A heterogeneous catalyst for the hydrochlorination of acetylene using gaseous HCl was obtained by prior mechanical activation of K2PdCl4 powder in an atmosphere of acetylene or propylene. Active sites are formed during the mechanical treatment in the surface layers of the catalyst, which are Pd(II) complexes with a coordination vacancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
One area in the development of green chemistry, which has recently become the predominant trend in the advancement of science [1, 2], is the chemistry of mechanically activated reactions involving solids. Examples of such processes include stoichiometric [2, 3] and catalytic reactions [4, 5]. In the latter case, a heterogeneous catalyst undergoes mechanical treatment. Catalytically activated states are generated on the surface of solid-state catalysts as a result of mechanical activation. Some of these states are retained on the relaxed surface, which permits us to carry out catalytic transformations on a catalyst subjected to prior mechanical activation without the continual input of mechanical energy, thereby significantly simplifying the process [6].
In previous work [7], we discovered the catalysis of the hydrochlorination of acetylene using gaseous HCl on the surface of K2PtCl4 subjected to prior mechanical activation in an atmosphere of acetylene, ethylene, or propylene. The role of the active states is carried out by complexes with a coordination vacancy generated by prior mechanical activation of the catalyst:

Such species are capable of coordinating acetylene virtually at the rate of diffusion to give π-complexes, which are key intermediates in the catalytic transformations of acetylene. In the mechanical activation of the platinum salt in an atmosphere of the gases mentioned above, equilibrium (1) is shifted to the right due to stabilization of the coordination-unsaturated species by means of π-coordination of the unsaturated hydrocarbons to them.
In recent work [8], we found that mechanical activation of a similar palladium salt, namely, K2PdCl4, also leads to the formation of an acetylene hydrochlorination catalyst. As in the system with K2PdCl4, the reaction on the surface of K2PdCl4 mechanically activated in the air proceeds very slowly. Prior mechanical activation of K2PdCl4 in an atmosphere of acetylene, ethylene, or propylene is necessary to obtain a more active catalyst. In the present work, we elucidated the nature of the active states of the catalyst generated by prior mechanical activation of K2PdCl4
Experimental
The preparation of the catalyst and the kinetic study with gas-liquid monitoring of the substrate and product concentrations were carried out as in our previous work [8]. The relative acetylene concentration φ(RH) was defined as the ratio of the areas of the chromatographic peaks for acetylene and the internal standard (ethane). The observed reaction rate constant was found using the equation k ef = –d(ln φ(RH))/dt. The specific dose of the mechanical energy absorbed by the powder (D sp) was determined using the equation D sp = It [9], where I is the specific energy intensity of the mill (∼15 W/kg [8]) and t is the mechanical activation time. The specific surface was determined by the BET method relative to argon desorption.
Gaseous CO was obtained according to Rapoport and Il’inskaya [10]. At the end of the mechanical activation, the reactor holding the K2PdCl4 powder was flushed with argon. Then, 60 mL CO was introduced. The reactor was sealed and maintained for 1.5 h. Then, the reactor contents were removed. The diffuse scattering IR spectra of palladium carbonyl complexes were identified using a Bruker Tensor 27 spectrometer with a Specac attachment and a program permitting integration of the IR signal.
The surface morphology was studied using a JEOL JSM-6490LV scanning electron microscope manufactured in Japan. The chemical analysis was carried out using an Oxford Instruments INCA Penta FET×3 energy dispersion spectrometer manufactured in Great Britain. The X-ray studies were carried out on a DRON-3 diffractometer with monochromatic CuK α radiation and a nickel filter.
The pyridinium NMR spectra of were taken on a Bruker BioSpin Avance-II-400 spectrometer at 400 MHz. A sample of 10 mL pyridine was introduced into the sealed reactor containing 0.25 g K2PdCl4 subjected to prior activation in an acetylene atmosphere and flushed with dry argon. The reactor was left for 1 h. Then, the reactor contents were removed and a weighed portion of the powder was dissolved in 1 M DCl in D2O. A commercial sample of sodium benzoate was used as the internal weighting standard.
