Abstract
A new monogenean species, Kannaphallus leptosomus n. sp., from the gills of the diamond trevally, Scyris indica Rüppell, caught off Terengganu, Peninsular Malaysia is described with molecular data. The new species differs from other species of the genus by the morphology of the largest clamp, the presence of a penis gun-associated needle, the unarmed genital atrium size, and the presence of two independent vaginal tubes. Previous records of Kannaphallus species were compiled, and four nominal species including one species incertae sedis (K. virilis Unnithan, 1957; K. lateriporis Mamaev, 1988; K. leptosomus n. sp.; and K. mochimae Fuentes Zambrano, 1998 incertae sedis) and two undescribed species recorded as invalid names were listed. Cemocotylelloides carangis Ramalingam, 1969 was considered a junior objective synonym of K. univaginalis Ramalingam, 1960; this species was treated as Cemocotylelloides univaginalis n. comb. In addition, Unnithan’s materials including type specimens of K. virilis could not be found in specimen repositories in India, and it appears that the specimens were probably not deposited in any institution or have been subsequently lost. Based on the phylogenetic analysis of 28S rDNA sequences, Heteraxinidae was broadly divided into two clades, and Heteraxininae and Cemocotylinae were shown as polyphyletic groups, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The diamond trevally, Scyris indica Rüppell, a carangid fish belonging to the subfamily Caranginae, is widely distributed in the tropic and sub-tropic of the Indo-Pacific Ocean (Froese & Pauly, 2021). This species is one of the commercially important and cultured in Singapore (Chou & Lee, 1997) and one of the targets for sport fishing (Froese & Pauly, 2021).
The genus Kannaphallus Unnithan, 1957 (Monogenea: Heteraxinidae) was established as monotypic for K. virilis Unnithan, 1957 from the gills of Atropus atropos (Bloch & Schneider) (as Caranx atropus) (Perciformes: Carangidae) off Trivandrum, Kerala, South India (Unnithan, 1957). This genus is distinguishable from the other heteraxinid genera by the presence of asymmetric clamps, paired vaginas, and a sclerotized ejaculatory duct named the ‘penis gun’ that is associated with the cirrus (Price, 1962; Mamaev, 1988). Price (1962) classified Kannaphallus into the subfamily Heteraxininae under the family Heteraxinidae. However, Mamaev (1988) transferred the genus into the subfamily Cemocotylinae based on the common morphological features with species of Cemocotyle Sproston, 1946, Cemocotylella Price, 1962, and Cemocotylelloides Ramalingam, 1969.
There are four species included in Kannaphallus: K. virilis, K. lateriporis Mamaev, 1988 described from Caranx sexfasciatus Quoy & Gaimard in the Gulf of the Mannar, South India (Mamaev, 1988), K. mochimae Fuentes Zambrano, 1998 from Acanthurus coeruleus Bloch & Schneider (Perciformes: Acanthuridae) in Mochima, Venezuela (Fuentes Zambrano, 1998), and K. univaginalis Ramalingam, 1960 from Ca. sexfasciatus in South India (Ramalingam, 1960). However, K. univaginalis was poorly described in the original description and considered as invalid by Price (1962). Nevertheless, Fuentes Zambrano (1998) treated it as a valid taxon without any further discussion.
During our parasitological survey of commercially important fishes in coastal waters of Malaysia, specimens of Kannaphallus species were collected from Scyris indica off Terengganu, Peninsular Malaysia. This is the first record of Kannaphallus species occurrence in Malaysia. We herein describe those specimens as a new species of Kannaphallus with molecular analysis. In addition, the results of an investigation for type specimens of K. virilis in specimen repositories of India are reported.
Materials and methods
One specimen of Scyris indica caught by local fishermen off Terengganu on the east coast of the Peninsular Malaysia was purchased from a local fish market at Kuala Terengganu, Malaysia on 8 December 2019. The specimen was transported on ice to the Aquatic Science Laboratory of Universiti Sultan Zainal Abidin, Besut Campus and examined for parasites. Monogeneans were removed from the gills using forceps under a dissecting microscope and fixed in 70% ethanol in vials.
Haptor and anterior body were excised from one specimen, dehydrated through a graded ethanol series, cleared in xylene, and mounted in Canada balsam, and the remaining posterior body were stored in 99% ethanol for molecular analysis. The other monogenean specimens were soaked in distilled water for 3 min, flattened under a coverslip on glass slides. Those specimens were stained in Heidenhain’s iron hematoxylin, dehydrated through a graded ethanol series, cleared in xylene, and mounted in Canada balsam (see Kuramochi, 2003). Drawings were made using a drawing tube fitted on a Nikon Optiphot-2 compound microscope equipped with phase contrast (Nikon, Tokyo, Japan). Measurements were obtained from images taken by a CANON EOS Kiss X2 digital camera (Canon, Tokyo, Japan) fitted onto the compound microscope using ImageJ software (version 1.48i, http://rsb.info.nih.gov/ij/) and were represented by straight-line distances between extreme points, but the length of the ovo-vitelline duct was measured curve length. All measurements in the description and Table 2 are in micrometres and given as the range followed by the mean ± standard deviation and number (n) of specimens examined in parentheses. This study was conducted under the Collaborative Research Agreement between Hiroshima University and Universiti Sultan Zainal Abidin, which was established on 10 February 2020, and all specimens examined were deposited in the South China Sea Repository and Reference Center, University Malaysia Terengganu, Terengganu, Malaysia (UMTPlaty). The host fish was identified morphologically based on Matsunuma et al. (2011); additionally, to confirm the host’s identification, molecular analysis was carried out following Ohtsuka et al. (2020). The scientific names of fish used in this paper follow Kimura et al. (2022) for Scyris, Platycaranx and Turrum species, and Froese & Pauly (2021) for other fishes.
