number. The two exceptions were: ATP synthase CF0 A chain (atpI; DQ908946), whose expression in both mutants was not reduced even though the copy number was reduced; ribosomal protein S19 (rps19; DQ908949), whose expression was reduced or it was not expressed at all even though there was no difference in genomic copy number between the wild-type and mutant plants. The genomic PCR results showed that chloroplast genome aberrations do occur in multiple shoot proliferation, and this phenomenon may be involved in the generation of albino mutants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spontaneous appearance of albino regenerants is a frequently occurring phenomenon in bamboo tissue culture (Rout and Das 1994; Lin and Chang 1998). Analyses have revealed that the plastids in these albino cells are morphologically highly abnormal, an example of which are the thylakoids, which are reduced in number and poorly developed (Caredda et al. 2004). Caredda et al. (2004) demonstrated that due to a lack of functional chloroplasts, albino plants cannot photosynthesize and become heterotrophic.
In Gramineae anther culture, one mechanism of albino regenerant formation has been shown to consist of the large-scale deletion and consequent loss of photosynthesis-related genes from plastid DNA (Day and Ellis 1984; Dunford and Walden 1991; Harada et al. 1991, 1992). The subsequent replication of the aberrated chloroplast genome could then result in the formation of albino plants (Day and Ellis 1984; Dunford and Walden 1991; Harada et al. 1991, 1992). However, this proposed mechanism is based on a protocol involving anther development and somatic embryogenesis in which the number of chloroplasts and chloroplast genome copy number had been reduced during pollen development. Consequently, the single cell that contained the chloroplast genome aberration could develop to be an albino plantlet by means of somatic embryogenesis (Day and Ellis 1984). Lin and Chang (1998) demonstrated that albino mutants also regenerated during bamboo multiple shoot proliferation. Unlike in vitro regeneration by means of somatic embryogenesis in which a single cell is the starting material, the multiple shoots obtained by Lin and Chang (1998) were derived from the in vitro culture of axillary buds, which consist of multiple cells and, consequently, multiple chloroplasts and multiple chloroplast DNA genomes in each chloroplast. However, there is no reference to explain the formation of albino plants in the multiple shoot system.
During 8 years of subculturing Bambusa edulis, we have identified two albino mutants: ab1 and ab2. These mutants were derived from multiple shoots (Lin et al. 2004) incubated in MS (Murashige and Skoog 1962) medium supplemented with 0.1 mg/l thidiazuron (TDZ). The aim of the investigation reported here was to apply PCR methodology to these mutants in order to study the mutation of albino plant regeneration during multiple shoot proliferation of B. edulis. We also wished to investigate the differences between the ab1 and ab2 mutants.
Materials and methods
Plant materials
Bambusa edulis wild-type and mutant (ab1 and ab2) multiple shoots were incubated in MS medium supplemented with 0.1 mg/l TDZ (Sigma, St. Louis, Mo, USA; Lin and Chang 1998) for proliferation. Explants were maintained at 26°C under a 16/8-h (light/dark) photoperiod with light supplied by daylight fluorescent tubes (FL-30D/29, 40 W, China Electric Co., Taipei, Taiwan) at an intensity of 54 mol/m2/s.
