Abstract
A new parasitic copepod, Ergasilus yandemontei n. sp., is described based on 10 adult females collected from the gills of the atherinid silverside Odontesthes hatcheri (Eigenmann), in Lake Pellegrini, Neuquén Province, Patagonia, Argentina. This new copepod species is characterized by having: (i) a 2-segmented endopod on leg 1; (ii) a semi pinnate seta on the terminal segment of the exopod of leg 1; (iii) a reduced leg 5 with a single seta; (iv) aesthetascs on antennule, 1 aesthetasc on the sixth segment and 1 on the fourth segment. Ergasilus yandemontei n. sp. represents the first species described from Patagonian freshwaters. Ergasilus sieboldi var. patagonicus Szidat, 1956 described from Lake Pellegrini, should be considered a synonym of the new species described herein.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ergasilidae von Nordmann, 1832, is one of the main families of cyclopoid copepods, with more than 260 described species; most are found in freshwater environments, although some inhabit estuarine or coastal marine waters (Boxshall & Halsey, 2004). According to Boxshall & Defaye (2008), the Neotropical region harbours the highest ergasilid richness, with more than 60 freshwater species recorded. However, knowledge of ergasilids is markedly patchy in this region and the number of species is expected to increase with further studies (Suárez-Morales & Santana-Piñeros, 2008; Muriel-Hoyos et al., 2015). The main site of infection in fishes is the gill filaments, although in some cases other microhabitats are used, such as fins, nasal cavity, and urinary bladder (Boxshall & Halsey, 2004; Rosim et al., 2013; Varella et al., 2019). Some ergasilids are considered a threat in aquaculture as they have the potential to cause fish mortalities (Piasecki et al., 2004). The life-cycle usually involves six naupliar stages, five copepodids and the adults; all the developmental stages and the adult males are free living. Only adult, fertilized females have a parasitic phase in their life-cycle, typically infecting freshwater fishes as hosts, although a few can be found on bivalve molluscs (Boxshall & Defaye, 2008).
Within the family Ergasilidae, Ergasilus von Nordmann, 1832 is the largest genus, with over 180 nominal species (Boxshall & Halsey, 2004). In South America, most of the species registered are from Brazil, with nearly 30 species described. Very few species have been reported from other countries: three from Colombia; two from Chile; one from Guyana; one from Bolivia; one from Ecuador; and one from Peru (El-Rashidy & Boxshall, 1999, 2002; Muriel-Hoyos et al., 2015; Taborda et al., 2016; Marques et al., 2017). In Argentina, a total of four species of Ergasilus have been reported outside Patagonia: Ergasilus atafonensis Amado & Rocha, 1997 from Mugil liza Valenciennes (see Montes & Martorelli, 2015); Ergasilus chelangulatus Thatcher & Brasil-Sato, 2008 from Luciopimelodus pati (Valenciennes), Pimelodus albicans (Valenciennes) and Pimelodus maculatus Lacépède (see Chemes & Takemoto, 2014); Ergasilus sieboldi Nordmann, 1832 from Jenynsia lineata Jenyns (see Montes & Martorelli, 2017), and Ergasilus versicolor Wilson, 1911 from Mugil liza Valenciennes and Mugil platanus Günther (see Alarcos & Etchegoin, 2010; Montes & Martorelli, 2015). Additionally, Ergasilidae sp. was registered parasitising Prochilodus lineatus (Valenciennes) and Ergasilus sp. was registered parasitising Odontesthes argentinensis (Valenciennes) (see Alarcos & Etchegoin, 2010; Chemes & Gervasoni, 2013).
In Patagonia, Szidat (1956) reported the presence of an ergasilid species, which was named Ergasilus sieboldi var. patagonicus Szidat, 1956, parasitising Odontesthes hatcheri, Odontesthes bonaeriensis (Valenciennes) and Percichthys trucha (Valenciennes) in Lake Pellegrini (Neuquén River Basin). Moreover, free larval stages and males from Andean lakes were reported as E. sieboldi (see Modenutti & Balseiro 1989). There are other records of this copepod parasitising O. hatcheri from freshwater environments in Chubut, Neuquén and Río Negro Provinces (Ruiz, 2007; Flores et al., 2016). Ergasilus sp. has also been registered in several Andean lakes for Galaxias maculatus (Jenyns) and P. trucha (Ortubay et al., 1994; Viozzi et al., 2009).