DNA was extracted from the body of a paratype using a DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany). Partial fragments of the 28S rDNA sequence were amplified using the polymerase chain reaction (PCR) U178 (5′-GCA CCC GCT GAA YTT AAG-3′: Lockyer et al. 2003) and LSU1200R (5′-GCA TAG TTC ACC ATC TTT CGG-3′: Littlewood et al. 2000). Partial fragments of the mitochondrial cytochrome c oxidase subunit 1 gene (cox1) were amplified using the primer pair JB3 (5′-TTT TTT GGG CAT CCT GAG GTT TAT-3′: Bowles et al. 1993) and CO1-R trema (5′-CAA CAA ATC ATG ATG CAA AAG G-3′: Miura et al. 2005). PCR was performed in a total volume of 15 μL, containing 7.5 μL KOD One PCR Master Mix (Toyobo, Osaka, Japan), 0.45 μL of each 10 μM primer, 0.7 μL of extracted DNA, and 5.9 μL of distilled water. The cycling for PCR consisted of an initial denaturation at 94°C for 2 min followed by 35 cycles of 98°C for 10 s, 56°C for 30 s, and 68°C for 30 s, with a final extension of 1 min at 68°C. The PCR products were purified using an ExoSAP-IT (USB Corporation, Cleveland, USA), and the sequencing was performed commercially (Macrogen Japan Corp., Kyoto, Japan). Sequence data and electropherograms were inspected and edited manually using MEGA7 (Kumar et al., 2016). The sequences obtained were submitted to the DNA Data Bank of Japan (DDBJ).
The obtained nucleotide sequence of cox1 was subjected to a BLAST (http://www.ncbi.nlm.nih.gov/) search on 9 December 2021 for comparison with other sequences deposited in the International Nucleotide Sequence Databases (INSD). The phylogenetic relationships of Heteraxinidae species including the new species were estimated using maximum likelihood (ML) and Bayesian inference (BI) analyses. Sequences obtained in the present study for 28S rDNA was aligned with other heteraxinid sequences and two sequences of Diclidophoridae retrieved from INSD (Table 1). The alignment was performed using MAFFT version 7 (Katoh et al., 2019) using the “unalignlevel: 0.8” and “Leave gappy regions” options under the G-INS-i strategy. Ambiguous sites in the aligned datasets were removed with Gblocks ver. 0.91b (Castresana, 2000). The best-fit models were determined based on the Bayesian information criterion using IQ-TREE version 2.0.4. (Kalyaanamoorthy et al., 2017; Minh et al., 2020). The ML phylogeny was constructed under the TPM3+F+G4 model using IQ-TREE version 2.0.4 with 1,000 bootstrap replicates. BI phylogeny and Bayesian posterior probabilities were estimated using MrBayes 3.2.6 (Ronquist et al., 2012) under the GTR+G+I model. Two independent runs of four Markov chains were conducted for 1,000,000 generations and the tree was sampled every 100 generations. Parameter estimates and convergence were checked using Tracer v. 1.6.0 (Rambaut & Drummond, 2013); the first 25,000 samples from each run were discarded as burn-in and the remaining samples were analyzed.
Surveys of the type specimens of K. virilis Unnithan, 1957 were made by P. T. Aneesh (Department of Aquatic Biology & Fisheries, University of Kerala) at the following five institutions where Unnithan’s specimens may have been deposited in India: the Indian Museum, Kolkata, the Department of Aquatic Biology & Fisheries, University of Kerala, Trivandrum, Cochin University of Science and Technology, the Western Ghat Field Research Centre of Zoological Survey of India, Kozhikode (ZSI/WGRC), and the Central Marine Fisheries Research Institute (CMFRI), Vizhinjam, Trivandrum during November 2021.
Mazocraeidea Bychowsky, 1957
Heteraxinidae Unnithan, 1957
Cemocotylinae Price, 1962
Kannaphallus Unnithan, 1957
Type species. K. virilis Unnithan, 1957.
Other species. K. lateriporis Mamaev, 1988; K. leptosomus n. sp.
Species incertae sedis. K. mochimae Fuentes Zambrano, 1998.
Kannaphallus leptosomus n. sp.
Type-host: Scyris indica Rüppell (Carangiformes: Carangidae: Caranginae).
Type-locality: Off Kuala Terengganu, Terengganu, Malaysia, east coast of the Peninsular Malaysia.
Type-materials: Holotype (UMTPlaty 0014) and 12 paratypes (UMTPlaty 0015–0026, 0029). All adult specimens.