Transmission electron microscopy (TEM)
Leaves were fixed in 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.2, at 4°C overnight. After three 20-min rinses in buffer, with the buffer changed between rinses, the tissues were postfixed in 1% OsO4 in the same buffer for 4 h at room temperature and then rinsed three times in fresh buffer (20 min each rinse; fresh buffer each time). The leaves were dehydrated in an acetone series, embedded in Spurr’s resin (Spurr 1969), and sectioned with a Leica Ultracut E ultramicrotome. Semi-thin sections (1 μm) for light microscopy were placed on slides and stained with 0.1% toluidine blue for 1 min at 60°C on a hot plate. Thin sections on grids were stained with uranyl acetate and lead citrate (Reynolds 1963) and examined with a Philips CM 100 or JEM 1,200 EX II transmission electron microscope (TEM) at 80 KV
Genomic DNA purification, RNA purification, and reverse transcription (RT)-PCR
Total genomic DNA was isolated from the bamboo leaves using urea extraction buffer (Sheu et al. 1996) and used in a PCR analysis of the chloroplast genome. Total RNA was isolated from the bamboo using the Plant total RNA Miniprep Purification Kit (GeneMark, Taipei, Taiwan) and digested by DNase I to eliminate DNA contamination. For RT-PCR, first-strand cDNAs were synthesized using 1 μg RNA isolated from green and albino tissues, M-MLV reverse transcriptase (RNase H Minus, Point mutant; Promega, Madison, WI, USA), and a poly (dT) primer. The total reaction mix (15 μl) was diluted to 40 μl, and 1-μl aliquots of the dilution were used as the PCR template, with the RNA as the negative control template. Primers specific for each gene were then used to amplify each transcript by means of a PCR amplification series consisting of 25 PCR cycles of 30 s at 94°C, 30 s at 55°C, and 1 min at 72°C. (The concentration of the template and the amplification parameters had been determined in preliminary experiments; data not shown). The primers used are shown in Table 1. The same experiments were repeated three times. Densitometric determination of the amounts of the RT-PCR product was carried out using TINA 2.09e software (Raytest, Straubenhardt, Germany). Based on microarray data, we used tubulin as the external control. The ratio of the gene expression was calculated by the following formula: (X-gene /wt-gene)/(X-tublin /wt-tubulin). Variance analysis was conducted using Costat (CoHort software, Minneapolis, MN, USA). Duncan’s multiple range test was used for separation of the means when significant differences between treatments existed (Duncan 1955).
cDNA sequencing and analysis
DNA sequencing was carried out with the BigDye Terminator Cycle Sequencing kit using an ABI Prism 3,700 DNA analyzer (Applied Biosystems, Foster City, CA, USA). The predicted unigene sequences were used to query the non-redundant nucleotide database at GenBank (http://www.ncbi.nlm.nih.gov/) using BLASTX.
Results and discussion
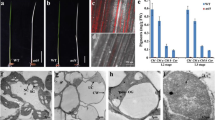
The mutant shoots maintained their albino mutant characteristic consistently during proliferation and subculturing in the medium supplemented with TDZ (Fig. 1), thereby providing a good source of experimental material for subsequent analyses. The albino phenotype was also maintained following the transfer of the multiple shoots to medium containing auxin or when cultured under different light conditions. The mesophyll cells of both the wild-type and albino mutant shoots were observed by TEM. The chloroplasts of the wild-type explants were diamond-shaped (Fig. 2a) and morphologically normal in that they contained both the thylakoid membrane sacs stacked and folded into grana and the products of photosynthesis, starch grains. In contrast, thylakoids were absent from the chloroplasts of both albino mutants and there was no formation of starch grains (Fig. 2b, c). Plastoglobules had accumulated in the plastids of both mutants.
Plastid features in wildtype and albino plantlet leaves which were incubated in 0.1 mg/l TDZ. The higher magnification shows typical chloroplasts in the wild type; note the organized thylakoids, grana, and starch grain. (s; bar = 1 μm, A: wild type). Plastids in albino leaves had plastoglobules (p; B: ab1, Bar = 1 μm; C: ab2, Bar = 0.5 μm; w = cell wall)
The number of plastoglobules present in a chloroplast usually decreases during chloroplast biogenesis and dramatically increases during chloroplast senescence. Plastoglobules contain lipoproteins and have been indicated in the formation of lipid storage structures for plant plastids (Caredda et al. 2004). Thylakoid membranes are the site of the photosynthetic light reactions. As such, they give the chloroplast a green color. Based on our TEM observations, we suggest that the albino mutants are still able to produce the phospholipids for thylakoid formation, however due to an aberrant chloroplast genome resulting from deletion, the thylakoids cannot form and the phospholipids accumulate in the plastids as plantoglobules (Caredda et al. 2004). Our suggestion is supported by earlier observations in albino mutants of rice and barley in which aberrated plastids were also found. Chen et al. (1988) investigated the plastids of albino plants derived from rice anther culture and found that the plastids were abnormal in shape and that the thylakoid structure was also reduced and abnormal. Some of plastids contained plastoglobules and prolamellar bodies. Many vesicles were found in the abnormal plastids, some of which were vacuolized. Caredda et al. (2004) found that the structure and development of plastids were different between a low-G/A (green/albino) barley cultivar (Cork) and a high-G/A cultivar (Igri). Plastid density, number of times the plastid underwent division, number of thylakoids, and lengths of the plastid were all reduced in the low-G/A cultivar, and not only in the somatic embryo derived from a microspore, but also in regenerants.