The copepod E. sieboldi occurs in the Palaearctic region and parasitises a wide range of freshwater fishes from many families, mainly cyprinids (Kabata, 1979; Kearn, 2004). The specific identity of the Patagonian specimens is in doubt, as several morphological differences were found between Patagonian copepods and the Palearctic E. sieboldi. This paper provides a description of a new species of Ergasilus from Patagonian freshwater fishes.
Materials and methods
In summer 2016, specimens of O. hatcheri and P. trucha were collected from Lake Pellegrini (38°42′16.69″S, 67°58′39.98″W) using gill nets. Specimens of G. maculatus and P. trucha were also captured, using baited traps and gill nets respectively, from Lake Morenito (41°3′25.14″S, 71°30′56.07″W). The fishes were transported to the laboratory alive or on ice, and immediately examined. Gills were removed from the host, and under a stereoscopic microscope the copepods were gently isolated from the filaments using needles, special care being taken not to damage the antennae. Copepods were cleared and mounted using Hoyerʼs medium, then measured under a microscope with a graded eyepiece, and photographed using a Sony digital camera. Illustrations were prepared with the aid of a drawing tube. Other specimens were stored in 96% alcohol for molecular studies. Some specimens were dehydrated using graded ethanol, sputter-coated with gold, and examined with a Phillips 515 10 kV scanning electron microscope (Amsterdam, The Netherlands) at the Centro Atómico Bariloche, Argentina. Other specimens were dehydrated through a graded ethanol series, critical-point dried and sputter-coated with gold and examined using a JEOL JSM 100 SEM at the Museo Argentino de Ciencias Naturales Bernardino Rivadavia, Buenos Aires, Argentina. Terminology follows Boxshall & Halsey (2004). All measurements are expressed in micrometres and are presented as the range followed by the mean, with the number of specimens measured in parenthesis. Specimens were deposited in the Colección Nacional de Parasitología, Museo Argentino de Ciencias Naturales Bernardino Rivadavia, Buenos Aires, Argentina (MACN-Pa). Specimens of Ergasilus sieboldi var. patagonicus (317/1; 317/2; 317/3) deposited by Szidat (1956) in MACN-Pa were examined for comparison.
The 18S rDNA fragment was amplified with primers 18SF (5′-AAG GTG TGM CCT ATC AAC T-3′) (Littlewood & Olson, 2001) and 18SR (5′-TTA CTT CTA AAC GCT C-3′) (Song et al., 2008). PCR reactions were performed in a final volume of 25 μL containing a mixture of: 10-30 ng of DNA extract, 1.5 μl MgCl2 (50mM), 0.4 μL dNTP (25mM), 2.5μL PCR-Buffer (10x), 0.3 μl of each primer (50pmoles/μL), 1U Platinum Taq DNA-polymerase (Invitrogen) and ultrapure water to complete the total volume of the reaction. The PCR program consisted of initial denaturation at 95°C for 5 min, 40 amplification cycles (94°C for 30 s, annealing temperatures for 45 s, 72°C for 1 min), and final step at 72°C for 5 min. Negative controls were used to test possible contamination of the reagents. After amplification, the products of the reactions were submitted to electrophoresis in agarose gel and visualized under ultraviolet light and positively amplified samples were purified with Minelute kit (Qiagen®), following the manufacturer directions. Sequencing with the BigDye® kit (Applied Biosystems) followed the manufacturer’s protocol. The sequencing products were purified with Sephadex G-50 (GE) and sequenced with an ABi 3130 automatic sequencer (Applied Biosystems). DNA sequences were edited in the programs Geneious v.4.5 (http://www.geneious.com/, Drummond et al., 2011).
Ergasilidae von Nordmann, 1832
Ergasilus von Nordmann, 1832
Ergasilus yandemontei n. sp.
Syn. Ergasilus sieboldi var. patagonicus Szidat, 1956
Type-host: Odontesthes hatcheri Eigenmann (Atheriniformes: Atherinopsidae).
Other hosts: Percichthys trucha Valenciennes (Perciformes: Percichthyidae); Galaxias maculatus Jenyns (Osmeriformes: Galaxiidae).
Type-locality: Lake Pellegrini (38°42’16.69”S, 67°58’39.98”W), Neuquén River Basin, Neuquén Province, Patagonia, Argentina.
