Abstract
Gefitinib is an epidermal growth factor tyrosine kinase inhibitor used as a targeted chemotherapeutic agent in the treatment of lung cancer and other solid malignancies. Unlike other tyrosine kinase inhibitors, gefitinib is not recognized as having significant cardiotoxicity though it has been reported to be capable of potentiating ADP-induced activation and thromboxane A2 generation in platelets which could promote thrombosis. We report a case of recurrent myocardial infarction with angiographically documented vulnerable plaque rupture in a patient receiving chronic gefitinib therapy for metastatic carcinoid tumor. Platelet function studies revealed marked ADP-induced platelet activation that was only suppressed by high-dose clopidogrel. Measurement of urine 11-dehydro-thromboxane B2 also indicated persistent thromboxane A2 generation despite aspirin therapy, an emerging risk factor for adverse cardiovascular events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Gefitinib is a targeted chemotherapeutic agent that inhibits tumor cell proliferation and survival by blocking epidermal growth factor tyrosine kinase (EGF-TK) [1]. Initially approved for the treatment of non-small cell lung cancer, it is now being evaluated for the treatment for a variety of solid tumors [2]. Gefitinib is administered orally and is generally well-tolerated. Common side effects include anorexia, diarrhea, acne-like rash and mild-moderate elevation of hepatic transaminases [3]. Administration of low-dose aspirin therapy has been shown to ameliorate some of these side effects [4]. Gefitinib is not known to be associated with significant cardiotoxicity that has been observed with other tyrosine kinase inhibitors, such as heart failure, conduction abnormalities or thrombosis [5]. We report a case of recurrent myocardial infarction in a patient with metastatic carcinoid tumor treated with gefitinib.
Case report
The patient was a 47-year-old man in 1993 with no significant prior medical history when he underwent right hemicolectomy for abdominal pain and bowel obstruction. The pathology revealed a multicentric carcinoid tumor involving the ileocecal valve and appendix with metastases to regional lymph nodes and peri-intestinal soft tissue. Two years later he underwent exploratory laparotomy for abdominal pain and was found to have recurrent disease involving the anterior abdominal wall. This was resected and he subsequently underwent chemotherapy with streptozocin and doxorubicin. Serial CT scans performed over the next several years demonstrated slowly progressive disease with hepatic and pulmonary metastases. In 2004, he was started on 40 mg octreotide monthly and relatively large doses of ibuprofen for persistent fever, body aches and night sweats. In 2005, he was started on gefitinib 250 mg daily as part of a phase II clinical trial. An echocardiogram obtained immediately prior to the start of treatment revealed an ejection fraction of 55% without wall motion or valvular abnormalities. He tolerated gefitinib therapy well and serial CT scans performed over the ensuing years revealed no significance progression in tumor burden.
In October 2008, at the age of 62, he developed crescendo angina. During an exercise stress test, he developed severe chest discomfort at a maximum workload of 8.0 METS associated with 2–3 mm ST segment depressions on ECG. The chest discomfort persisted into recovery and he was referred for urgent cardiac catheterization. At the time of admission, the patient was on his 42nd monthly cycle of gefitinib, did not smoke and had never had hypertension or diabetes. His fasting lipid profile on no lipid-lowering therapy was notable for LDL and HDL cholesterol levels of 98 and 44 mg/dl, respectively. His family history was notable for a father who was a heavy smoker and died of a myocardial infarction at age 57. Coronary angiography revealed a long 95% hazy stenosis of the proximal left anterior descending (LAD) coronary artery with moderate stenoses of the mid and distal vessel (Fig. 1a). There were no significant stenoses observed in either the left circumflex or right coronary arteries. Aspirin, clopidogrel, unfractionated heparin and eptifibatide were administered and the culprit proximal LAD stenosis was successfully stented with an everolimus drug-eluting stent with an excellent angiographic result. The initial cardiac enzymes were positive with a CPK-MB of 14 μg/l (normal range 0–7 μg/l) that peaked at 36 μg/l after the procedure. Discharge medications included aspirin 325 mg daily, clopidogrel 75 mg daily, pantoprazole 40 mg daily, and pravastatin 40 mg daily in addition to his preadmission medications of ibuprofen, gefitinib, and octreotide.
