Abstract
Previous studies have reported that left ventricular (LV) thrombus is a complication in 10–56% of ST-segment elevation acute anterior wall myocardial infarctions (AWMI). Data suggest that changes in acute myocardial infarction management such as early anticoagulation, thrombolysis, and most recently, primary percutaneous coronary intervention (PCI), may decrease thrombus occurrence. Early time to reperfusion has been shown to decrease mortality and improve LV function recovery. To determine if door-to-balloon time (DTBT) affects the incidence of LV thrombus, we retrospectively analyzed data on 43 consecutive patients who underwent successful PCI of a primary acute ST-segment elevation AWMI. Transthoracic echocardiography was performed for detecting LV thrombus and measuring LV ejection fraction (EF) within 5 days on all patients (average time: 2.17 days post event). Nineteen patients underwent PCI within 2 h of arrival to the Emergency Department (Group A, average 88 min) and 24 patients underwent PCI with DTBT of more than 2 h (Group B, average 193 min). Clinically significant LV thrombus was detected in 35% of all patients. The incidence of LV thrombus formation in Group A was not significantly different from that in Group B (42.1% vs. 29.0%, respectively; P = 0.52). The risk of LV thrombus was independent of in-hospital anticoagulation and medical management, peak enzyme levels, and LVEF but did relate to age (odds ratio = 1.96, 95% CI 1.03–3.73, P = 0.04 per decade). No embolic events in hospital were observed (average hospital stay 9.2 days). We conclude that the incidence of LV thrombus remains high despite PCI. Also, we find that DTBT in patients presenting with an ST-segment elevation AWMI does not affect the incidence of LV thrombus formation. Increased age, however, does appear to increase the risk of LV thrombus development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Left ventricular (LV) thrombus is a common complication of anterior wall myocardial infarction (AWMI) and is of great concern due to the associated risk of systemic embolization. Anticoagulation, the current standard therapy for LV thrombus, exposes the patient to the risk of bleeding. The incidence of LV thrombus has been reported at rates up to 56% [1–7]. Recent studies suggest a decrease in the rate of occurrence of LV thrombi, which have been attributed to changes in acute myocardial infarction (AMI) management. Studies show that early anticoagulation with heparin decreases the risk of LV thrombus formation [8–12]. Reperfusion therapy with thrombolysis has also been shown to decrease the incidence of LV thrombus thought to be secondary to decreased infarct size and preservation of LV function [13–17]. Two studies have evaluated the effect of percutaneous coronary intervention (PCI) on LV thrombus formation and report an incidence post AWMI of 10% and 15% [18, 19].
Early time to reperfusion, specifically the time from initial presentation to a hospital to first PCI (door-to-balloon time (DTBT)) has been shown to decrease mortality and improve recovery of LV function [20–23]. The object of this study was to evaluate the effect of time to reperfusion by primary PCI on the incidence of LV thrombus by retrospectively analyzing data on 43 consecutive patients who underwent successful PCI of a primary ST-segment elevation AWMI.
Methods
Approval was obtained by the Columbia University Institutional Review Board. Basic demographics were collected on 175 consecutive ST-segment elevation myocardial infarctions (STEMI) that presented to Columbia University Medical Center Emergency Department (ED) from December 2000 to April 2003. STEMI was defined as chest pain at rest for >20 min and an ST-segment elevation of greater or equal to 1 mm in two contiguous leads or new or not known to be old left bundle branch block. Of the patients that presented and underwent cardiac catheterization, 53 had an AWMI. Of the 53, 43 had a primary event with a culprit lesion identified and treated with successful angioplasty and coronary stenting of the culprit lesion.
Once the patient arrived to the hospital, a timeline of events from time of evaluation by ED triage (“door” time) until coronary balloon inflation was recorded. Patients in group A had a DTBT of <2 h, and group B consisted of those patients with DTBT ≥2 h and <6 h. A chart review was conducted for each patient to obtain baseline patient demographics, past medical history, lab data, and electrocardiograms. The administration of medications including intravenous heparin, platelet glycoprotein IIbIIIa inhibitors, clopidogrel, aspirin, beta-blockers, ACE-inhibitors, statins, and warfarin was noted. Catheterization data including lesion site and residual stenosis was collected.