There was no starch grain formation in the ab1 and ab2 chloroplasts. Caredda et al. (2004) found that there were more starch grains in the embryo cells of the low-G/A barley cultivar (Cork) than in those of the high-G/A barely cultivar (Igri). These investigators concluded that the plastids of the green cultivar Igri had digested the starch grains, subsequently divided, and then formed the thylakoid before the incubation. However, the plastids of the albino barley cultivar Cork had developed into amyloplasts, and the plastids had failed to divide and form thylakoids because starch had accumulated.
The shoots of the albino ab1 and ab2 mutants were shorter than those of the wild type (Fig. 1). Given the key position of the well-formed chloroplast in the photosynthetic process, it is obvious that the chloroplast genome directly influences plant growth and development. Damasco et al. (1996) found that one random amplified polymorphic DNA (RAPD) product was missing from the dwarf off-type banana; sequencing revealed that this DNA is chloroplast-encoded (Damasco et al. 1996). The growth rate of homoplasmic ycf9 knockout plants was found to be significantly reduced under low-light conditions when compared with that of the wild-type (Ruf et al. 2000). This information is useful for molecular breeding and chloroplast studies on the control of plant growth.
We used simple genome PCR to exam the specific gene copy number of the wild type and albino mutants. The levels of the genomic PCR products of ndhE and rps7 were the same in the ab1 and ab2 mutants as in the wild type (Fig. 3). The deletions in the chloroplast genome of these two mutants were different: ten genes were deleted in the chloroplast genomic aberration in ab1 and 11 genes in ab2. No correlation was found between gene function and deletion. Deletions were found in genes encoding photosystem I, photosystem II, ATPase, and ribosomal protein. The genomic copy numbers of ndhD, rpl14, and atpB were reduced in both albino mutants (Fig. 4). The copy numbers of photosystem II protein D1 (psbA; DQ908939), photosystem II 44-kDa protein (psbC; DQ908937), photosystem II protein D2 (psbD; DQ908936), ATP synthase CF0 C chain (atpH; DQ908947), ribosomal protein S2 (rps2; DQ908932), and NAD(P)H-quinone oxidoreductase chain 6 (ndhG; DQ908942) were only slightly reduced in the ab2 mutant (Fig. 5). The copy number of photosystem II 47-kDa protein (psbB; DQ908938), cytochrome b559 alpha chain (psbE; DQ908935), cytochrome f (petA; DQ908941), cytochrome b6/f complex subunit IV (petD; DQ908940), ribosomal protein S11 (rps11; DQ908930), and NAD(P)H-quinone oxidoreductase chain 3 (ndhC; DQ908945) were only reduced in the ab1 mutant (Fig. 6). Based on these results, we conclude that albino mutants ab1 and ab2 are the result of different genetic mutations. In the investigation of Harada et al. (1992), the albino mutant derived from different subcultures also had different gene deletions, although in general most of their photosynthesis genes were deleted. In these mutants, some ribosome and ATPase genes were absent in the albino mutants, but the gene for porphyrin synthesis, trnE, was preserved (Harada et al. 1992).