Other locality: Lake Morenito (41°3’25.45”S, 71°30’59.46”W), Limay River Basin, Río Negro Province, Patagonia, Argentina.
Type-material: Holotype: adult female (MACN-Pa 699). Paratypes: 9 female specimens (MACN-Pa 700/1-9).
Comparative material examined: Fifteen specimens in three permanent mounts of ex Ergasilus sieboldi var. patagonicus: 317/1; 317/2; 317/3.
Site in host: Gill filaments.
ZooBank registration: To comply with the regulations set out in Article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Ergasilus yandemontei n. sp. is urn:lsid:zoobank.org:act: F47889CA-9F66-44F1-BA7F-BEF182095E6F.
Representative DNA sequences: MT969345.
Etymology: The species name is formed as a noun in the genitive case in memory of the late Yan De Monte, a student at the Universidad Nacional del Comahue, who studied the biology of this species in Patagonian Andean lakes.
Description
Adult female. [Based on 10 specimens; Figs. 1, 2]. Mean body length measured from anterior margin of prosome to posterior margin of caudal rami 969–1,065 (1,038) (n = 10). Body comprising prosome and urosome, bearing small sensillae along dorsal cephalic shield (Fig. 2c). Prosome consisting of triangular cephalosome with antennule and antenna visible in dorsal view and 4 pedigerous somites. Cephalosome and first pedigerous somite not fused. Cephalosome longer than wide. Urosoma consisting of 5th pedigerous somite, double genital somite, and 3 free abdominal somites (Figs. 1e, 2e). Double-genital somite barrel-shaped, with ventral surface ornate with spinules and 2 rows of spinules along posterior ventral margin. Free abdominal somites with row of spinules on posteroventral margins, decreasing in width posteriorly; first and second nearly equal in length. Anal somite bipartite, smaller than preceding two. Caudal rami longer than wide, each ramus armed with long medial seta, median lateral seta and 2 smaller setae, spinule rows present ventrally anterior to setae. Egg-sacs 2, long, much longer than wide, each with 2–3 rows of eggs.
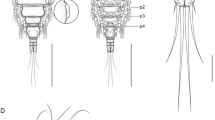
Scanning electron micrographs of Ergasilus yandemontei n. sp., adult female. A, Lateral view of entire female body, carrying egg sacs; B, Antenna and antennule, dorsal view; C, Lateral dorsal view of antennule; arrows indicate the small sensilla distributed on the cephalosome; D, Ventral view of interpodal plates ornamented with spinules and legs 1; arrow indicates 2-segmented endopod; E, Ventral view of abdomen and caudal rami, ornamented with spinules; F, Mouthparts. Scale-bars: A, 200 µm; B, 100 µm; C, D, 50 µm; E, F, 20 µm
Antennule (Figs. 1c, 2c) 6-segmented, tapering distally, armed with simple setae (s) and aesthetascs (ae); setal formula from proximal to distal segments as follows: 1s, 10s, 5s, 3s + 1ae, 2s, 6s + 1ae.
Antenna (Figs. 1d, 2b) comprising coxobasis, 3-segmented endopod with curved terminal claw. Coxobasis short, widest proximally; membrane between coxobasis and first endopodal segment not inflated. First endopodal segment nearly 3 times longer than coxobasis; sensillum near mid-length on inner margin. Second endopodal segment curved, similar in length to first segment, with a sensillum near mid-length and small distal sensillum. Third endopodal segment small, bearing single seta. Claw with small pit (fossa) distally on concave margin.
Mouthparts (Figs. 1f, g, 2f) comprising mandible, maxilla and maxillule; maxilliped absent. Mandible with 3 blades; anterior delicate, ornamented with teeth on anterior margin, median blades robust, ornamented with teeth on posterior margin. Maxillule with 2 setae. Maxilla 2-segmented, distal segment with numerous teeth on anterior margin.
Interpodal plates of all legs ornamented with spinules ventrally (Fig. 2d). Swimming legs 1–4 biramous and with separate coxa and basis. Armature of legs is given below.