Two-dimensional echocardiograms were done on all patients within 5 days of the event. The clinical echocardiographic study, interpreted by an experienced echocardiographer, was used to determine the presence of thrombus. The presence of an LV thrombus was defined as an echogenic mass with a convex surface which was clearly distinct from the endocardium and was located in a region of abnormal wall motion. Clinically significant LV thrombus was defined as evidence or suggestion of a thrombus that resulted in the decision to institute long-term anticoagulation with warfarin.
Statistical analysis was performed using the SAS 8.2 (SAS Institute, Cary, NC). Continuous variables were compared between subjects in Groups A and B by unpaired t-test. Categorical variables were compared by Fisher’s exact test. Comparisons were also performed between those with and without LV thrombus in order to assess the effect of the measured independent variables upon outcome. Multivariable logistic regression analysis was performed including variables that were significant at a 0.10 level using a backwards elimination variable selection technique. For all tests a P value of <0.05 was considered significant.
Results
Clinical characteristics are shown in Table 1. Overall, group A (DTBT <2 h) had significantly more male patients than group B (89.5% vs. 58.3%) and had fewer diabetic patients (15.8% vs. 41.7%). The patients in the two groups were similar in age, and in percentage of patients who were smokers and had hypertensive disease. Also, there was no statistically significant difference in peak troponin levels (group A = 837.5, group B = 760.6, P = 0.24).
Average DTBT for patients in group A was 88.8 min and group B was 193.2 min. The number of patients who developed clinically significant LV thrombus in group A and group B was 8 (42.1%) and 7 (29.0%) respectively. This was not significantly different (P = 0.52). All catheterizations were described as successful with 86% of left anterior descending (LAD) coronary lesions stented open to 0% residual stenosis (group A + group B) and the rest were stented open to at least 30%, with no significant difference between the two groups. The distribution of the LAD coronary stenosis site was also similar.
All echocardiograms were performed within 5 days of presentation (average = 2.17, group A = 1.9, group B = 2.4). There was no significant difference in the average ejection fraction (EF) between groups (group A = 37.9, group B = 38.8, P = 0.53). All LV thrombi were described as mural and/or apical with no evidence of mobility or protrusion. There was no significant difference between end LV diastolic dimensions in the two groups with the majority being within normal limits.
One patient in each of groups A and B required the use of an intra-aortic balloon pump. One patient in group A suffered anoxic brain injury and one patient in group B died within 48 h after event. All other patients left the hospital with an average hospital stay of 9.2 days. Warfarin was started on 15 patients with evidence or suggestion of a thrombus on echocardiogram. Warfarin was also started on one patient in group B with no evidence of a thrombus but with reduced EF and apical akinesis. There were no systemic emboli in either group during the hospitalization.
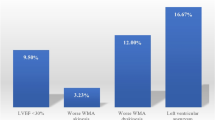
Univariate analysis showed that the development of an LV thrombus was not related to tobacco use, hypertension, diabetes and LVEF after AWMI (Table 2). There was a trend towards significance for peak troponin as a risk factor for LV thrombus. The development of LV thrombus did relate to age (odds ratio = 1.96, 95% CI 1.03–3.73, P = 0.04 per decade). The use of heparin and GPIIbIIIa inhibitors was similar in all patients. Aspirin, clopidogrel, B-blockers, statins, and ACE-inhibitors were started in all but one patient by day 5 unless contraindicated (data not shown). In multivariate analysis peak troponin was not significant, leaving age as the only variable predictive of LV thrombus.
Discussion
This study demonstrates that there is no statistical difference in the development of LV thrombus within the first few days after an ST-segment elevation AWMI based on time to reperfusion by primary PCI. Patients with DTBT of <2 h were more likely to male and less likely to have diabetes, similar to previously reported data [20]. However, it appears that LV thrombus development is independent of these factors. Statistical analysis shows a relation between age and incidence of LV thrombus. Given the study design, this relationship is not confounded by previous events or delayed time to treatment. This relationship has not been previously observed.
