Abstract
We aimed to characterize the independent predictors of LVT following STEMI and the association with outcomes. The clinical predictors of left ventricular thrombus (LVT) formation after ST-segment elevation myocardial infarction (STEMI) are not well-defined in the contemporary era. We performed a retrospective analysis of STEMI patients at Duke from 2000 to 2011 who had a transthoracic echocardiogram within 90 days post-STEMI and compared patients with and without LVT (LVT+ vs. LVT−). Univariate Cox proportional hazards regression models of baseline characteristics were examined and significant variables were used in a multivariable model to assess adjusted relationships with LVT. A multivariable Cox PH survival model with covariate adjustments was used for assessment of LVT and long-term mortality. Of all eligible patients, 1734 patients met inclusion criteria and 4.3 % (N = 74) had a LVT. LVT+ patients tended to have a history of heart failure (HF) and higher initial troponin compared to LVT- patients. After adjustment, higher heart rate, non-white race, HF severity, and presence of left anterior descending artery (LAD) disease were independent predictors of LVT. There was a trend toward an association between LVT and increased all-cause mortality (HR 1.36; 95 % CI 0.84–2.21, P = 0.22), however this was not statistically significant. LVT was seen in over 4 % of this contemporary post-STEMI population. Several baseline characteristics were independently associated with LVT: Heart rate, HF severity, LAD disease, and non-white race. Prospective studies are warranted to determine whether anticoagulation in patients at increased risk for LVT improves outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Left ventricular thrombus (LVT) formation following acute myocardial infarction (AMI) is associated with increased morbidity and possibly mortality [1, 2]. The reports of LVT prevalence post-AMI vary markedly in the literature. For instance, data from the pre-percutaneous intervention (PCI) era demonstrated a prevalence ranging from 7 to 46 % [3–7], while data in the PCI-era suggest a prevalence ranging from 2.5 to 15 % [8–14]. Anterior myocardial infarction, reduced ejection fraction (EF) and larger infarct size have previously been identified as predictors of LVT formation [9–15]. However, beyond these variables, few other predictors have been identified in the present era.
Given the limited data available related to LVT in the contemporary, primary-PCI era, we aimed to evaluate the prevalence and outcomes associated with LVT formation post-AMI at a large tertiary care hospital. A secondary aim was to identify independent predictors of LVT formation post-AMI. We hypothesized that the prevalence of LVT formation would be on the lower-end of recently reported rates and that additional predictors of LVT formation, such as administration of post-AMI evidenced-based medications and the presence of comorbidities, would be identified. We also hypothesized that the presence of LVT post-STEMI would be associated with higher mortality in contrast to previous acute coronary syndrome cohorts which included patients with non-STEMI.
Methods
Study population
We performed a retrospective analysis of patients that underwent cardiac catheterization for ST-segment elevation MI (STEMI), from January 2000 through December 2011 at Duke University Medical Center (DUMC). Patients were identified within the Duke Databank for Cardiovascular Disease (DDCD), an ongoing databank of all patients who undergo diagnostic cardiac catheterization at DUMC [16]. In order to document the presence or absence of LVT within 90 days following STEMI, we merged these data with the Duke Echocardiography database, which is an ongoing database of all patients that receive an echocardiogram at DUMC [17]. Patients in whom LVT was detected were compared with patients without LVT.