Site of infection: Gill filament.
Representative DNA sequences: DDBJ accession numbers LC664021 (28S rDNA) and LC664022 (cox1). The sequences were obtained from a paratype (UMTPlaty 0029 [UMTGen 2479]).
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Kannaphallus leptosomus n. sp. is urn:lsid:zoobank.org:act: 993E5C7E-AEAD-4531-931C-A099C570A468.
Etymology: The new scientific name is from Greek words (leptós = slender + sôma = body) and refers to the relatively slender body of the new species within the genus.
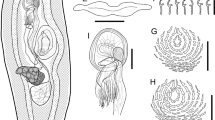
Description (Fig. 1)
Kannaphallus leptosomus n. sp. parasitizing Scyris indica Rüppell off Terengganu, east coast of the Malay Peninsula. A–E, G: holotype, ventral view (UMTPlaty 0014); F: paratype (UMTPlaty 0017). A, whole body; B, penis gun; C, anterior part of reproductive system; D, anterior end; E, posterior part of reproductive system; F, egg in uterus; G, closed clamp.
Body (Fig. 1A) elongate, slightly expanding from the anterior end to the posterior end, total length 5441–8162 (6536 ± 761.3, n = 13), maximum width 442–946 (612 ± 139.7, n = 13) in front of the haptor, length: width ratio 1: 0.064–0.158 (0.095 ± 0.03, n = 13). Haptor (Fig. 1A) asymmetrical with 34–42 (39 ± 3.0, n =8) clamps in total; long row 1325–1874 (1514 ± 166.3, n = 8) in length with 24–32 (28 ± 2.8, n = 8) clamps; short row 632–1061 (853 ± 133.3, n = 8) in length with 10–12 (11 ± 0.9, n = 8) clamps. Clamps (Fig. 1G) Microcotyle-type, largest clamp 76–92 × 82–97 (85 ± 4.4×88 ± 6.0, n = 11), length: width ratio 1: 0.94–1.11 (1.04 ± 0.05, n = 11), slightly asymmetrical, consisting of a pair of antero-lateral and postero-lateral sclerites, mid sclerite, accessory sclerite, and thin muscular base; antero-lateral and postero-lateral sclerites slender, taped toward both ends, left sclerites both slightly longer; mid sclerite with dorsal and ventral T-shaped terminations; slender trident-shaped accessory sclerite at dorsal termination of median sclerite. Anchor absent.
Mouth (Fig. 1A, D) subterminal, wide; its margin irregular, wavy, and anterior margin associated with grand cells. Eye-spots absent. A pair of prohaptoral suckers (Fig. 1D) oval, muscular, lying in each lateral wall of buccal cavity, 50–69 × 49–95 (61 ± 5.7 × 73 ± 10.4, n = 13). Pharynx (Fig. 1D) oval to elliptical, 50–76 × 39–53 (59 ± 6.2 × 45 ± 3.9, n = 14). Esophagus (Fig. 1A, D) with small lateral diverticula. Intestinal bifurcation dorsal to genital atrium. Intestinal ceca with lateral and median diverticula, extending into haptor; not confluent posteriorly.
Testes (Fig. 1A) numerous, 100–151 (120 ± 16.8, n = 11) in number, spherical to oval, 69–94 (81 ± 6.8, n = 12) long, 40–60 (51 ± 6.5, n = 12) wide; testicular zone intercecal, posterior to germarium, entering anterior side of haptor, 1864–2823 × 201–420 (2221 ± 303.1 × 274 ± 71.9, n = 11). Vasa efferentia (Fig. 1E) with thin wall, traveling up while merging each other, then becoming vas deferens at the top of the testicular zone. Vas deferens (Fig. 1A, C, E) running anteriorly, heavily coiled on anterior of germarium, surrounded by accessory grands, meandering slightly along the midline and traveling anteriorly, expanded seminal vesicle. Seminal vesicle (Fig. 1C) elongate, muscular, 158–268 × 45–62 (225 ± 27.1 × 56 ± 5.4, n = 13), containing prostatic cells, lying postero-lateral to part of ejaculatory bulb; its duct meandering, ascending to anterior part of ejaculatory bulb, looping downwards and entering base of ejaculatory bulb. Ejaculatory bulb (Fig. 1C) muscular, ovate, 46–62 × 46–74 (56 ± 5.4×60 ± 7.9, n = 13), connecting base of sclerotized ejaculatory duct, ‘penis gun’, as named by Unnithan (1957). Penis gun (Fig. 1B, C) straight, tapering towards anterior end; extreme tip slightly expanded and slightly bifurcated from which needle associated with base of cirrus arises; 199–228 (212 ± 8.0, n =14) long including the needle part, 169–182 (176 ± 4.1, n = 14) long excluding the needle part, 20–26 (24 ± 1.8, n = 14) wide at base, needle part 35–54 (48 ± 6.0, n = 14) long. Cirrus muscular, round bowl-shaped, unarmed, 12–27 (19 ± 4.4, n = 14) in diameter, opening posterior to uterine opening in genital atrium. Genital atrium (Fig. 1C) muscular, ovate to pyriform, unarmed, 104–189 × 75–134 (151 ± 22.7 × 101 ± 16.7, n = 14), length: width ratio 1: 0.50–0.75 (0.67 ± 0.08, n = 14), opening mid-ventrally at level of the intestinal bifurcation.