Results and discussion
X-ray Phase Analysis. Studies of the crystal structure of the samples of the initial palladium salt and samples obtained after mechanical activation in the air (sample 1) and in an acetylene atmosphere (sample 2) showed the presence of all the X-ray diffraction maxima corresponding to the monophasic K2PdCl4 salt. Comparison of the diffraction patterns of the K2PdCl4 samples mechanically activated in the air and in an acetylene atmosphere suggests that the composition of the dispersion medium does not significantly alter the texture of the samples. Mechanical activation in an acetylene atmosphere leads only to a decrease in particle size and partial amorphization of the surface layer. Estimation using the Scherrer equation and the widths of the corresponding X-ray reflections indicates that the crystallites sample 2 are smaller than in sample 1 by a factor of 1.6, which corresponds to an increase in the interparticle surface by a factor of 2–3.
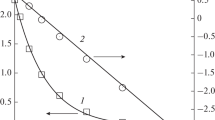
Catalyst activity at the end of the mechanical activation of the initial palladium salt increases with increasing time of the prior mechanical activation of K2PdCl4 in an acetylene atmosphere (Table 1). This finding may result from an increase in the specific catalyst surface and increase in the number of active sites generated by mechanical treatment. The increase in the specific surface relative to the specific dose of mechanical energy absorbed by the catalyst (Fig. 1a) corresponds to the equation
where S0 is the specific surface of the initial K2PdCl4 powder, \( {S_0} + S_{\text{sp}}^* \) is the limiting value of the specific surface, and ηs is a term inverse to the work of forming the new surface.
a) Dependence of the specific surface S sp of the catalyst (1) and the amount of vinyl chloride liberated during treatment ν (2) on the specific dose of mechanical energy absorbed by the K2PdCl4 powder (the points on curve 1 correspond to experimental values of S sp, the envelope curve was obtained by calculation using Eq. (2) with ηs =(8 ± 2)∙10–2 g/J and \( S_{\text{sp}}^* = 6.7 \pm 0.3\;{{{{\text{m}}^2}} \mathord{\left/{\vphantom {{{{\text{m}}^2}} {\text{g}}}} \right.} {\text{g}}}) \), b) dependence of the observed rate constant for acetylene consumption k* relative to a unit of catalyst surface area (1) and fraction ε of the surface palladium complexes deficient in chloride ligands (2) on the specific dose of mechanical energy absorbed by the K2PdCl4 powder (the points on curves 1 and 2 correspond to experimental values of k* and ε, the envelope curves were obtained by calculation using Eqs. (3) and (4) for η = (3.2 ± 0.6)∙10–2 g/J, k* = 6.8 ± 0.4 and ηε = (2.2 ± 0.6)∙10–2 g/J, εmax = 0.41 ± 0.04, respectively).
In order to exclude the effect of the increase in surface on the catalytic activity of K2PdCl4, let us consider the reaction rate constant relative to a unit of catalyst surface, k* = k ef(S sp m)–1, where m is the catalyst mass. The dependence of k* on the specific dose of mechanical energy absorbed by the powder corresponds to the following equation (Fig. 1b)
where \( k_{{ \max }}^* \) is the maximum value of k* and h is a constant inverse to the work of formation of the active catalyst sites during prior mechanical activation.
Equations (2) and (3) satisfy the experimental values for ηs =(8 ± 2)∙10–2 g/J and η = (3.2 ± 0.6)∙10–2 g/J. The finding that ηs is about three times larger than η indicates that the increase in the specific surface of the salt and the increase in the amount of active catalyst sites are independent processes.
The mechanical activation of K2PdCl4 powder in an atmosphere of acetylene or propylene is accompanied by the release of vinyl chloride or isopropyl chloride. The source of the chlorine atoms in the organic chlorides can only be the K2PdCl4 complex. In other words, the formation of palladium(II) complexes deficient in chloride ligands (Pd*) along with the indicated products is a stoichiometric consequence of such tribochemical reactions. The typical kinetics of vinyl chloride accumulation in the tribochemical reaction of K2PdCl4 with acetylene is sigmoid in shape (Fig. 1a). The initial portion of the kinetic curve corresponds to the period, in which the introduced mechanical energy is transformed predominantly into development of the surface. At the end of the initial period of vinyl chloride accumulation, the mechanical activation proceeds more efficiently, which indicates the transformation of the mechanical energy mainly into the formation of Pd* complexes.
These observations suggest that catalyst activity is predominantly a function not of the increase in the specific surface of the catalyst but rather by the amount of active sites generated during the prior mechanical activation of K2PdCl4 represented by palladium(II) complexes deficient in chloride ligands.