Patients were excluded from analysis if they had primary valvular heart disease (defined as severe aortic or mitral insufficiency or severe stenosis of any heart valve), congenital heart disease, acquired immunodeficiency syndrome, metastatic cancer, or underwent CABG within 90 days of AMI. Remaining patients were excluded if they did not have a STEMI (i.e., those with non-STEMI or inadequate MI diagnosis). The patients with NSTEMI were excluded given lower likelihood of LVT formation in this patient population. Patients were included in the present analysis if they received a post-AMI TTE within 90 days of left heart catheterization. Data from the index catheterization were prospectively collected as part of routine patient care and compiled into the DDCD database. Given the limited number of patients with STEMI that did not undergo PCI and due to the confines of the database, only those that underwent catheterization were included in the analysis. Baseline clinical variables for each patient were stored in the DDCD using methods previously described [16]. Follow-up was obtained through self-administered questionnaires, with telephone follow-up of non-responders. Patients not contacted through this mechanism had vital status determined through a search of the National Death Index [18]. Death, stroke and acute MI were determined using methods previously described [16]. No extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Study definitions
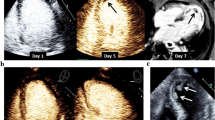
Acute STEMI was diagnosed by electrocardiogram changes consistent with STEMI along with associated symptoms of ischemia, peri-PCI cardiac biomarkers, and coronary angioplasty findings as used in previous analyses and consistent with current guidelines [12, 19–22]. LVT on TTE was identified using at least 2 views, typically long and short-axis views with or without an echocardiographic contrast agent. TTE images were read by a consensus of 2 experienced readers. Based on previous studies, LVT was defined as an “echo-dense mass” located in the LV cavity with “distinct margins” that are clearly differentiated from artifact or papillary muscles, but thought to be contiguous with the endocardium [9, 11, 13, 23, 24].
Endpoints
The primary end-point was the presence of LVT on TTE within 90 days of STEMI. The secondary end-point was long-term all-cause mortality.
Statistical methods
Baseline characteristics are described with medians and interquartile ranges (IQRs) for continuous variables and percentages for discrete variables in patients with versus those without LVT. These characteristics were compared using the Wilcoxon rank sum test for continuous variables and Chi square tests for categorical variables unless otherwise noted.
Univariate Cox proportional hazards regression models of baseline characteristics were examined and significant variables were included in a multivariable model to assess adjusted relationships with LVT within 90 days. Variables with p ≤ 0.1 in the unadjusted setting and those thought to have clinical importance were candidates for selection into the final model. Stepwise and backwards elimination techniques were examined for the final multivariable model. Using a landmark view at 90 days, a multivariable Cox Proportional Hazards survival model with covariate adjustments was used for assessment of the relationship of LVT and long-term mortality. A comprehensive set of covariates was used for the adjustment analysis based on clinical relevance and data from previous investigations [25]. A p value of <0.05 was used to indicate statistical significance for all comparisons. Statistical analyses were performed independently by the Duke Clinical Research Institute (Durham, North Carolina) using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina). The protocol was performed under the oversight of the Duke University institutional review board.
Results
Baseline characteristics of patients with and without LVT are presented in Table 1. Of all eligible patients, 1734 patients met inclusion criteria and 4.3 % (n = 74) had an LVT detected on TTE within 90 days following STEMI (Fig. 1). Median time to TTE from left heart catheterization was 3 days (Q1: 1 day, Q3: 20 days, mean: 15). LVT was detected in 48 of the 1261 patients (3.8 %) that had a PCI performed as compared with 26 patients (5.5 %) of the 473 that did not have any PCI performed (p = 0.086). Dividing the data into two-year subsets, LVT prevalence declined in recent years as follows: 7.4 % in 2001–2002, 7.3 % in 2003–2004, 2.9 % in 2005–2006, 3.0 % in 2007–2008, 3.3 % in 2009–2010 and 4.1 % in 2011. Adjusting for year of cardiac catheterization did not change the relationships of the baseline variables associated with the development of LVT. When monitoring for resolution of LVT on follow-up TTE, 24 % (18 pts) had resolution of a prior detected LVT within 90 days, 34 % within 180 days, and 43 % resolved within one year of detection.
Among patients diagnosed with a LVT, there was a greater proportion of non-white race, history of HF prior to STEMI, higher heart rate, lower EF, more significant LAD disease, and higher troponin levels compared with patients without LVT. There was no difference between the LVT and non-LVT groups with respect to the use of evidence-based post-AMI and HF medications, including ACE-inhibitors and beta-blockers. The diagnosis of LVT was less likely in those taking clopidogrel, but more likely in those taking diuretics on presentation. There was no significant difference between rates of echocardiography contrast use in the LVT (8.2 %) versus no LVT (7.2 %) patient groups (p = 0.7).