Coxa | Basis | Exopod | Endopod | |
|---|---|---|---|---|
Leg I | 0-0 | 0-1 | I-0;0-1;II*-4 | 0-1;II-5 |
Leg II | 0-0 | 0-1 | I-0;0-1;I-6 | 0-1;0-2;I-4 |
Leg III | 0-0 | 0-1 | I-0;0-1;I-6 | 0-1;0-2;I-4 |
Leg IV | 0-0 | 0-1 | 0-0;I-5 | 0-1;0-2;I-3 |
Leg 1 (Figs. 1h, 2d). Coxa lacking spinules; basis with proximal outer seta. Exopod with 3 segments, first segment pilose on inner margin, lacking inner seta, distal spine present on outer margin; second segment with spinules on outer margin, with 1 inner seta, distal spine absent; third segment with spinules distally on outer margin, with 1 pectinate seta and 4 pilose setae, 2 distal spines spinulated. Endopod with 2 segments; first segment with spinules on entire outer margin with 1 seta; second segment with spinules on entire outer margin, with 5 setae and 2 spines, only outer spine spinulated.
Legs 2 and 3 similar (Fig. 1i). Coxa ornamented with spinules; basis lacking spinules, with proximal seta. Exopod with 3 segments; first segment pilose on inner side, with spinules on entire outer margin and distal spine, lacking seta; second segment with spinules on outer margin, with 1 seta, lacking spine; third segment with spinules on outer margin, with 6 setae and 1 spine. Endopod with 3 segments; first segment with spinules on entire outer margin, with 1 seta; second segment with spinules on entire outer margin with 2 setae, distal spine absent; third segment with spinules on entire outer margin with 4 setae and 1 distal spine.
Leg 4 (Fig. 1j). Coxa ornamented with spinules. Basis with external seta. Exopod with 2 segments; first segment not pilose, lacking seta, distal outer spine absent; second segment with 5 setae, distal spine present. Endopod with 3 segments; first segment with spinules on entire outer margin with one seta; second segment with spinules on entire outer margin, with 2 setae; third segment with spinules on entire outer margin, with 3 setae, distal outer spine present.
Leg 5 represented by small lobe with small seta.
Remarks
The present specimens were identified as Ergasilus due to several diagnostic characters according to Boxshall & Montú (1997), Boxshall & Halsey (2004) and Suárez-Morales & Santana-Piñeros (2008). These include: (i) biramous leg 4 with 2-segmented exopod and 3-segmented endopod; (ii) 6-segmented antennule; and (iii) antenna with a single claw.
Ergasilus yandemontei n. sp. has several characters shared with other species of the genus in the Neotropics: pinnate seta on the third exopodal segment of the first pair of legs accompanied by 4 pilose setae, 2-segmented first endopod, presence of a single seta on the first antennular segment, triangular cephalothorax not fused with the first pedigerous somite (El-Rashidy & Boxshall, 2002; Muriel-Hoyos et al., 2015; Marques et al., 2017). The interpodal plates of the new species are ornamented with spinules, similar to E. pitalicus Thatcher, 1984, E. leporinidis Thatcher, 1981, E. hypophthalmi Boeger, Martins & Thatcher, 1993, E. ecuadorensis El-Rashidy & Boxshall, 2002 and E. turkayi Marques, Clebsh, Cordova & Boeger, 2017. Only a small group of species of this genus parasitising freshwater fishes in the Neotropical region, has leg 4 with a 3-segmented endopod, a character shared with the new species from Patagonia: E. colomesus Thatcher & Boeger, 1983; E. pitalicus Thatcher, 1984; E. trygonophilus Domingues & Marques, 2010; and E. sinefalcatus Marques, Boeger & Brasil-Sato, 2015. However, E. colomesus differs from the new species by having an inflated membrane between coxa and the first segment endopod of the antenna. The new species can be distinguished from E. pitalicus by having a robust antenna, a different setal formula of the antennule, and a pectinated seta in leg 1. E. trygonophilus differs from the new species by having leg 1 with a terminal endopodal segment with a rosette-like array of blunt spinules and E. sinefalcatus can be easily differentiated by the presence of a robust spiniform process on the coxae of legs 2, 3 and 4.
Based on body shape and morphology of the legs and antennae, Ergasilus yandemontei n. sp. shows greatest similarity to E. turkayi, described from the characid Serrasalmus hollandi Jégu, 2003 from River Paragua, Bolivia. However, it can be differentiated by: (i) the number of setae and aesthetascs in the antennule; (ii) the lack of seta on the coxobasis of the antenna; (iii) leg 4 with 3-segmented endopod; and (iv) the lack of a dorsal pseudoperculum in the anal somite, a structure which has been reported only for E. turkayi.