Coronary arteriograms obtained a at the initial presentation showing a complex 95% stenosis of the left anterior descending coronary artery (LAD black arrow). b 1 week after successful LAD stent implantation, c 6 weeks after LAD stent implantation showing plaque rupture causing a new 95% lesion in the left circumflex coronary artery (LCX white arrow), and d immediately following successful circumflex stent implantation
One week after stent placement, the patient experienced atypical chest discomfort associated with facial numbness, lightheadedness, and shortness of breath. The ECG was unremarkable. Repeat coronary angiography revealed a widely patent stent in the proximal LAD with no significant changes elsewhere (Fig. 1b). Tests were performed to evaluate the patient’s responsiveness to aspirin and clopidogrel (Table 1). While there was suppressed platelet aggregation to arachidonic acid (3%) and a prolonged PFA-100 collagen/epinephrine closure time (>300 s) suggesting an appropriate aspirin-mediated antiplatelet effect, urine level of 11-dehydro-thromboxane B2 was elevated (>1500 pg/mg creatinine), indicating persistent thromboxane generation despite aspirin therapy. There appeared to be an adequate antiplatelet effect of clopidogrel based on the VerifyNow P2Y12 assay (<240 PRU), though by light transmission aggregometry there remained robust platelet aggregation in response to 5 and 10 μM ADP (>70%). Based on this, the clopidogrel dose was increased to 150 mg daily. Repeat platelet function testing performed one week after increasing the clopidogrel dose revealed no platelet aggregation in response to 5 and 10 μM ADP and <50% aggregation in response to 20 μM ADP.
The patient was well until 6 weeks after LAD stent placement when he developed severe substernal chest pain at rest. Although the ECG revealed non-specific ST segment changes, he ruled in for a myocardial infarction with a peak CPK-MB fraction of 41 μg/l. Coronary angiography revealed a widely patent stent in the proximal LAD. There was, however, a new 95% hazy stenosis in the circumflex marginal coronary artery (Fig. 1c) at the site of a non-significant narrowing observed on the previous angiograms. The new culprit lesion was successfully stented with an everolimus drug-eluting stent with an excellent angiographic result (Fig. 1d). Transthoracic echocardiography demonstrated a left ventricular ejection fraction of 45–50% with inferolateral akinesis. The gefitinib was discontinued.
Over the subsequent 2 years, the patient has not had any recurrent cardiovascular events and an interval dobutamine stress echocardiogram revealed no evidence of inducible ischemia. He continues on aspirin 325 mg daily, clopidogrel 150 mg daily, octreotide 40 mg monthly and ibuprofen as needed to control persistent fevers and body aches. Serial CT scans reveal no significant progression of hepatic and pulmonary metastatic disease.
Discussion
We report the first case of recurrent coronary thrombosis in a patient undergoing treatment with gefitinib. Based on the Naranjo et al. [6] adverse drug reaction probability scale, the association can be classified as possible. At the time of the index cardiovascular event, our patient had only a moderate 10-year cardiovascular risk with a Framingham risk score of 11%. Yamaguchi et al. [7] previously reported a case of myocardial infarction in a 75-year-old woman with a history of diabetes and hypertension 2 months after initiating treatment with gefitinib for relapsing lung adenocarcinoma. In contrast to that report, our patient underwent serial coronary angiography which revealed the second myocardial infarction to have been clearly caused by the rupture of a vulnerable atherosclerotic plaque in a vessel with minimal prior luminal narrowing.