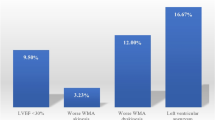
Using Cox Proportional Hazards time to event methods, the multivariable model for LVT within 90 days was reduced to 4 significant variables and a fifth borderline significant variable. The c-index for our final model was 0.73. Independent predictors of LVT formation are presented in Fig. 2. The strongest association with LVT in the Cox model was heart rate (p = 0.0003), followed by race (p = 0.0004). The presence of LAD disease was also associated with an increased risk in LVT formation with a hazard ratio (HR) of 8.17 (95 % confidence interval (CI), (2.00–33.32), p = 0.003). Additional independent predictors of LVT were HF severity (HR 1.26; 95 % CI 1.03–1.53) and non-white race (HR 2.32; 95 % CI 1.46-3.68). Despite the association with LAD disease, there was not a difference in LVT detection based on the degree/severity of CAD detected.
Using a landmark analysis at 90 days, the unadjusted relationship between LVT and mortality was not significant (Fig. 3). This remained true after adjusting for age, history of HF, heart rate, Charlson Comorbidity Index (removing HF and MI in order to be examined as covariates), gender, PCI in prior 90 days, history of peripheral vascular disease, cerebrovascular disease, diabetes mellitus, hyperlipidemia, hypertension, smoking, BMI, CAD severity, creatinine, hemoglobin, and BUN (LVT HR = 1.36, 95 % CI 0.84–2.21, p = 0.22). One hundred seventy-four patients died during the first 90 days and were not used in the long-term survival assessment. While there was a trend toward an association between LVT and increased all-cause mortality, statistical significance was not achieved.
Discussion
In a contemporary cohort of post-STEMI patients, we found that the prevalence of LVT formation within 90-days was 4.3 %. Several baseline characteristics such as heart rate, HF severity, LAD disease, and non-white race were independently associated with LVT. There was a trend that did not reach statistical significance toward an association between LVT and increased all-cause mortality (HR 1.36; 95 % CI 0.84–2.21, p = 0.22). These data support and extend previous research by documenting the prevalence and independent predictors of LVT formation in a large cohort of patients at a tertiary care hospital over more than 10 years.
This study supports a lower prevalence of LVT post-STEMI in the modern era compared with earlier studies. In order to assess for a change in the incidence of LVT formation and predictors over time, we divided the data into two-year groups (2001–2002, 2002–2003, etc.). There was a trend towards lower LVT detection over time (average incidence of 7.3 % in years 2001–2004 vs. 3.3 % from 2005 to 2011). This change in incidence over time may be attributable to more prompt and complete reperfusion interventions as well as advancements and more consistent use of evidence-based STEMI treatments. However, our analysis did not show any clear association between the use of evidence-based heart failure medications and LVT detection as discussed below. Despite the change in incidence of LVT over time, the predictors of LVT formation remained consistent during the study period.
Although the incidence of LVT formation in STEMI patients is modest, the overall number of patients who experience this complication may be substantial given that 77 out of 100,000 patients in the US have a STEMI annually and 41 % of first acute MI events in a European and US registry were STEMI [26–28]. This risk is significant given one American has a coronary event every 34 seconds, according to 2014 AHA statistical update [29].
The variability in detection rates in previous studies, especially in the earlier studies, is likely due, in part, to the different treatments utilized in the thrombolysis and pre-PCI era. Although there was a trend toward lower LVT detection within the patients that underwent PCI compared to those that did not, this difference did not reach statistical significance. Interestingly, the largest study thus far (GISSI-3) evaluating the risk of LVT formation following AMI also reported a lower prevalence rate of approximately 5 % [8]. An additional component of variability in the reported prevalence rates of LVT post-AMI can further be explained by undetected LVT formation following hospital discharge [12, 30]. Previous studies have suggested that late LVT formation risk is significant, with more than 40 % of all post-AMI LVT detected following discharge [5, 10]. Solheim et al. also reported a prevalence of LVT as high as 15 % within 3 months of AMI [11].