Genomic PCR and RT-PCR of chloroplast-encoded genes which were same in wild-type and albino plants. Wild-type and albino multiple shoots harvested after 21 days in vitro were analyzed by Genomic PCR and RT-PCR. A fragment of a constitutively expressed tubulin gene was amplified as a nucleus control for the amount of genomic DNA and RNA template in the reactions. Three independent samples for the same harvest were included to document biological variation. Means followed by the same letter are not significantly different (Least significant difference test, P < 0.05; Duncan 1955)
Genomic PCR and RT-PCR of chloroplast-encoded genes for which the copy number was reduced in both mutants. In ab1, gene expressions were correlated with the genome copy number. The expression of rpl14 and atpB were not significantly different between ab2 and the wild type even the genome copy number was reduced. Wild-type and albino multiple shoots harvested after 21 days in vitro were analyzed by Genomic PCR and RT-PCR. A fragment of a constitutively expressed tubulin was amplified as a nucleus control for the amount of genomic DNA and RNA template in the reactions. Three independent samples for the same harvest were included to document biological variation. Means followed by the same letter are not significantly different (Least significant difference test, P < 0.05; Duncan 1955)
Genomic PCR and RT-PCR of chloroplast-encoded genes which were slightly reduced in ab2. Wild-type and albino multiple shoots harvested after 21 days in vitro were analyzed by Genomic PCR and RT-PCR. A fragment of a constitutively expressed tubulin gene was amplified as a nucleus control for the amount of genomic DNA and RNA template in the reactions. Three independent samples for the same harvest were included to document biological variation. Means followed by the same letter are not significantly different (Least significant difference test, P < 0.05; Duncan 1955)
Genomic PCR and RT-PCR of chloroplast-encoded genes which were significant reduced in ab1 but not or only slightly reduced in ab2. Wild-type and albino multiple shoots harvested after 21 days in vitro were analyzed by Genomic PCR and RT-PCR. A fragment of a constitutively expressed tubulin gene was amplified as a nucleus control for the amount of genomic DNA and RNA template in the reactions. Three independent samples for the same harvest were included to document biological variation. Means followed by the same letter are not significantly different (Least significant difference test, P < 0.05; Duncan 1955)
Our analyses revealed that most of genes were only reduced in copy number and not completely deleted (Figs. 3–6). Unlike the nucleus genes, there were between ten and 20 copies in each chloroplast. These data indicate that the chloroplast genomes of the albino mutants were heterozygous. Similar results were obtained by Day and Ellis (1984) in barley anther culture.
We applied RT-PCR to investigate the transcription of these 20 genes and found that for most of the genes (18/20) there was a correlation between the number of transcripts and the copy number: there were fewer transcripts when there was a reduced copy number. However, for two genes—atpI, and rps19—the number of transcripts was unexpected (Fig. 7).
Genomic PCR and RT-PCR of chloroplast-encoded genes which were not correlated with the genomic PCR data. The expression of atpI in both mutants was not reduced, even the copy number was reduced. The expression of rps19 was reduced or there was no expression even the genomic copy number was not different between the wild type and mutants. Wild-type and albino multiple shoots harvested after 21 days in vitro were analyzed by Genomic PCR and RT-PCR. A fragment of a constitutively expressed tubulin was amplified as a nucleus control for the amount of genomic DNA and RNA template in the reactions. Three independent samples for the same harvest were included to document biological variation. Means followed by the same letter are not significantly different (Least significant difference test, P < 0.05; Duncan 1955)
For atpI, the copy number was also reduced in the albino mutants, but there was no difference between the wild type and the mutants. According to these data, not all the chloroplast-encoded genes or photosynthesis-related genes showed a reduced transcript number in the albino mutants. This phenomenon has also been observed in cyanobacteria. Some of photosystem II genes are up-regulated in various photosystem II mutants. For example, the transcript number of psbA was higher in the psbC mutant (psbC -), the expression of psbDI/DII increased in the psbB - mutant, and the expression of psbB increased in the psbDI/DII - mutant (Yu and Vermaas 1990). For rps19, there was no difference in gene copy number between the albino mutants and the wild type, but the number of transcripts was lower in the mutants, or undetectable (Fig. 7). The same phenomenon was observed in tobacco chloroplast mutant. The tobacco ≥ycf6 mutant also displays this aberration in that the transcription of petA was inhibited in this mutant (Hager et al. 1999). According to these data, not only the gene copy number but also the transcription is regulated by other genes.