Germarium (Fig. 1E) elongate in the shape of a question mark, intercecal, middle third of the body, width at midline 32–62 (44 ± 9.6, n = 9). Oviduct (Fig. 1E) slightly muscular, arising to the right of the anterior terminal of germarium, running downwards, turning left while branching to genitointestinal canal, then entering the vitelline duct to form the ovo-vitelline duct on the midline near the proximal part of germarium, 131–191 (151 ± 20.9, n = 7) long. Ovo-vitelline (Fig. 1E) duct running sinistrally, turning upwards and forming oötype surrounded Mehlis’ grand and continuing to uterus, 92–118 (107 ± 10.8, n = 7) long. Uterus (Fig. 1C, E) running midline anteriorly on dorsal side; opening vertical slit-shaped, anterior to cirrus in genital atrium. Two vaginae (Fig. 1A, C), individually opening on both sides of dorsal surface at interceca, level of anterior one-fifth between posterior margin of seminal vesicle and anterior margin of germarium; opening cup shape, weakly sclerotized, 49–74 × 23–37 (62 ± 7.0 × 32 ± 4.5, n = 13); each vaginal duct possessing small muscular sphincter near their openings, flowing into the longitudinal vitelline ducts. Sperm receptacle absent. Vitelline reservoir (Fig. 1A, E) Y-shaped, slender, median, ventral to germarium; vitellarium coextensive with intestinal ceca, extending into haptor. Genitointestinal canal (Fig. 1E) dextral, connecting to the right of intestinal cecum. Egg (Fig. 1F) spindle-shaped, 279–369×54–65 (314×59, n = 3) with short filament.
Remarks
The new species is assigned to Kannaphallus due to the presence of the genus characteristics defined by Unnithan (1957), Price (1962), and Yamaguti (1963), such as the presence of asymmetric clamps, paired vaginas, and a penis gun associated with the cirrus. Morphological measurements and features of Kannaphallus species, including invalid ones, are presented in Table 2. Kannaphallus leptosomus n. sp. is readily separated from K. mochimae incertae sedis by the possession of an armed cirrus and lack of a penis gun of the latter (Fuentes Zambrano, 1998). The new species is distinguished from K. virilis by the following features: the presence of a needle associated with the tip of the penis gun (Unnithan, 1957; Lebedev et al., 1970) and a larger clamp (76–92 × 82–97 μm vs. 32–52 × 40–60 μm: Unnithan, 1957). The clamp of the new species is also larger than that of K. lateriporis (vs. 65 × 62 μm: Mamaev, 1988). Furthermore, the new species differs from K. lateriporis by a longer penis gun associated with the needle and larger genital atrium (199–228 μm vs. 74–82 μm: Mamaev, 1988 and 104–189 × 78–134 μm vs. 40–41 × 37–40 μm: Mamaev, 1988, respectively). The vertical duct of the vagina connecting each vaginal duct reported by Mamaev (1988) in K. lateriporis is absent from K. leptosomus n. sp. The new species is also distinguished from the two Kannaphallus species reported by Pillai (1968) (see the Discussion) by the sizes of the clamp, penis gun, and egg (see Table 2).
Kannaphallus univaginalis described by Ramalingam (1960) was considered an invalid taxon by Price (1962), but Fuentes Zambrano (1998) treated it as a valid taxon. Ramalingam (1960) named the monogenean as “Kannaphallus univaginalis n. sp.” with a short and limited description with figures and the development of functional asymmetry. Although the description is certainly inadequate, this paper fully meets the criteria for establishing a new scientific name (ICZN, 1999), and as a result, Kannaphallus univaginalis is remained as a valid name to this date. Nevertheless, Ramalingam (1969) used the same specimens of Ramalingam (1960) to describe Cemocotylelloides carangis Ramalingam, 1969 and admitted that he had already reported it as K. univaginalis (Ramalingam, 1969: p. 231). Therefore, Cemocotylelloides carangis is considered as a junior objective synonym. The species is henceforth treated as Cemocotylelloides univaginalis n. comb. which is readily separated from Kannaphallus species due to the presence of only one vagina (Ramalingam, 1969).
The partial cytochrome c oxidase subunit I gene sequence (538 bp) from the host was deposited in GenBank (accession number: OM868132). The sequence showed 99.43% similarity to sequences of the same species in GenBank (KU179066) and in the BOLD identification engine (Ratnasingham & Hebert, 2007). Scyris is composed of 2 species: S. indica and S. alexandrina (Geoffroy Saint-Hilaire) (Kimura et al., 2022), and the similarity of cox1 sequences of each species registered in INSD is less than 90%, thus this confirms the identification of the host species.