Another explanation for the variability regarding prevalence reports is related to overall clinical detection techniques. In general, TTE has modest sensitivity and excellent specificity (23–60 and 96 % respectively) for the detection of LVT and is dependent upon the experience of the reader as well as the size of the thrombus. Notably, the sensitivity of TTE for LVT is significantly increased when TTE is ordered primarily for evaluation of LVT and when contrast is utilized [2, 31, 32]. A recent retrospective study found similar LVT formation rates of 4 % following STEMI utilizing TTE as the primary modality with contrast used in inconclusive cases [14]. The use of contrast with TTE in this study was not associated with increased LVT detection rates. Furthermore, our study focused predominantly on LVT detection with early TTE (IQR from STEMI to TTE was approximately 1-3 days) when the process of thrombus formation may be more dynamic and conceivably LV thrombi may be transient and or smaller limiting detection.
Previous studies have also demonstrated that presence of LAD disease or anterior MI, larger infarct size (by imaging or peak cardiac marker levels), and heart failure are associated with increased risk for LVT formation [5, 10–13, 15, 23]. In this analysis, independent predictors of LVT formation were the presence of LAD disease, non-white race, heart rate, and heart failure severity.
Ejection fraction (EF) obtained by either contrast ventriculography or echocardiogram at the time of left heart catheterization, based on availability within our database, was not included in the multivariate analysis due to missing data points among our analytic cohort, despite reaching a significant association in our univariate analysis (LR 29.5 (Pr > Chi sq < 0.001), HR 0.74, 95 % CI 0.66–0.83). Missing data points are likely explained by non-numerical reporting of EF, especially those with normal values, which prevented this data from being accessed via our TTE database and the DDCD. As demonstrated in aforementioned prior studies, it has been shown to be a significant predictor of LVT formation.
Unique to our study was the finding that non-white race, particularly black and Native Americans, was independently associated with higher rates of LVT formation. This has not been demonstrated in prior studies and suggests genetic variables, socioeconomic factors, or environmental risk factors that may place this patient population at higher risk of LVT formation. Our analysis utilized the DDCD, which includes a good representation of minorities and therefore likely enabled the detection of a potential difference in LVT formation risk among minority populations as compared to the white race population.
Recent studies have demonstrated that African Americans and also other minorities are less likely to undergo invasive cardiac procedures/revascularization than white patients, which was independent of services available and severity of disease [33–35]. While this might partially explain the higher risk of LVT detection with non-white race in clinical practice, it is unlikely to have influenced these results, as cardiac catheterization for STEMI was an inclusion criterion for all patients regardless of race. Differences in LVT formation between races are more likely attributed to genetic differences. A recent small study was able to demonstrate higher platelet reactivity and aggregation among black subjects compared to whites explained by increased activity of a platelet activating receptor (PAR4) due to genetically different RNA expression, which could be potential mechanisms behind the racial differences in thrombus formation [36]. Furthermore, higher platelet reactivity and CYP2C19 allele expression in African-Americans compared to similar white patients undergoing PCI has also been demonstrated [37].
The present analysis also demonstrated an independent association with increased HR at the time of AMI and LVT formation. Tachycardia in the peri-MI period implies more compromised myocardium, larger infarct size, and likely diminished ejection fraction, all of which have been identified as drivers to LVT formation [38, 39]. Furthermore, the increased risk associated with tachycardia may be attributable to the higher risk of supraventricular tachyarrhythmia associated with larger infarcts, anterior MI, and decreased left ventricular function [39–41].
Despite our hypothesis that improved medical management of heart failure would reduce the risk of LVT formation, we did not find an association between the use of ACE-inhibitors/ARBs, beta-blockers, or nitrates and lower risk for LVT. While the risk of LVT formation may be modified by evidenced-based HF therapy in the early post-STEMI period, we believe that our analyses may have been limited to detect the independent effect of medications on LVT due to high overall use in our cohort. Nevertheless, our analyses suggest that even in the context of high routine use of guideline-recommended medical therapies in the post-STEMI population, a significant number of patients are still at risk for LVT formation.