Both transcription and translation are regulated by chloroplast genes. In ≥ycf3, the plastid encoded protein psaC and the nucleus-encoded protein PsaD and PsaF are not expressed (Ruf et al. 1997). Based on our results and those of these previous investigations, we suggest that the cell has different pathways for regulating the transcription and translation of chloroplast-encoded genes.
We have been able to demonstrate here that the albino mutants derived from multiple shoots of B. edulis have major deletions in their chloroplast genome. However, not all albino mutants have a deletion in the chloroplast genome. We identified three albino mutants in Bambusa oldhamii from 2, 7, and 20 years ago and found that was no difference between the mutants and the wild type with respect to the 33-gene copy number (data no shown). Conversely, Ruf et al. (1997) observed that a single chloroplast-encoded gene mutation could induce albino mutant formation in tobacco: a homoplasmic ≥ycf3 is present in the albino phenotype under standard light conditions (Ruf et al. 1997). The ≥ycf6 also appeared in the albino phenotype under the standard light conditions (Hager et al. 1999). Apparently, not only the mutation in the chloroplast genome had induced the albino phenotype, but the nucleus-encoded gene mutation had also induced it. Many chloroplast proteins are encoded in the nucleus, translated in the cytosol, and finally transferred to the chloroplast (Martin et al. 1998). This process is mediated by the translocon. The ppi1 mutant, which was a translocon protein atTOC33 knockout mutant, has the albino phenotype (Kubis et al. 2003). These investigations indicate that the formation of a functional chloroplast is required for the cooperation of chloroplast and nucleus genes. Consequently, if any of the genes involved in these processes are mutated, the photosynthetic ability of the cell could be reduced.
Albino mutants derived from a genetic mutation may develop into chlorophyll-defective plantlets. The present investigation has shown that plastids of the mesophyll cells of the bamboo albino mutants, ab1 and ab2, had undergone serious damage and that the thylakoids were reduced in number or deleted entirely, a result also supported by the investigation of Caredda et al. (2004). Albino mutant plants have been derived from tissue culture, most specifically by anther culture. Genovesi and Magill (1979) showed that all of the regenerants in their rice anther culture system were albino mutants if the anthers were treated by a 3-week cold treatment. Because these albino plants could not process the products of photosynthesis, they could not survive in vivo. The regeneration of albino mutants during tissue culture is a costly waste of resources with respect to time, effect, and expenses. While such a system as that used by Genovesi and Magill (1979) may reduce the percentage of albino regeneration; it remains very difficult to separate albino and wild-type callus. By gaining an understanding of the mutation(s) of albino regeneration, we may be able to develop technology for detecting albino cells and, consequently, adapt the technology for reducing albino regeneration in tissue culture. In addition, there are many ornamental plants having leaves with undesirable albino stripes or spots. Our results may be one step in the direction of developing such techniques.
In this report, we characterize two albino mutants of B. edulis with respect to the aberrations in their chloroplast genome. These mutants are heterozygous with respect to multiple chloroplast-encoded gene deletions—, i.e., the two mutants have different gene mutations. While most of the chloroplast-encoded gene expressions were correlated with the copy number of genome, the expression of three of the genes was regulated in these mutants.