The new species differs from Ergasilus sieboldi, the only species of Ergasilus that has been cited from freshwater fish in Patagonia, in the possession of (i) a more robust sensilla near mid-length of the first endopodal segment and on the second endopodal segment of the antenna; (ii) a single seta on the first segment of the antennule, and aesthetascs in segments 4 and 6 (vs 3 setae on the first segment in E. sieboldi); and (iii) a 2-segmented endopod of leg 1, and a pinnate seta on the exopod of the first pair of legs.
Specimens of Ergasilus sieboldi var. patagonicus deposited by Szidat (1956) in MACN-Pa were examined and showed no differences from specimens of Ergasilus yandemontei n. sp. (Fig. 3).
Ergasilus sieboldi var. patagonicus deposited by Szidat (1956) in Colección Nacional de Parasitología of the Museo Argentino de Ciencias Naturales Bernardino Rivadavia (MACN-Pa 317/1; 317/2; 317/3). A, Ventral view of cephalosome; B, Antenna; C, Leg 1; arrow indicates 2-segmented endopod; D, Caudal rami, dorsal view. Scale-bars: A, 100 µm; B–D, 50 µm
Discussion
The new species described herein shares several characters with a regional monophyletic group of the genus Ergasilus described from the Amazon River Basin (Engers et al., 2000), suggesting a common origin and regional dispersal and isolation processes (Muriel-Hoyos et al., 2015).
In all cases, copepods were found attached to the gills of the host, the most common site of infection among ergasilids. The presence of Ergasilus yandemontei n. sp. in fish species belonging to different orders (Atheriniformes, Galaxiformes and Perciformes) indicates that these hosts offer adequate conditions for the parasite and highlights its low host specificity, as in other Ergasilus spp. (Jiménez-García & Suárez-Morales, 2017).
The possible occurrence of the Eurasian copepod E. sieboldi on other continents has been reported in some surveys (Szidat, 1956; Johnson & Rogers, 1973). However, according to Kabata (1979), these suggestions could not be confirmed due to the lack of sufficiently convincing evidence. In particular, in Argentina E. sieboldi var. patagonicus described from Lake Pellegrini should be considered a synonym of the new species described herein, as there were no morphological differences between specimens of E. sieboldi var. patagonicus from the Colección de Parasitología del Museo Argentino de Ciencias Naturales Bernardino Rivadavia, Buenos Aires, Argentina and specimens of Ergasilus yandemontei n. sp. The records of Ergasilus sp. parasitising fishes from Patagonian freshwater environments in Neuquén, Río Negro and Chubut provinces probably correspond to E. yandemontei.
In recent years, fish parasitological research has increased mainly in Brazil and Mexico, while other Latin American countries have rich biodiversity but lack the resources necessary to perform biodiversity surveys (Luque & Poulin, 2007). The parasitic copepod fauna is still underestimated, and there is a great potential for the discovery of new species and new records of parasitic crustaceans in the Neotropical region (Suárez Morales & Santana Piñeros, 2008; Luque et al., 2013). Ergasilus yandemontei n. sp. is the first species described from Patagonian freshwater environments. Further studies are needed to confirm the identity of the specimens parasitising fishes from other environments in Patagonia.
Data availability statement
All data generated or analysed during this study are included in this published article.
References
Alarcos, A. J., & Etchegoin, J. A. (2010). Parasite assemblages of estuarine-dependent marine fishes from Mar Chiquita coastal lagoon (Buenos Aires Province, Argentina). Parasitology Research, 107, 1083–1091.
Boxshall, G. A., & Halsey, S. H. (2004). An introduction to copepod diversity. London: The Ray Society.
Boxshall, G. A., & Montú, M. A. (1997). Copepods parasitic on Brazilian coastal fishes: a handbook. Nauplius, 5, 1–225.
Boxshall, G. A., & Defaye, D. (2008). Global diversity of copepods (Crustacea: Copepoda) in freshwater. Hydrobiologia, 595, 195–207.