Recent studies have shown a trend toward a decreasing incidence of LV thrombus, attributed to the changes in STEMI management. Heparin therapy, now one of the standard therapies of care during a STEMI, has been shown to decrease the risk of LV thrombus formation after STEMI [8, 9]. However, a meta-analysis of four studies could only show a trend toward statistical significance [10]. There are conflicting data regarding the use of thrombolysis and the incidence of LV thrombus. Data suggest that though the use of thrombolysis may not be associated with a lower incidence of LV thrombus, successful reperfusion with TIMI 2–3 flow does correlate with a decreased incidence [13, 17]. In addition, the use of thrombolysis has been associated with a lower incidence of systemic embolization [16]. Data suggest that there is a positive relation between systemic embolization and thrombus morphology, such as protrusion into the LV and mobility [23, 24]. After thrombolysis, the decreased incidence of systemic embolization may be attributed to morphologic changes seen on echocardiogram [16].
There is limited data on the effect PCI has on LV thrombus and systemic embolization. Our study reports an incidence higher than the recently reported data of 10–15% after PCI. Greaves et al. reported an LV thrombus post all STEMI incidence of only 3.7% by day 11 in a population of patients where approximately 80% of patients received thrombolysis or primary PTCA [14]. Kalra and Jang also reported a 10% (3/30) incidence of LV thrombus after an AWMI and PCI [18]. Nayak et al. showed a 15% incidence of LV thrombus post AWMI and PCI in subgroup analysis [19]. Such data suggest that PCI may decrease the risk of LV thrombus.
Past studies assessing LV function and thrombus formation after STEMI have used symptom onset to balloon time as time to reperfusion, which may also affect reported differences from our findings. Symptom onset time was not collected in our study as it is a difficult point to define. Early data suggest that time from ED arrival until time to PCI is a better predictor of mortality than time from symptom onset until PCI [20]. However, a recent study suggests that this indeed may be a significant variable [25]. TIMI flow has also been reported in previous studies and may potentially affect LV thrombus formation. While DTBT clearly affects mortality in primary PCI for STEMI, this relationship may be independent of the recovery of LV function and thrombus formation. Indeed, it is possible that the recovery of the LV function and thrombus formation may be more closely linked to the symptom onset time.
On univariate analysis, we discovered a trend toward significance of peak troponin levels and the development of LV thrombus (P = 0.07). However, this was not true for LVEF which has been shown to correlate with LV thrombus formation in a past study of AMI without PCI [15]. EF has been shown to correlate with troponin levels >12–48 h after presentation when enzyme levels plateau [26, 27]. Peak troponin levels vary as a result of coronary reperfusion and could be a marker for LV thrombus formation. This would need to be further evaluated with a larger sample size powered to show statistical significance. In this regard, it is noteworthy that peak creatine kinase level on day one post Q wave anterior wall MI has emerged as the strongest independent predictor of subsequent recovery of ventricular function by multivariate analysis in a study measuring several clinical and echocardiographic measures [28].
In our study, the development of LV thrombus did relate to age on univariate and multivariate analysis. The average age of the patients in our data set was 64 years as compared to 61 years in the Kalra and Jang study, which may partially account for the percentage difference in LV thrombus incidence. In addition, differences in echo timing as well as more sensitive echo techniques may have contributed to this discrepancy. One prior study showed age was not a significant risk factor in multivariate logistic regression analysis for LV thrombus after a first AWMI where 63% had revascularization therapy with either thrombolytics or PCI [29]. No previous studies have assessed the relationship between patient age and formation of LV thrombus after PCI. It is possible that age, independent of prompt reperfusion therapy, contributes to LV thrombus development. Indeed, the GUSTO-1 angiographic experience revealed that elderly patients have increased LV dysfunction despite successful thrombolysis [30]. Moreover, elderly patients had increased damage in the infarct zone and persistently increased mortality despite sustained infarct related patency after successful thrombolysis [30]. The activity of Cardiac 5′-nucleotidase, the enzyme which mediates the conversion of adenosine monophosphate to adenosine, increases with age and is inversely related to functional recovery of the myocardium after ischemia [31].