Note of the type specimen of Kannaphallus virilis Unnithan, 1957
Unnithan (1957) erected the genus Kannaphallus Unnithan, 1957 for placing the new species Kannaphallus virilis Unnithan, 1957, based on three specimens collected from the gill of the marine fish Caranx atropus (now Atropus atropos), collected at Trivandrum in Kerala, India from 10 November 1955. Unfortunately, Unnithan (1957) did not mention the deposition of the type materials. The survey of specimens housed at the Indian Museum, Kolkata, the Department of Aquatic Biology & Fisheries, University of Kerala, Trivandrum, and Cochin University of Science & Technology failed to reveal any of Unnithan’s (1957) specimens including the types for K. virilis. The survey of repositories of the Western Ghat Field Research Centre of Zoological Survey of India, Kozhikode (ZSI/WGRC) (established in April 1980) and the Central Marine Fisheries Research Institute (CMFRI), Vizhinjam (well established after 1960) also failed to reveal the materials. Further, if the author indeed deposited the types in any repositories, he would have been required to mention the registration number. However, since there were no museum records of Unnithan’s (1957) materials in any of the repositories surveyed, it appears that those specimens were probably never deposited in any institution or have been subsequently lost.
Molecular data analysis
Comparing the cox1 sequence of K. leptosomus n. sp. from other monogeneans available on INSD by BLASTn search, the closest hits were Microcotyle sebastis Goto, 1894 (DQ412044: Park et al., 2007, MT876115–MT876119: Song et al., 2021, MW730642: direct submission, 76.42–78.14% identity with 85–99% coverage), Microcotyle caudata Goto, 1894 (MT180126: Nam et al., 2020, LC472527–LC472531: Ono et al., 2020, MW730633–MW730634: direct submissions, 76.52–78.40% identity with 77–99% coverage), Microcotyle kasago Ono, Matsumoto, Nitta & Kamio, 2020 (LC472525–LC472526: Ono et al., 2020, 77.16–77.25% identity with 83% coverage) and Zeuxapta seriolae (Meserve, 1938) (KP119183–KP119357: Sepúlveda & González, 2015, 77.01–77.39% identity with 82% coverage).
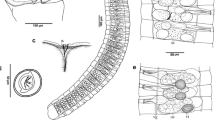
The trimmed multiple sequence alignment of 28S rDNA fragments consisted of 544 base pairs. Sequences of two diclidophorids were used as the outgroups following Tambireddy et al. (2016). The topologies of the trees constructed by ML and BI analysis were almost identical (Fig. 2). Kannaphallus leptosomus n. sp. was placed as a sister group with another Cemocotylinae species, Cemocotylelloides carangis. This clade showed affinity with two species of Hetaraxononae, Zeuxapta seriolae and Hetaraxinoides atlanticus. On the other clade, Bicotyle reticulata (Lintaxininae) diverged first, then Probursatra brasiliensis (Heteraxininae) and Cemocotyle carangis (Cemocotylinae) formed a clade.
Discussion
The present collection from S. indica represents the first record of Kannaphallus in Malaysia. In total, 10 monogenean species from Caranginae were recorded in Southeast Asia (Lim, 1998). However, the list of Southeast Asian monogeneans completed by Lim (1998) overlooked K. virilis reported from Vietnam by Lebedev (1970) and Lebedev et al. (1970).
Lebedev (1970) and Lebedev et al. (1970) reported K. virilis from S. indica (as Alectis indicus) and Platycaranx malabaricus (Bloch & Schneider) (as Caranx malabaricus) in the Gulf of Tonkin, North Vietnam, and Lebedev et al. (1970) provided a short description but no figure. There were two major differences between this redescription and the original description by Unnithan (1957), but Lebedev et al. (1970) did not mention them: the accessory sclerite shape (conical: Unnithan, 1957 vs. trident: Lebedev et al., 1970) of the clamp and the penis gun length (150 μm: Unnithan, 1957 vs. 267 μm: Lebedev et al., 1970). The former difference may result from an observational mistake by Unnithan (1957) because the accessory sclerite is a small and thin structure, whereas those of other congeners have trident-shaped structure (Table 2). On the other hand, the difference in penis gun length (117 μm) is too long to be considered intraspecific variation because the variation in the length is less than 30 μm in other species (see Table 2). Furthermore, the specimens reported by Lebedev et al. (1970) were substantially longer than those of other species, including the new species, K. leptosomus n. sp. (Table 2). Therefore, the record of Lebedev et al. (1970) may not be K. virilis and it cannot be confirmed if the specimens reported by Lebedev et al. (1970) included K. leptosomus n. sp. A reexamination of their specimens or Kannaphallus species that parasitize the reported hosts in the region is required.