Chemes, S. B., & Gervasoni, S. H. (2013). Gill parasites of Prochilodus lineatus (Valenciennes, 1836) (Pisces; Curimatidae; Prochilodontinae) in the Middle Paraná System (Argentina). Revista Brasileira de Parasitologia Veterinária, 22, 619–622.
Chemes, S. B., & Takemoto, R. M. (2014). Nuevos registros de ectoparásitos en peces pimelódidos (Siluriformes) del Paraná Medio (Argentina). Revista Argentina de Parasitología, 2, 24–30.
Drummond, A. J., Ashton, B., Buxton, S., Cheung, M., Cooper, A., Duran, C., et al. (2011). Geneious v5.4. http://www.geneious.com
El-Rashidy, H., & Boxshall, G. A. (1999). Ergasilid copepods (Poecilostomatoida) from the gills of primitive Mugilidae (grey mullets). Systematic Parasitology, 42, 161–186.
El-Rashidy, H. H., & Boxshall, G. A. (2002). New species and new records of Ergasilus Nordmann (Copepoda: Ergasilidae) from the gills of grey mullet (Mugilidae). Systematic Parasitology, 51, 37–58.
Engers, K. B., Boeger, W. A., & Brandão, D. A. (2000). Ergasilus thatcheri n. sp. (Copepoda, Poecilostomatoida, Ergasilidae) from the gills of Rhamdia quelen (Teleostei, Siluriformes, Pimelodidae) from Southern Brazil. Journal of Parasitology, 86, 945–947.
Flores, V., Semenas, L., Rauque, C., Vega, R., Fernandez, V., & Lattuca, M. (2016). Macroparasites of silversides (Atherinopsidae: Odontesthes) in Argentina. Revista Mexicana de Biodiversidad, 87, 919–927.
Jiménez-García, M. I., & Suárez-Morales, E. (2017). Complementary description of Ergasilus arthrosis Roberts, 1969 (Copepoda: Poecilostomatoida: Ergasilidae), a new parasite of cichlid teleosts in southeast Mexico. Systematic Parasitology, 94, 81–90.
Johnson, S. K., & Rogers, W. A. (1973). Distribution of the Genus Ergasilus in Several Gulf of Mexico drainage basins. Bulletin Agricultural Expetiment Station Auburn University, 445, 1–74.
Kabata, Z. (1979). Parasitic Copepoda of British Fishes. London: The Ray Society.
Kearn, G. C. (2004). Leeches, lice and lampreys. A natural history of skin and gill parasites of fishes. The Netherlands: Springer.
Littlewood, D. T. J., & Olson, P. (2001). Small subunit rDNA and the Platyhelminthes: signal, noise, conflict and compromise. In D. T. J. Littlewood & Bray, R. A. (Eds.), Interrelationships of the Platyhelminthes (pp. 262–278). London: Taylor and Francis Publishing Co.
Luque, J. L., & Poulin, R. (2007). Metazoan parasite species richness in Neotropical fishes: hotspots and the geography of biodiversity. Parasitology, 134, 865–878.
Luque, J. L., Vieira, F. M., Takemoto, R. M., Pavanelli, G. C., & Eiras, J. C. (2013). Checklist of Crustacea parasitizing fishes from Brazil. Checklist, 9, 1449–1470.
Marques, T. M., Clebsh, L., Córdova, L., & Boeger, W. A. (2017). Ergasilus turkayi n. sp. (Copepoda, Cyclopoida, Ergasilidae): a gill parasite of Serrasalmus hollandi Jégu, 2003 (Characiformes, Serrasalmidae) from the Paragua River. Bolivia. Nauplius, 25, 1–6.
Modenutti, B. E., & Balseiro, E. G. (1989). Presencia de Ergasilus sieboldi en el plancton de un lago Andino Argentino. Anales del Museo de Historia Natural de Valparaíso, 20, 29–30.
Montes, M. M., & Martorelli, S. R. (2015). An ecological and comparative analysis of parasites in juvenile Mugil liza (Pisces, Mugilidae) from two sites in Samborombón bay, Argentina. Iheringia, Série Zoologia, 105, 403–410.
Montes, M. M., & Martorelli, S. R. (2017). A Bayesian analysis of the parasitic ecology in Jenynsia multidentata (Pisces: Anablepidae). Iheringia, Série Zoologia, 107, 1–10.