Although a direct causal relationship cannot be established, there is intriguing circumstantial evidence suggesting gefitinib might be capable of increasing the risk of myocardial infarction. In a study of 20 aspirin-naïve patients, Nomura’s group found that gefitinib appeared to potentiate ADP-induced platelet activation [8]. One week after LAD stent implantation, our patient manifested surprisingly robust ADP-induced platelet activation despite being on clopidogrel 75 mg daily. Such high on-treatment platelet reactivity is a recognized risk factor for both stent thrombosis and recurrent myocardial infarction [9]. Fortunately, ADP-induced platelet activation was significantly suppressed after doubling the clopidogrel dose to 150 mg daily.
Nomura’s group also showed that gefitinib significantly increased the capacity of platelets to generate thromboxane A2 (TXA2) during clot formation as measured by serum levels of its stable metabolite, thromboxane B2 (TXB2) [4]. Aspirin, which suppresses platelet TXA2 formation by irreversibly inhibiting the cyclooxygenase-1 (COX-1) enzyme, significantly reduced serum TXB2 levels in these patients. Arachidonic acid-induced platelet aggregation is known to be inhibited when platelet TXA2 generation is suppressed >90% by aspirin [10, 11]. Our patient had essentially no arachidonic acid-induced platelet aggregation, indicating a high degree of platelet COX-1 inhibition by aspirin. Despite this, urine levels of the TXB2 metabolite, 11-dehydro-TXB2, were significantly elevated in our patient (>1500 pg/mg creatinine). Unlike levels of serum TXB2, urine 11-dehydro-TXB2 levels reflect TXA2 synthesis that occurs throughout the body, not just in platelets. In addition to stimulating COX-1-mediated TXA2 synthesis in platelets, it is conceivable that gefitinib also potentiates TXA2 synthesis from non-platelet sources, such as via an inducible COX-2 pathway in inflammatory and endothelial cells. Whatever the source, persistent TXA2 generation despite aspirin therapy is emerging as a potent cardiovascular risk factor. In a cohort of 3,261 subjects enrolled in the CHARISMA trial on aspirin who were randomized to clopidogrel or placebo, subjects with the highest quartile of urine 11-dehydro-TXB2 at baseline had a 1.7-fold increased risk (CI 1.1–2.6, P = 0.03) of myocardial infarction, stroke and cardiovascular death at 28 months compared to those with the lowest quartile of UTXB2 [12]. Our group also recently found that aspirin-insensitive thromboxane generation was associated with a 2.6-fold (CI 1.2–5.6, P = 0.015) increased odds of early vein graft thrombosis after coronary artery bypass surgery [13].
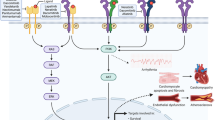
It is also conceivable that gefitinib may have contributed to vulnerable plaque rupture in our patient. EGF is a potent chemoattractant and mitogen for vascular smooth muscle cells whose expression is increased in human atherosclerotic plaques [14] and induced after arterial balloon injury where it mediates neointima formation [15]. Smooth muscle cells are a major source of collagen synthesis and are thus critical to maintain the integrity of the fibrous cap of atherosclerotic plaques [16]. Gefinitib is known to be capable of inhibiting EGF-induced smooth muscle cell proliferation [15] and thus may impair the integrity of the fibrous cap leaving atherosclerotic plaques more prone to rupture.
In conclusion, we report an intriguing case of recurrent myocardial infarction in a patient with metastatic carcinoid tumor treated with chronic gefitinib therapy. The known biologic effects of the EGF signaling pathway raise the possibility that an EGF-TK inhibitor such as gefitinib could predispose to atherothrombosis. Further studies are needed to determine if gefitinib use is associated with an increase risk of adverse cardiovascular events and the mechanism by which this might occur.