Scanning Electron Microscopy. The mean diameters of the K2PdCl4 powder particles obtained by the mechanical activation of the initial palladium salt in the air (sample 1) and in acetylene (sample 2) are 220 and 160 nm, respectively. The difference in the diameter of the microparticles by a factor of approximately 1.4 corresponds to an approximate doubling of the specific surface of sample 2. This result is similar to the value obtained by X-ray diffraction, which is clearly a consequence of change in the same direction of crystallite size and microparticle size.
Microelemental analysis showed the presence of elements corresponding to K2PdCl4 in the composition of the catalyst samples. Increasing the time of mechanical activation of the starting salt in an acetylene atmosphere leads to a steady decrease in the chlorine/palladium ratio in the catalyst surface layer (Table 1). The dependence of the fraction of surface palladium complexes deficient in chloride ligand ε = N(Pd*)/[N(PdCl 2-4 ) + N(Pd*)] on the specific dose of mechanical energy absorbed by the powder is a curve with saturation (Fig. 1b) and corresponds to the equation
where εmax is the maximum value of parameter ε, ηε is a constant characterizing the efficiency of the transformation of mechanical energy upon mechanical activation of the catalyst into the formation of Pd(II) complexes deficient in chloride ligands. Equation (4) corresponds to the experimentally obtained equation ηε = (2.2 ± 0.6)∙10–2 g/J. The values of constants ηε and η are identical within experimental error. Thus, there should be a linear correlation between k* and ε, which is indeed observed (Fig. 2a). The existence of such a correlation between catalyst activity and the fraction of surface Pd* complexes is in accord with the assumption that these complexes are the active sites of the heterogeneous catalysts.
Linear correlations between: a) the surface concentration of chemisorbed pyridine c (1), observed acetylene consumption rate constant k* reduced to a unit of catalyst surface area (2), and value of the fraction of surface palladium complexes deficient in chloride ligands ε; b) integral optical density of the stretching band of carbonyl ligands A and amount of coordinated pyridine υ(PyH).
Nature of the Active States of Pd*. Complexes of palladium with coordination vacancies may be, as in the K2PtCl4 system, the active states of the palladium catalyst. Another possibility is tetracoordinated palladium(II) complexes containing a ligand other than a chloride ion or a bridging chloride ligand. Distinguishing between these two possibilities is feasible, for example, by exposing the surface of the freshly-treated catalyst in an atmosphere of gas containing M molecules, which may coordinate with the metal ion to give strong complexes.Footnote 1 Surface palladium complexes with coordination vacancies should chemisorb gas to form species [PdCl3M]–. Carbon monoxide and pyridine vapor were used as such gases.
Such chemisorption actually occurs. The diffuse scattering IR spectra of the catalyst samples exposed in a carbon monoxide atmosphere show bands at 1907 and 1972 cm–1, corresponding to CO stretching vibrations. The IR spectra of similarly prepared samples but maintained for8hat120 °C or maintained at room temperature for six months contain only the band at 1907 cm–1. We may conclude that the high-frequency band corresponds to stretching vibrations of physically adsorbed CO molecules, while the band at 1907 cm–1 corresponds to stretching vibrations of carbonyl ligands. The value for the integral optical density of the band at 1907 cm–1, A, steadily increases with increasing specific dose of mechanical energy absorbed by the catalyst (Table 1).
Pyridine adsorbed by the catalyst samples was extracted with 1 M DCl in D2O and identified as the pyridinium cation using 1H NMR spectroscopy. The amount of extracted pyridine is the same within experimental error for freshly-prepared samples and samples maintained at 120 °C for 1.5 h, which indicates strong bonding of this compound. The amount of extracted pyridine increases with increasing time of prior mechanical activation of K2PdCl4 (Table 1). In order to exclude the effect of the increase in the surface on pyridine adsorption, let us examine the amount of pyridine reduced to a unit of catalyst surface c = υ(PyH)/S sp. The linear dependence of the surface pyridine concentration c on ε (Fig. 2a) is in accord with the assumption that the palladium(II) complexes with coordination vacancies are the active catalyst sites. As expected, there is a correlation between the integral optical density of the carbonyl ligand bands A and the amount of coordinated pyridine (Fig. 2b).
We note that propylene and acetylene, whose molecules are capable of occupying vacant coordination sites, were used as gases in systems with K2PtCl4 in our previous work [7]. In the case of acetylene, the formation of π-acetylene complexes was confirmed by diffuse scattering IR spectroscopy as a band at 2095 cm–1, corresponding to stretching vibrations of the C—C triple bond. This band is lacking in the IR spectrum for the system with K2PdCl4. This discrepancy can probably be attributed to the lower stability of palladium(II) π-acetylene complexes [12] so that such complexes could not be detected directly.