Muriel-Hoyos, F., Santana-Piñeros, A. M., Cruz-Quintana, Y., & Suárez-Morales, E. (2015). A new species of Ergasilus Nordmann, 1832 (Copepoda: Cyclopoida: Ergasilidae) from Bryconops giacopinii Fernández-Yépez (Characidae) in the Vichada River Basin, Colombia. Systematic Parasitology, 92, 241–249.
Ortubay, S., Semenas, L. G., Úbeda, C. A., Quaggiotto, A., & Viozzi, G. (1994). Catálogo de peces dulceacuícolas de la Patagonia Argentina y sus parásitos metazoos. San Carlos de Bariloche: Dirección de Pesca, Subsecretaria de Recursos Naturales de la provincia de Río Negro.
Piasecki, W., Goodwin, A. E., Eiras, J. C., & Nowak, B. F. (2004). Importance of Copepoda in freshwater Aquaculture. Zoological Studies, 43, 193–205.
Rosim, D. F., Boxshall, G. A., & Ceccarelli, P. S. (2013). A novel microhabitat for parasitic copepods: A new genus of Ergasilidae (Copepoda: Cyclopoida) from the urinary bladder of a freshwater fish. Parasitololy International, 62, 347–354.
Ruiz, A. E. (2007). Biología del Pejerrey Patagónico en el embalse Florentino Ameghino, Chubut, Argentina. Córdoba: Editorial Científica Universitaria Universitas.
Song, Y., Wang, G. T., Yao, W. J., Gao, Q., & Nie, P. (2008). Phylogeny of freshwater parasitic copepods in the Ergasilidae (Copepoda: Poecilostomatoida) 190 based on 18S and 28S rDNA sequences. Parasitology Research, 102, 299–306.
Suárez-Morales, E., & Santana-Piñeros, A. M. (2008). A new species of Ergasilus (Copepoda: Cyclopoida: Ergasilidae) from coastal fishes of the Mexican Pacific. Folia Parasitologica, 55, 224–230.
Szidat, L. (1956). Über die Parasitenfauna von Percichthys trucha (Cuv. & Val.) Girard del patagonishen Gew asser und die Beziehungen des Wirtsfisches und seiner Parasiten zur palaarktischen Region. Archiv fur Hydrobiologie, 51, 542–577.
Taborda, N. L., Paschoal, F., & Luque, J. L. (2016). A new species of Ergasilus (Copepoda: Ergasilidae) from Geophagus altifrons and G. argyrostictus (Perciformes: Cichlidae) in the Brazilian Amazon. Acta Parasitologica, 61, 549–555.
Varella, A. M. B., Morey, G. A. M., & de Oliveira Malta, J. C. (2019). Ergasilus tipurus n. sp. (Copepoda: Ergasilidae), A parasite of Brazilian Amazon fish species. Acta Parasitologica, 64, 187–194.
Viozzi, G., Semenas, L., Brugni, N., & Flores, V. (2009). Metazoan Parasites of Galaxias maculatus (Osmeriformes: Galaxiidae) from Argentinean Patagonia. Comparative Parasitology, 76, 229–239.
Funding
This study was funded by the following projects: “Invasiones ocultas: Los parásitos y la introducción de peces en la cuenca del Río Negro”. CONICET. PIP 2015-2017 GI. “Parasitismo en ecosistemas patagónicos: invasiones y zoonosis. Universidad Nacional del Comahue”. UNCo B/225.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed. Sampling was carried out with permission from the Provincial and Country authorities of the Neuquén and Río Negro provinces; and with the permission from the authorities of Subsecretaría de Medio Ambiente de la Municipalidad de San Carlos de Bariloche (N° 108 AP-16).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as urn:lsid:zoobank.org:pub: C7C7A87A-B2EB-4709-B218-37B727070A73 . This article was published as an Online First article on the online publication date shown on this page. The article should be cited by using the doi number. This is the Version of Record.
This article is part of the Topical Collection Arthropoda.
Rights and permissions
About this article
Cite this article
Waicheim, M.A., Mendes Marques, T., Rauque, C.A. et al. New species of Ergasilus von Nordmann, 1832 (Copepoda: Ergasilidae) from the gills of freshwater fishes in Patagonia, Argentina. Syst Parasitol 98, 131–139 (2021). https://doi.org/10.1007/s11230-021-09966-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-021-09966-4