References
Lurje G, Lenz HJ (2009) EGFR signaling and drug discovery. Oncology 77:400–410
Reck M (2009) Gefitinib in the treatment of advanced non-small-cell lung cancer. Expert Rev Anticancer Ther 9:401–412
AstraZeneca Pharmaceuticals. Iressa summary of product characteristics. http://www.iressa.com/_mshost5259502/content/6906430/Gefitinib-PI
Kanazawa S, Yamaguchi K, Kinoshita Y, Muramatsu M, Komiyama Y, Nomura S (2006) Aspirin reduces adverse effects of gefitinib. Anticancer Drugs 17:423–427
Chen MH, Kerkela R, Force T (2008) Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics. Circulation 118:84–95
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ (1981) A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30:239–245
Yamaguchi K, Kanazawa S, Kinoshita Y, Muramatsu M, Nomura S (2005) Acute myocardial infarction with lung cancer during treatment with gefitinib: the possibility of gefitinib-induced thrombosis. Pathophysiol Haemost Thromb 34:48–50
Kanazawa S, Yamaguchi K, Kinoshita Y, Muramatsu M, Komiyama Y, Nomura S (2005) Gefitinib affects functions of platelets and blood vessels via changes in prostanoids balance. Clin Appl Thromb Hemost 11:429–434
Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, Bhatt DL, Cattaneo M, Collet JP, Cuisset T, Gachet C, Montalescot G, Jennings LK, Kereiakes D, Sibbing D, Trenk D, Van Werkum JW, Paganelli F, Price MJ, Waksman R, Gurbel PA (2010) Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 56:919–933
Pulcinelli FM, Riondino S, Celestini A, Pignatelli P, Trifiro E, Di Renzo L, Violi F (2005) Persistent production of platelet thromboxane A2 in patients chronically treated with aspirin. J Thromb Haemost 3:2784–2789
Armstrong PC, Truss NJ, Ali FY, Dhanji AA, Vojnovic I, Zain ZN, Bishop-Bailey D, Paul-Clark MJ, Tucker AT, Mitchell JA, Warner TD (2008) Aspirin and the in vitro linear relationship between thromboxane A2-mediated platelet aggregation and platelet production of thromboxane A2. J Thromb Haemost 6:1933–1943
Eikelboom JW, Hankey GJ, Thom J, Bhatt DL, Steg PG, Montalescot G, Johnston SC, Steinhubl SR, Mak KH, Easton JD, Hamm C, Hu T, Fox KAA, Topol EJ (2008) Incomplete inhibition of thromboxane biosynthesis by acetylsalicylic acid. determinants and effect on cardiovascular risk. Circulation 118:1690
Gluckman TJ, McLean RC, Schulman SP, Kickler TS, Shapiro EP, Conte JV, et al. (2011) Effects of aspirin responsiveness and platelet reactivity on early vein graft thrombosis after coronary artery bypass graft surgery. J Am Coll Cardiol. (in press)
Miyagawa J, Higashiyama S, Kawata S, Inui Y, Tamura S, Yamamoto K, Nishida M, Nakamura T, Yamashita S, Matsuzawa Y (1995) Localization of heparin-binding EGF-like growth factor in the smooth muscle cells and macrophages of human atherosclerotic plaques. J Clin Invest 95:404–411
Dahal BK, Cornitescu T, Tretyn A, Pullamsetti SS, Kosanovic D, Dumitrascu R, Ghofrani HA, Weissmann N, Voswinckel R, Banat GA, Seeger W, Grimminger F, Schermuly RT (2010) Role of epidermal growth factor inhibition in experimental pulmonary hypertension. Am J Respir Crit Care Med 181:158–167
Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R (2010) Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol 30:1282–1292
Ben Dor I, Kleiman NS, Lev E (2009) Assessment, mechanisms, and clinical implication of variability in platelet response to aspirin and clopidogrel therapy. Am J Cardiol 104:227–233
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lynch, D.R., Kickler, T.S. & Rade, J.J. Recurrent myocardial infarction associated with gefitinib therapy. J Thromb Thrombolysis 32, 120–124 (2011). https://doi.org/10.1007/s11239-010-0539-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-010-0539-4