Thus, mechanical activation of the heterogeneous catalyst for acetylene hydrochlorination in the palladium system examined, as well as in the previously studied system with K2PtCl4, leads to the formation of active sites which are complexes with coordination vacancies:

Dispersion of the catalyst in an atmosphere of acetylene or propylene in contrast to dispersion in the air creates a higher surface concentration of complexes with coordination vacancies due to shifting of Eq. (5) to the right as a consequence of binding of the chloride ions into the corresponding organic chlorides, namely, vinyl chloride or isopropyl chloride. This hypothesis may account for the differences between the catalytic activities of K2PdCl4 samples treated in an atmosphere of these “active” gases and samples treated in the air. The question concerning the origin of the protium entering vinyl chloride or isopropyl chloride formed in the tribochemical reaction of acetylene or propylene with K2PdCl4 remains open. The protium source could be, for example, water molecules adsorbed in the reactor and on the catalyst surface. However, we could not detect monodeuterated vinyl chloride by 1H NMR spectroscopy. The formation of monodeuterated vinyl chloride would be expected in the tribochemical reaction of acetylene with K2PdCl4 in the presence of 1 μLD2O: the vinyl chloride isolated did not contain deuterium. Nevertheless, this result may be the result of a high kinetic isotope effect of the tribochemical reaction and small amount of accumulated product.
This work was carried out with the support of the National Academy of Sciences of Ukraine (Grant No. 0101U008137) and the Ukraine Ministry of Education and Science (Grant No. 0103I003614).
Notes
Ligand exchange is usually accomplished through a dissociative mechanism but the dissociation of ligands is unlikely in the absence of salvation [11].
References
P. T. Anastas and J. C. Wamer, Green Chemistry: Theory and Practice, Oxford Science Publ., Oxford (1998).
V. D. Pokhodenko and V. V. Pavlishchuk, Teor. Éksp. Khim., 38, No. 2, 67–83 (2002). [Theor. Exp. Chem., 38, No. 2, 69–87 (2002) (Engl. Transl.).]
A. M. Dubinskaya, Usp. Khim., 68, No. 8, 708 (1999).
G. Mulas, M. Monagheddu, G. Cocco, et al., J. Mater. Synth. Proc., 8, Nos. 5/6, 385 (2000).
K. Domen, S. Ikeda, T. Takata, et al., Appl. Energy, 67, 159 (2000).
S. A. Mitchenko, Teor. Éksp. Khim., 43, No. 4, 199–214 (2007). [Theor. Exp. Chem., 43, No. 4, 211–228 (2007) (Engl. Transl.).]
S. A. Mitchenko, T. V. Krasnyakova, R. S. Mitchenko, and A. N. Korduban, J. Mol. Catal. A, 275, 101 (2007).
S. A. Mitchenko, T. V. Krasnyakova, and I. V. Zhikharev, Teor. Éksp. Khim., 44, No. 5, 306–309 (2008). [Theor. Exp. Chem., 44, No. 5, 316–319 (2008) (Engl. Transl.).]
P. Yu. Butyagin, A. R. Kuznetsov, and I. K. Pavlychev, Prib. Tekhn. Éksp., No. 6, 201 (1986).
F. M. Rapoport and A. A. Il’inskaya, Laboratory Methods for the Preparation of Pure Gases [in Russian], Goskhimizdat, Moscow (1963).
F. Basolo and R. G. Pearson, Mechanisms of Inorganic Reactions [Russian translation], Mir, Moscow (1971).
M. Herberhold, Metal π-Complexes. Complexes with Monolefinic Ligands [Russian translation], Mir, Moscow (1975), p. 134.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoreticheskaya i Éksperimental’naya Khimiya, Vol. 46, No. 1, pp. 32–37, January–February, 2010.
Rights and permissions
About this article
Cite this article
Mitchenko, S.A., Krasnyakova, T.V. & Zhikharev, I.V. Effect of mechanicochemical treatment on the activity of K2PdCl4 in the heterogeneous catalytic hydrochlorination of acetylene. Theor Exp Chem 46, 32–38 (2010). https://doi.org/10.1007/s11237-010-9117-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-010-9117-2