Kannaphallus mochimae is the only Kannaphallus species that parasitizes an acanthurid host (Fuentes Zambrano, 1998). However, the original description of this species lacks the morphological features possessed by Kannaphallus species: the longitudinal vaginal ducts, asymmetrical clamp, accessory sclerite, unarmed genital atrium, penis gun, and conspicuous seminal vesicle. Furthermore, other Kannaphallus species have been recorded from carangids in the western Indo-Pacific, whereas only K. mochimae has been obtained from the Atlantic Ocean (Unnithan, 1957; Pillai, 1968; Young, 1970; Lebedev, 1970; Lebedev et al., 1970; Mamaev, 1988; Fuentes Zambrano, 1998; present study). Therefore, we consider K. mochimae regarded as incertae sedis in the genus. Kannaphallus mochimae is morphologically similar to Probursata species (Heteraxinidae: Heteraxininae) reported from the Western Atlantic, and Probursata species also possesses two vaginae (Bravo-Hollis, 1984; Takemoto et al., 1993). However, K. mochimae lacks two features of Probursata: the accessory sclerite of the clamp and the muscular auricular expansions of the genital atrium (Bravo-Hollis, 1984; Takemoto et al., 1993; Fuentes Zambrano, 1998). Unfortunately, Fuentes Zambrano (1998) did not provide information on the deposit of type specimens and its whereabouts are unknown. We believe that re-examination of some characteristics (e.g., the morphology of the clamp and genital atrium) can determine the generic position of this species based on newly collected specimens.
Pillai (1968) described two Kannaphallus species in his doctoral dissertation, which has so far been overlooked by subsequent researchers: K. bivagina Pillai, 1968 from Turrum gymnostethus (Cuvier) (as Carangoides gymnostethoides Bleeker) and K. carangis Pillai, 1968 from Caranx sp. in Trivandrum and Neendakara, Kerala, South India, respectively. However, this did not satisfy the publication criteria defined by the International Commission on Zoological Nomenclature (ICZN, 1999, 2012: Articles 8.1.2 and 8.1.3). Thus, all scientific names described in this paper and its authorship were judged to be invalid, and those two species were regarded Kannaphallus sp. 1 and sp. 2 in this paper. According to the descriptions of Pillai (1968), these two species can be distinguished from other Kannaphallus species by the following features: the vaginal opening position, clamp size, and the unarmed genital atrium (see Table 2). Therefore, they may be independent taxa. Re-examinations of these specimens and a formal publication of descriptions are expected.
In the 28S rDNA-based phylogenetic analysis presented in this study (Fig. 2), Heteraxinidae was broadly divided into two clades, and Heteraxininae and Cemocotylinae were shown as polyphyletic groups, respectively. This family consists of 5 subfamilies, 16 genera, and c. 60 species (Mamaev, 1990; Gibson, 2021), and the molecular information of limited species is insufficient to change the classification based on the present phylogenetic analysis. Broad taxon sampling of Heteraxinidae is required for a detailed understanding of the phylogenetic relationships and reconstruction of the family.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aiken, H. M., Bott, N. J., Mladineo, I., Montero, F. E., Nowak, B. F., & Hayward, C. J. (2007). Molecular evidence for cosmopolitan distribution of platyhelminth parasites of tunas (Thunnus spp.). Fish and Fisheries, 8, 167–180.
Bowles, J., Hope, M., Tiu, W. U., Liu, X., & McManus, D. P. (1993). Nuclear and mitochondrial genetic markers highly conserved between Chinese and Philippine Schistosoma japonicum. Acta Tropica, 55, 217–229.
Bravo-Hollis, M. (1984). Monogenea (van Beneden, 1858) Carus, 1863 de peces marinos del Golfo de México y del Mar Caribe. IX. Descripción de un género y una especie nuevos de la familia Heteraxinidae Price, 1962, subfamilia Heteraxininae Unnithan, 1957. Anales del Instituto de Biología, Universidad Nacional Autónoma de México. Serie Zoología, 54, 1–11.
Castresana, J. (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution, 17, 540–552.
Camargo, A. C. A., & Santos, C. P. (2020). Morphological and molecular analyses of Pseudomazocraes sulamericana n. sp., Pseudomazocraes selene Hargis, 1957, Cemocotyle carangis (MacCallum, 1913) and Zeuxapta seriolae (Meserve, 1938) (Monogenea: Mazocraeidea) from carangid fishes in the south-western Atlantic Ocean. Journal of Helminthology, 94, e28.
Chou, R., & Lee, H. B. (1997). Commercial marine fish farming in Singapore. Aquaculture Research, 28, 767–776.
Froese, R., & Pauly, D. (Eds) (2021). FishBase. World Wide Web electronic publication. www.fishbase.org, version (2/2021). Accessed on 16 May 2021.
Fuentes Zambrano, J. L. (1998). Descripción de dos nuevas especies de monogéneos microcotiloideos, parásitos de peces marinos de la costa noreste de Venezuela. Scientia Marina, 62, 65–72.
Gibson, D. (2021). WoRMS: Heteraxinidae Unnithan, 1957. Available at: https://marinespecies.org/aphia.php?p=taxdetails&id=518772. Accessed 10 February 2022.
Hossen, M. S., Barton, D. P., Zhu, X., Wassens, S., & Shamsi, S. (2020). Re-description and molecular characterisation of Choricotyle australiensis Roubal, Armitage & Rohde, 1983 (Monogenea: Diclidophoridae) infecting Chrysophrys auratus (Forster) (Perciformes: Sparidae). Systematic Parasitology, 97, 815–825.
ICZN. (1999). International Code of Zoological Nomenclature, Fourth Edition. International Trust for Zoological Nomenclature, London. p. xxix+ 306.