In our study, no LV thrombi had morphologic characteristics that are associated with increased risk of systemic emboli. There were no in-hospital emboli, the time period where emboli are most common. Previous data does suggest that successful reperfusion may change the morphologic characteristics of LV thrombi, making them less likely to embolize [10, 16, 24]. This effect should be further characterized for PCI. Also with PCI, many anticoagulant agents are instituted, including the early use of heparin and GP IIbIIIa inhibitors, as well as long term agents such as clopidogrel and aspirin. With these changes in STEMI management the risk of bleeding versus benefit of adding warfarin in the setting of LV thrombus should also be addressed. Indeed, the higher incidence of LV thrombus in elderly patients has important clinical ramifications; in that elderly patients post primary PCI with LV thrombus treated with aspirin, clopidogrel, and coumadin are at increased risk for bleeding complications.
In summary, LV thrombus formation after primary PCI for an acute ST-segment elevation AWMI was not affected by time to reperfusion but was influenced by advanced age. Further studies are needed to define the optimal antiplatelet and anticoagulant regimen for primary PCI patients who develop LV thrombus.
Study limitations
As a retrospective study, we were unable to obtain certain data that might have been useful. A prospective study would have allowed us to collect symptom onset and TIMI flow data pre and post intervention. Echocardiographic studies were obtained before the widespread use of echo contrast agents which improve the sensitivity and specificity of the echocardiographic diagnosis of LV thrombus. We would have liked to have repeat echocardiography as some point after initial assessment to identify possible subsequent development or interval resolution of LV thrombus. Finally, our study would have benefited from a larger study population.
References
Asinger RW, Mikell FL, Elsperger J, Hodges M (1981) Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. N Engl J Med 305:297–302
Weinreich DJ, Burke JF, Pauletto FJ (1984) Left ventricular thrombi complicating acute myocardial infarction. Long-term follow-up with serial echocardiography. Ann Intern Med 100:789–794
Domemicucci S, Belotti P, Chiarella F, Lupi G, Vecchio C (1987) Spontaneous morphologic changes in left ventricular thrombi: a prospective two-dimensional echocardiographic study. Circulation 75:737–743
Lamas GA, Vaughan DE, Pfeffer MA (1988) Left ventriculcar thrombus formation after first anterior wall acute myocardial infarction. Am J Cardiol 62:31–35
Keren A, Goldberg S, Gottlieb S, et al (1990) Natural history of left ventricular thrombi: their appearance and resolution in the post-hospitalization period of acute myocardial infarction. J Am Coll Cardiol 15:790–800
Keating EC, Gross SA, Schlamowitz RA, et al (1983) Mural thrombi in myocardial infarction: prospective evaluation by two-dimensional echocardiography. Am J Med 74:989–995
Keeley E, Hillis LD (1996) Left ventricular mural thrombus after acute myocardial infarction. Clin Cardiol 19:83–86
Turpie AG, Robinson JG, Doyle DJ, et al (1989) Comparison of high-dose with low-dose subcutaneous heparin to prevent left ventricular mural thrombosis in patients with acute myocardial infarction. N Engl J Med 320:352
Kontny F, Dale J, Abildgaard U, Pedersen TR (1997) Randomized trail of low molecular weight heparin (dalteparin) in prevention of left ventricular thrombus formation and arterial embolism after acute myocardial infarction: The Fragmin in Acute Myocardial Infarction (FRAMI) Study. J Am Coll Cardiol 30:962–969
Vaitkus PT, Barbathan ES (1993) Embolic potential, prevention, and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol 22:1004–1009
Cregler LL (1992) Antithrombotic therapy in the left ventricular thrombosis and systemic embolism. Am Heart J 123:1110–1114
Mooe T, Teien D, Karp K, Eriksson P (1995) Left ventricular thrombosis after anterior myocardial infarction with and without thrombolytis therapy. J Intern Med 237:563–569
Pizzetti G, Belotti G, Margonato A, et al (1996) Thrombolytic therapy reduces the incidence of left ventricular thrombus after anterior myocardial infarction Relationship to vessel patency and infarct size. Eur Heart J 17:421–426