References
Caredda S, Devaux P, Sangwan RS, Proult I, Clément C (2004) Plastid ultrastructure and DNA related to albinism in androgenetic embryos of various barley (Hordeum vulgare) cultivars. Plant Cell Tissue Organ Cult 76:35–43
Chen YR, Lin TL, Huang YF (1988) Variations in the plastids of albino plants derived from rice anther culture. Proc Natl Sci Counc B 12:62–68
Damasco OP, Graham GC, Henry RJ, Adkins SW, Smith MK (1996) Random amplified polymorphic DNA (RAPD) detection of dwarf off-types in micropropagated Cavendish (Musa spp. AAA) bananas. Plant Cell Rep 16:118–123
Day A, Ellis THN (1984) Chloroplast DNA deletions associated with wheat plant regenerated from pollen: possible basis for maternal inheritance of chloroplasts. Cell 39:359–368
Duncan DB (1955) Multiple range and multiple F test. Biometrics 11:1–42
Dunford R, Walden RM (1991) Plastid genome structure and plastid-related transcript levels in albino barley plants derived from anther culture. Curr Genet 20:339–347
Genovesi AD, Magill CW (1979) Improved rate of callus and green plant production from rice anther culture following cold shock. Crop Sci 19:662–664
Hager M, Biehler K, Illerhaus J, Ruf S, Bock R (1999) Targeted inactivation of the smallest plastid genome-encoded open reading frame reveals a novel and essential subunit of the cytochrome b 6 f complex. EMBO J 18:5834–5842
Harada T, Ishikawa R, Niizeki M, Saito KI (1992) Pollen-derived rice calli that have large deletions in plastid DNA do not require protein synthesis in plastids for growth. Mol Gen Genet 233:145–150
Harada T, Sato T, Asaka D, Matsukawa I (1991) Large-scale deletions of rice plastid DNA in anther culture. Theor Appl Genet 81:157–161
Kubis S, Baldwin A, Patel R, Razzaq A, Dupree P, Lilley K, Kurth J, Leister D, Jarvis P (2003) The Arabidopsis ppi1 mutant is specifically defective in the expression, chloroplast import, and accumulation of photosynthetic proteins. Plant Cell 15:1859–1871
Lin CS, Chang WC (1998) Micropropagation of Bambusa edulis through nodal explants of field-grown culms and flowering of regenerated plantlets. Plant Cell Rep 17:617–620
Lin CS, Lin CC, Chang WC (2004) Effect of thidiazuron on vegetative tissue-derived somatic embryogenesis and flowering of Bambusa edulis. Plant Cell Tissue Organ Cult 76:75–82
Martin W, Stoebe B, Goremykin V, Hansmann S, Hasegawa M, Kowallik K (1998) Gene transfer to the nucleus and the evolution of chloroplasts. Nature 393:162–165
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–497
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 55:541–552
Rout GR, Das P (1994) Somatic embryogenesis and in vitro flowering of 3 species of bamboo. Plant Cell Rep 13:683–686
Ruf S, Biehler K, Bock R (2000) A small chloroplast-encoded protein as a novel architectural component of the light-harvesting antenna. J Cell Biol 149:369–377
Ruf S, Kössel H, Bock R (1997) Targeted inactivation of a tobacco intron–containing open reading frame reveals a novel chloroplast-encoded photosystem I–related gene. J Cell Biol 139:95–102
Sheu JJ, Yu TS, Tong WF, Yu SM (1996) Carbohydrate starvation stimulates differential expression of rice α-amylase genes that is modulated through complicated transcriptional and posttranscriptional processes. J Biol Chem 27:26998–27004
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Rev 26:31–43
Yu JJ, Vermaas WFJ (1990) Transcript levels and synthesis of photosystem II components in cyanobacterial mutants with inactivated photosystem II genes. Plant Cell 2:315–322
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, NT., Jane, WN., Tsay, HS. et al. Chloroplast genome aberration in micropropagation-derived albino Bambusa edulis mutants, ab1 and ab2 . Plant Cell Tiss Organ Cult 88, 147–156 (2007). https://doi.org/10.1007/s11240-006-9182-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-006-9182-3