In the present analysis, there was a trend toward increased mortality associated with LVT, but this did not meet statistical significance. Despite the lack of data on systemic embolization events within our database, previous studies have demonstrated increased risk of cerebrovascular events associated with LVT, but despite this risk, there was no change in associated mortality risk, which is in line with our results [1, 42]. Furthermore, a smaller study (N = 144) demonstrated ischemic cardiomyopathy as an independent predictor of LVT formation and systemic embolization, with embolization associated with a higher mortality [43]. None of these studies included patients in the peri-AMI period and therefore may be quite distinct from the post-STEMI population in the present cohort. However, a recent study in a post-STEMI population did not demonstrate any change in mortality risk with LVT presence [14].
Future prospective trials are needed to investigate these high-risk patient populations in order to determine whether they may benefit from prophylactic anticoagulation following STEMI. Currently, it is a class IIb recommendation from the ACCF/AHA guidelines on STEMI management that anticoagulation should be considered for patients following STEMI with anterior-apical akinesis or dyskinesis [22]. However, triple therapy anticoagulation following PCI is associated with as much as three times the bleeding risk compared to dual anti-platelet therapy alone [44]. Deciding whether or not to empirically anticoagulate this high-risk population has significant implications. The results of the current study may lead to future trials that will further explore patient populations with the aforementioned risk factors associated with higher risk for LVT formation.
Limitations
Our study also has several limitations including a retrospective design, a long accrual time, and a modest event rate for LVT formation. In addition, while echocardiography is the dominant imaging modality at the point of care available for post-STEMI care, it may underestimate LVT prevalence as compared with cardiac MRI [31, 32]. Additionally, we do not have inter- or intra-observer variability data on interpretation of the echocardiograms given the retrospective nature of the study. Furthermore, the majority of echocardiograms did not involve contrast as there was evolving guidelines regarding the safety of contrast use during the period of study; therefore, future prospective studies with routine use of contrast are required to validate these findings. Since our study required that patients must have had a TTE as part of routine clinical care, LVT may have been under-reported and the lack of association between LVT and increased mortality may represent a type 2 error. Furthermore, we do not have data regarding the specific characteristics of the LVT (e.g., size, location, mobility) detected on echocardiography, which may have a differential association with outcome. Also, our findings were not validated with a second cohort or prospective cohort such that further study is warranted to support these data. As mentioned above, despite a relatively low LVT event rate, this study represents one of the largest to date with regards to LVT event rates post-STEMI.
Conclusion
We demonstrated that the prevalence of LVT detected on TTE within 90 days of a STEMI was 4.3 % in a large contemporary population. These data suggest that the incidence of LV thrombus formation following STEMI remains high even in the primary PCI-era with high use of evidenced-based treatment of STEMI. Most paramount, non-white race was an independent predictor of LVT formation, which has not been previously noted. Therefore LVT formation post-STEMI might be a significant risk faced by minorities, potentially influenced by genetic variables or environmental risk factors. Tachycardia, anterior MI, and congestive heart failure severity were also associated with an increased incidence of LVT formation. These findings not only provide additional evidence that support previously documented risk factors for LVT formation, but also demonstrate unique risk factors not previously identified that could be utilized to identify STEMI patients at higher risk of LVT formation. These data support further exploration of novel strategies to mitigate adverse outcomes in STEMI patients at risk for LVT formation.