ICZN. (2012). International Commission on Zoological Nomenclature: Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Bulletin of Zoological Nomenclature, 69, 161–169.
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K., Haeseler, A. V., & Jermiin, L. S. (2017). ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods, 14, 587–589.
Katoh, K., Rozewicki, J., & Yamada, K. D. (2019). MAFFTonline service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics, 2019, 1160–1166.
Kimura, S., Takeuchi, S., & Yadome, T. (2022). Generic revision of the species formerly belonging to the genus Carangoides and its related genera (Carangiformes: Carangidae). Ichthyological Research. https://doi.org/10.1007/s10228-021-00850-1 (Published online).
Kumar, S., Stecher, G., & Tamura, K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870–1874.
Kuramochi, T. (2003). [Parasites]. In: National Museum of Nature and Science, Tokyo (Ed.) Collection Building and Management for Natural History. Hadano: Tokai University Press, pp. 70–77 (In Japanese).
Lebedev, B. I. (1970). Helminthes of epipelagical [sic] fishes of the South-Chinese Sea, in Helminths of animals from south-eastern Asia. In: Oshmarin, P. G., Mamaev, Yu, L, & Lebedev, B.I. (Eds.) Helminth of Animals from the South-Eastern Asia (pp. 191–216). Moscow: Nauka (In Russian).
Lebedev, B. I., Parukhin, A. M., & Roitman, V. A. (1970). [Monogenetic trematodes, Oligongchoinea (Monogenoidea), parasites of horse mackerel fishes of North Vietnam Gulf]. Biologya Morya, Kiev, 20, 167–187 (In Russian).
Lim, L. H. S. (1998). Diversity of monogeneans in Southeast Asia. International Journal for Parasitology, 28, 1495–1515.
Littlewood, D. T. J., Curini-Galletti, M., & Herniou, E. A. (2000). The interrelationships of Proseriata (Platyhelminthes: Seriata) tested with molecules and morphology. Molecular Phylogenetics and Evolution, 16, 449–466.
Lockyer, A. E., Olson, P. D., & Littlewood, D. T. J. (2003). Utility of complete large and small subunit rRNA genes in resolving the phylogeny of the Neodermata (Platyhelminthes): implications and a review of the cercomer theory. Biological Journal of the Linnean Society, 78, 155–171.
Mamaev, Ju[Yu]. L. (1988). Kannaphallus lateriporis sp. n. and its position in the system of higher monogeneans. Parazitologiya, 22, 354–358 (In Russian).
Mamaev, Yu. L. (1990). The systematical composition of the family Heteraxinidae and other allied families of Monogenea. Folia Parasitologica, 37, 225–230.
Matsunuma, M., Motomura, H., Matsuura, K., Shazili, N. A. M., & Ambak, M. A. (Eds) (2011). Fishes of Terengganu – east coast of Malay Peninsula, Malaysia. Tsukuba, Terengganu, and Kagoshima: National Museum of Nature and Science, Universiti Malaysia Terengganu, and Kagoshima University Museum, pp. ix + 251.
Minh, B. Q., Schmidt, H. A., Chernomor, O., Schrempf, D., Woodhams, M. D., von Haeseler, A., & Lanfear, R. (2020). IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution, 37, 1530–1534.
Miura, O., Kuris, A. M., Torchin, M. E., Hechinger, R. F., Dunham, E. J., & Chiba, S. (2005). Molecular-genetic analyses reveal cryptic species of trematodes in the intertidal gastropod, Batillaria cumingi (Crosse). International Journal for Parasitology, 35, 793–801.
Mollaret, I., Jamieson, B. G., Adlard, R. D., Hugall, A., Lecointre, G., Chombard, C., & Justine, J. L. (1997). Phylogenetic analysis of the Monogenea and their relationships with Digenea and Eucestoda inferred from 28S rDNA sequences. Molecular and Biochemical Parasitology, 90, 433–438.
Nam, U.-H., Whang, I., & Kim, J.-H. (2020). The complete mitochondrial genome sequence of Microcotyle caudata (Platyhelminthes: Monogenea) from dark-banded rockfish (Sebastes inermis) in Korea. Mitochondrial DNA Part B, 5, 1817–1819.
Ohtsuka, S., Piasecki, W., Ismail, N., & Kamarudin, A. S. (2020). A new species of Brachiella (Copepoda, Siphonostomatoida, Lernaeopodidae) from Peninsular Malaysia, with relegation of two genera Charopinopsis and Eobrachiella to junior synonyms of Brachiella. Parasite, 27, 40.
Olson, P. D., & Littlewood, D. T. J. (2002). Phylogenetics of the Monogenea – evidence from a medley of molecules. International Journal for Parasitology, 32, 233–244.
Ono, N., Matsumoto, R., Nitta, M., & Kamio, Y. (2020). Taxonomic revision of Microcotyle caudata Goto, 1894 parasitic on gills of sebastids (Scorpaeniformes: Sebastidae), with a description of Microcotyle kasago n. sp. (Monogenea: Microcotylidae) from off Japan. Systematic Parasitology, 97, 501–516.