Greaves S, Zhi G, Lee RT, et al (1997) Incidence and natural history of left ventricular thrombus following anterior wall acute myocardial infarction. Am J Cardiol 80:442–448
Chiarella F, Santoro E, Domeniccuci S, Maggioni A, Vecchio C (1998) Predischarge two-dimensional echocardiography evaluation of left ventricular thrombosis after acute myocardial infarction in the GISSI-3 study. Am J Cardiol 81:822–827
Domenicucci S, Chiarella F, Bellotti P, Belline P, Lupi G, Vecchio C (1999) Long term prospective assessment of left ventricular thrombus in anterior wall myocardial infarction and implications for a rational approach to embolic risk. Am J Cardiol 83:519–524
Ileri M, Tadogan I, Kosar F, Yetkin E, Buyukasik Y, Kutuk E (1999) Influence of thrombolytic therapy in the incidence of left ventricular thrombi after acute anterior myocardial infarction: role of successful reperfusion. Clin Cardiol 22:477–480
Kalra A, Jang IK (2000) Prevalence of early left ventricular thrombus after primary coronary intervention for acute myocardial infarction. J Thromb Thrombolysis 10:133–136
Nayak D, Aronow WS, Sukhija R, McClung JA, Monsen CE, Belkin RN (2004) Comparison of frequency of left ventricular thrombi in patients with anterior wall versus non-anterior wall acute myocardial infarction treated with antithrombotic and antiplatelet therapy with and without coronary revascularization. Am J Cardiol 93:1529–1530
Cannon CP, Gibson CM, Lambrew CT, et al (2000) Relationship of symptom-onset-to-balloon time and door-to-balloon time with mortality in patients undergoing angioplasty for acute myocardial infarction. JAMA 283:2941–2947
Berger P, Ellis SG, Holmes DR Jr et al (1999) Relationship between delay in performing direct coronary angioplasty and early clinical outcomes in patients with acute Myocardial infarction (GUSTO-IIb). Circulation 100:14–20
Juliard JM, Feldman LJ, Golmard JL et al (2003) Relation of mortality of primary angioplasty during acute myocardial infarction to door-to-Thrombolysis in Myocardial Infarction (TIMI) time. Am J Cardiol 91:1401–1405
Sheiban I, Fragasso G, Rosana GM et al (2001) Time course and determinants of left ventricular function recovery after primary angioplasty in patients with acute myocardial infarction. J Am Coll Cardiol 38:464–471
Visser CA, Kan G, Meltzer RS, Dunning AJ, Roelandt J (1985) Embolic potential of left ventricular thrombus after myocardial infarction: a two-dimensional echocardiographic study of 119 patients. J Am Coll Cardiol 5:1276–1280
De Luca G, Suryapranata H, Zijlstra F et al (2003) Symptom-onset-to-balloon time and mortality in patients with acute myocardial infarction treated by primary angioplasty. J Am Coll Cardiol 42:991–997
Rao AC, Collinson PO, Canepa-Anson R, Joseph SP Troponin (1998) T measurements after myocardial infarction can identify left ventricular ejection of less that 40%. Heart 80:223–225
Panteghini M, Cuccia C, Bonnetti G et al (2002) Single point cardiac troponin T at coronary care unit discharge after myocardial infarction correlates with infarct size and ejection fraction. Clin Chem 48:1432–1436
Solomon SD, Glynn RJ, Greaves S et al (2001) Recovery of ventricular function after myocardial infarction in the reperfusion era: the healing and early afterload reducing therapy study. Ann Intern Med 134(6):451–458
Anzia T, Yoshikawa T, Kaneko H et al (2004) Association between serum C-reactive protein elevation and left ventricular thrombus formation after first anterior myocardial infarction. Chest 125:384–389
Lesnefsky EJ, Lundergan CF, Hodgson JM et al (1996) Increased left ventricular dysfunction in elderly patients despite successful thrombolysis: the GUSTO-1 angiographic experience. J Am Coll Cardiol 28(2):331–337
Grosso MA, Banerjee A, St Cyr JA et al (1992) Cardiac 5′-nucleotidase activity increases with age and inversely relates to recovery from ischemia. J Thorac Cardiovasc Surg 103(2):206–209
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rabbani, L.E., Waksmonski, C., Iqbal, S.N. et al. Determinants of left ventricular thrombus formation after primary percutaneous coronary intervention for anterior wall myocardial infarction. J Thromb Thrombolysis 25, 141–145 (2008). https://doi.org/10.1007/s11239-007-0064-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-007-0064-2