Abbreviations
- LVT:
-
Left ventricular thrombus
- HF:
-
Heart failure
- AMI:
-
Acute myocardial infarction
- STEMI:
-
ST-segment elevation myocardial infarction
- TTE:
-
Transthoracic echocardiogram
- PCI:
-
Percutaneous coronary intervention
- EF:
-
Ejection fraction
- IQR:
-
Interquartile range
- DDCD:
-
Duke Databank for Cardiovascular Disease
References
Crawford TC et al (2004) Prognostic usefulness of left ventricular thrombus by echocardiography in dilated cardiomyopathy in predicting stroke, transient ischemic attack, and death. Am J Cardiol 93(4):500–503
Weinsaft JW et al (2008) Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol 52(2):148–157
Asinger RW et al (1981) Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med 305(6):297–302
Jugdutt BI, Sivaram CA (1989) Prospective two-dimensional echocardiographic evaluation of left ventricular thrombus and embolism after acute myocardial infarction. J Am Coll Cardiol 13(3):554–564
Keren A et al (1990) Natural history of left ventricular thrombi: their appearance and resolution in the posthospitalization period of acute myocardial infarction. J Am Coll Cardiol 15(4):790–800
Visser CA et al (1983) Left ventricular thrombus following acute myocardial infarction: a prospective serial echocardiographic study of 96 patients. Eur Heart J 4(5):333–337
Delewi R, Zijlstra F, Piek JJ (2012) Left ventricular thrombus formation after acute myocardial infarction. Heart 98(23):1743–1749
Ascione L et al (2002) Relation between early mitral regurgitation and left ventricular thrombus formation after acute myocardial infarction: results of the GISSI-3 echo substudy. Heart 88(2):131–136
Chiarella F et al (1998) Predischarge two-dimensional echocardiographic evaluation of left ventricular thrombosis after acute myocardial infarction in the GISSI-3 study. Am J Cardiol 81(7):822–827
Delewi R et al (2012) Left ventricular thrombus formation after acute myocardial infarction as assessed by cardiovascular magnetic resonance imaging. Eur J Radiol 81(12):3900–3904
Solheim S et al (2010) Frequency of left ventricular thrombus in patients with anterior wall acute myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy. Am J Cardiol 106(9):1197–1200
Shacham Y et al (2013) Frequency and correlates of early left ventricular thrombus formation following anterior wall acute myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol 111(5):667–670
Zielinska M, Kaczmarek K, Tylkowski M (2008) Predictors of left ventricular thrombus formation in acute myocardial infarction treated with successful primary angioplasty with stenting. Am J Med Sci 335(3):171–176
Gianstefani S et al (2014) Incidence and predictors of early left ventricular thrombus after ST-elevation myocardial infarction in the contemporary era of primary percutaneous coronary intervention. Am J Cardiol 113:1111–1116
Osherov AB et al (2009) Incidence of early left ventricular thrombus after acute anterior wall myocardial infarction in the primary coronary intervention era. Am Heart J 157(6):1074–1080
Harris PJ et al (1980) Outcome in medically treated coronary artery disease. Ischemic events: nonfatal infarction and death. Circulation 62(4):718–726
Velazquez EJ et al (2012) Survival in Patients with Moderate or Severe Mitral Regurgitation at High Risk for Surgery Treated With and Without the MitraClip: A Propensity Matched Comparison. J Am Coll Cardiol 59(13):A490
Boyle CA, Decoufle P (1990) National sources of vital status information: extent of coverage and possible selectivity in reporting. Am J Epidemiol 131(1):160–168
Thygesen K et al (2012) Third universal definition of myocardial infarction. J Am Coll Cardiol 60(16):1581–1598
Wagner GS (2007) Marriott’s practical electrocardiography, 11th edn. Lippincott Williams & Wilkins, Philadelphia
Sgarbossa EB et al (1996) Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. GUSTO-1 (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) investigators. N Engl J Med 334(8):481–487
O’Gara PT et al (2013) ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 61(4):e78–e140
Rehan A et al (2006) Incidence of post myocardial infarction left ventricular thrombus formation in the era of primary percutaneous intervention and glycoprotein IIb/IIIa inhibitors. A prospective observational study. Cardiovasc Ultrasound 4:20
Weinsaft JW et al (2011) LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging 4(7):702–712