Park, J. K., Kim, K. H., Kang, S., Kim, W., Eom, K. S., & Littlewood, D. T. J. (2007). A common origin of complex life cycles in parasitic flatworms: evidence from the complete mitochondrial genome of Microcotyle sebastis (Monogenea: Platyhelminthes). BMC Evolutionary Biology, 7, 1–13.
Pillai, V. S. (1968). Trematode Parasites of Fishes (Monogenea from Kerala Coastal Fishes). Trivandrum: Doctoral thesis, University of Kerala, pp. 367.
Price, E. W. (1962). North American monogenetic trematodes. XI. The family Heteraxinidae. Journal of Parasitology, 48, 402–418.
Ramalingam, K. (1960). The functional development of “compensating asymmetry” in the higher Monogenea. Zeitschrift fur Wissenschaftliche Zoologie, 164, 374–381.
Ramalingam, K. (1969). Morphological description of Cemocotylelloides gen. n. (Monogenea: Cemocotylidae), its life history and bionomics. Parazitologicheski Sbornik, 24, 220–241.
Rambaut, A., & Drummond, A. J. (2013). Tracer v 1.6. Available at http://tree.bio.ed.ac.uk/software/tracer/. Accessed 20 June 2017.
Ratnasingham, S., & Hebert, P. D. (2007). BOLD: The barcode of life data system (http://www.barcodinglife.org). Molecular Ecology Notes, 7, 355–364.
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M. A., & Huelsenbeck, J. P. (2012). MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542.
Sepúlveda, F. A., & González, M. T. (2015). Patterns of genetic variation and life history traits of Zeuxapta seriolae infesting Seriola lalandi across the coastal and oceanic areas in the southeastern Pacific Ocean: potential implications for aquaculture. Parasites & Vectors, 8, 282.
Song, J. Y., Kim, K. Y., & Choi, S. W. (2021). Occurrence and molecular identification of Microcotyle sebastis isolated from fish farms of the Korean rockfish, Sebastes schlegelii. The Korean Journal of Parasitology, 59, 89.
Takemoto, R. M., Amato, J. F. R., & Luque, J. L. (1993). A new species of Probursata Bravo-Hollis, 1984 (Monogenea: Heteraxinidae: Heteraxininae) parasite of Oligoplites spp. (Osteichthyes: Carangidae) from the coast of the state of Rio de Janeiro, Brazil. Memórias do Instituto Oswaldo Cruz, 88, 285–288.
Tambireddy, N., Gayatri, T., Gireesh-Babu, P., & Pavan-Kumar, A. (2016). Molecular characterization and phylogeny of some mazocraeidean monogeneans from carangid fish. Acta Parasitologica, 61, 360–368.
Unnithan, R. V. (1957). On the functional morphology of a new fauna of Monogenea on fishes from Trivandrum and environs part I. Axinidae fam. nov. Bulletin of the Central Research Institute of the University of Kerala, Trivandrum. Series C. Natural Sciences, 5, 27–122.
Verma, A. K., & Verma, J. (2021). Redescription and new host record of Heteraxinoides atlanticus (Monogenea: Heteraxinidae) from the gills of Nemipterus japonicus (Bloch) and its systematics. Agricultural Science Digest, online first, D-5293.
Yamaguti, S. (1963). Systema Helminthum IV. Monogenea and Aspidocotylea. New York: Interscience Publishers, pp. 699.
Young, P. G. (1970). The species of Monogenoidea recorded from Australian fishes and notes on the zoogeography. Anales del Instituto de Biologia Universidad Nacional Autonoma de Mexico, Zoologia, 41, 163–175.
Acknowledgments
We thank Dr. Takuya Sato (Kobe University) for providing laboratory facilities. We are grateful to Dr. P. T. Aneesh (University of Kerala), Dr. N. K. Sanil (Central Marine Fisheries Research Institute), the Director of Zoological Survey of India, the station in-charge of CMFRI, Vizhingam, the Officer in-Charge of the Western Ghat Field Research Centre of Zoological Survey of India, and staffs of the Cochin University for their help with the survey of Unnithan’s materials. We thank Dr. Mallory Eckstut (Edanz, https://jp.edanz.com/ac) for editing a draft of this manuscript. We would like to thank Dr. P. T. Aneesh and two anonymous reviewers for their valuable comments to improve the manuscript.
Funding
This study was partially supported by JSPS Bilateral Partnership Program (No. JPJSBP120209924, awarded to SO), JSPS KAKENHI Grants (No. 18J00466, to MN) and Special Research Grant Scheme for International Collaboration (UNISZA/2021/SRGS-IC/04) awarded to NI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national, and international guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as 02F186BB-F93D-4257-A48C-60D1D6ED71AC. This article was published as an Online First article on the online publication date shown on this page. The article should be cited by using the doi number. This is the Version of Record.
Rights and permissions
About this article
Cite this article
Nitta, M., Kondo, Y., Ohtsuka, S. et al. Kannaphallus leptosomus n. sp. (Monogenea: Heteraxinidae: Cemocotylinae) parasitizing Scyris indica (Carangiformes: Carangidae) from Malaysia. Syst Parasitol 99, 587–599 (2022). https://doi.org/10.1007/s11230-022-10048-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-022-10048-2