Mentz RJ et al (2012) Comparison of clinical characteristics and long-term outcomes of patients with ischemic cardiomyopathy with versus without angina pectoris (from the Duke Databank for Cardiovascular Disease). Am J Cardiol 109(9):1272–1277
Gale CP et al (2014) Trends in hospital treatments, including revascularisation, following acute myocardial infarction, 2003–2010: a multilevel and relative survival analysis for the National Institute for Cardiovascular Outcomes Research (NICOR). Heart 100(7):582–589
McManus DD et al (2011) Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am J Med 124(1):40–47
Rogers WJ et al (2008) Trends in presenting characteristics and hospital mortality among patients with ST elevation and non-ST elevation myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J 156(6):1026–1034
Go AS et al (2014) Executive summary: heart disease and stroke statistics–2014 update: a report from the american heart association. Circulation 129(3):399–410
Rabbani LE et al (2008) Determinants of left ventricular thrombus formation after primary percutaneous coronary intervention for anterior wall myocardial infarction. J Thromb Thrombolysis 25(2):141–145
Srichai MB et al (2006) Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 152(1):75–84
Weinsaft JW et al (2009) Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovasc Imaging 2(8):969–979
Kressin NR, Petersen LA (2001) Racial differences in the use of invasive cardiovascular procedures: review of the literature and prescription for future research. Ann Intern Med 135(5):352–366
Gregory PM et al (1999) Impact of availability of hospital-based invasive cardiac services on racial differences in the use of these services. Am Heart J 138(3 Pt 1):507–517
Fincher C et al (2004) Racial disparities in coronary heart disease: a sociological view of the medical literature on physician bias. Ethn Dis 14(3):360–371
Edelstein LC et al (2013) Racial differences in human platelet PAR4 reactivity reflect expression of PCTP and miR-376c. Nat Med 19:1609–1616
Pendyala LK et al (2013) Racial disparity with on-treatment platelet reactivity in patients undergoing percutaneous coronary intervention. Am Heart J 166(2):266–272
Crimm A et al (1984) Prognostic significance of isolated sinus tachycardia during first three days of acute myocardial infarction. Am J Med 76(6):983–988
Galcera Tomas J (1999) Incidence, clinical characteristics and prognostic significance of supraventricular tachyarrhythmias in acute myocardial infarction. Rev Esp Cardiol 52(9):647–655
Schmitt J et al (2009) Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J 30(9):1038–1045
Pizzetti F et al (2001) Incidence and prognostic significance of atrial fibrillation in acute myocardial infarction: the GISSI-3 data. Heart 86(5):527–532
Gottdiener JS et al (1983) Frequency and embolic potential of left ventricular thrombus in dilated cardiomyopathy: assessment by 2-dimensional echocardiography. Am J Cardiol 52(10):1281–1285
Sharma ND et al (2000) Left ventricular thrombus and subsequent thromboembolism in patients with severe systolic dysfunction. Chest 117(2):314–320
Nikolsky E et al (2012) Outcomes of patients treated with triple antithrombotic therapy after primary percutaneous coronary intervention for ST-elevation myocardial infarction (from the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction [HORIZONS-AMI] trial). Am J Cardiol 109(6):831–838
Funding
No extramural funding. Mentz, R: supported by Grant Number 5-T32GM086330 from the National Institute of General Medical Sciences. Fiuzat, M and O’Connor, C: Roche Diagnostics—research Grants; Velazquez, E: Consulting Fees/Honoraria: Novartis Pharmaceuticals Corp.; Research/Research Grants: Ikaria Pharmaceuticals; supported by grants from the National Institute of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. Garber completed the project while working at Duke, finished the manuscript after taking new position at VCU.
Rights and permissions
About this article
Cite this article
Garber, A.M., Mentz, R.J., Al-Khalidi, H.R. et al. Clinical predictors and outcomes of patients with left ventricular thrombus following ST-segment elevation myocardial infarction. J Thromb Thrombolysis 41, 365–373 (2016). https://doi.org/10.1007/s11239-015-1252-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-015-1252-0