Abstract
Halomonhystera parasitica n. sp. (Monhysterida: Monhysteridae) is described from the body-cavity and under the dorsal plates of the sandy beach amphipod Talorchestia brito Stebbing (Crustacea: Talitridae) in Portugal. The new species differs from previously described members of the genus by a combination of the following characters: four medium-sized cephalic setae; base of stoma with three blunt denticles; posterior dilated portion of stoma absent; amphids small, with width less than quarter of corresponding body width; amphids located less than two labial widths from anterior extremity; uterine eggs elliptical and unembryonated; gubernaculum lacks caudal process; and male tail with two separated pairs of postcloacal papillae and a single subterminal seta-like papilla. This is the first representative of the family Monhysteridae parasitic in the body-cavity of crustaceans. Approximately 48% of the amphipods examined contained various stages of H. parasitica.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
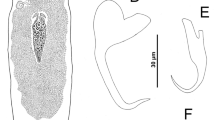
The sand-hopper Talorchestia brito Stebbing (Fig. 1) is one of the most common species of amphipods (Amphipoda: Gammaridea) along the northern coast of Portugal. It ranges along the entire European Atlantic coast, from the British Islands to the Mediterranean Sea, and occurs on sandy beaches among decaying seaweeds and debris (Lincoln, 1979). The present paper describes a new species of monhysterid nematode that parasitises this brackish water amphipod on the coast of Portugal.
Members of the nematode family Monhysteridae De Man, 1876 are typically free-living nematodes occurring in fresh, brackish or salt water as well as terrestrial environments (Fonseca & Decraemer, 2008). External associations with invertebrates include members of Gammarinema Kinne & Gerlach, 1953 on the gills of freshwater crustaceans (crayfish and crabs) (Allen, 1933; Chitwood, 1935; Coomans & Eyualem-Abebe, 2006) and other monhysterids in the gill-chambers of land crabs (Baylis, 1915) and freshwater crustaceans (Schneider, 1932; Osche, 1955; Steiner, 1958). In addition, the interesting species Odontobius ceti Roussel de Vauzème, 1834 lives in the baleen plates of whales (Lorenzen, 1986).
Parasitic representatives of the Monhysteridae are rare and thus far limited to Halomonhystera cameroni Steiner, 1958 parasitising the American oyster Crassostrea virginica (Gmelin, 1879) (see Meyers et al., 1985) and Monhystrella plectoides Cobb, 1918 from the oögonia of the freshwater plant Chara zeylanica (Klein in Willdenow) (see Harrington et al., 1967).
Materials and methods
During February and March, 2008, 150 individuals of Talorchestia brito were collected from the Aveiro estuary (40º38′N; 8º44′W), a brackish water lagoon located in Portugal near the sea. The amphipods were removed from dried, decayed seaweed buried in the sand in the intertidal zone. The specimens were maintained in a refrigerator in the laboratory in plastic containers containing algae and moist sand. The sand hoppers were identified using the key in Lincoln (1979) and compared with the species descriptions provided by Marques (1989) and Bellan-Santini et al. (1993).
The length of the amphipods (from the anterior end of the cephalon to the posterior tip of the telson) ranged from 5 to 15 mm, with a mean ± standard deviation of 9.9 ± 2.6 mm. After removing all external nematodes from their surfaces, the amphipods were dissected and any body-cavity nematodes were washed in seawater, fixed in 70% ethanol and processed to glycerine. Observations, measurements and photographs were made with a Nikon Optiphot light microscope. The measurements are in micrometres, unless otherwise indicated, and represent the mean followed by the range in parentheses.
-
Monhysterida Filipjev, 1929
-
Monhysteroidea de Man, 1876
-
Monhysteridae de Man, 1876
-
Halomonhystera Andrássy 2006
Halomonhystera parasitica n. sp.
Type-host: Talorchestia brito Stebbing (Crustacea: Talitridae).
Type-locality: Aveiro Estuary, Portugal (40º38′N; 8º44′W).
Site: Free in the host’s haemocoel and under the dorsal body-plates.
Type-material: Female holotype (accession no. T-636t) and male paratype deposited in the USDA Nematode Collection, Beltsville, Maryland. Other paratypes are deposited in the authors’ collections.
Description (Figs. 2–9)
Body cylindrical, nearly straight, tapered more towards posterior end; cuticle thin, smooth, apparently uni-layered; somatic setae absent or inconspicuous; head bluntly rounded, not offset, bearing only four medium-sized cephalic setae c.1/4 labial diameter in length; mouth surrounded by 6 partly fused lips; inner labial papillae indistinct; 6 outer labial papillae barely visible; ocelli not observed; walls of buccal cavity (stoma) sclerotised, with cheilorhabdons distinct in most specimens (in some individuals, up to 5 divisions of buccal wall were determined); buccal cavity slightly longer than wide, with 3 short denticles positioned near base; denticles blunt-tipped, project outwards more than upwards; posterior stomal chamber absent; amphids located within 2 labial widths posterior to head; amphidial apertures circular, small; excretory pore located between amphids and nerve-ring; excretory (ventral) gland large, ovoid, with distinct nucleus, positioned mid-ventrally 2–4 body widths posterior to base of pharynx; pharynx muscular, cylindrical throughout its length but narrowing slightly toward base, 1/5 – 1/7 of body length; anterior portion of pharynx surrounds base of buccal cavity; cardia narrow, with width less than widths of adjoining pharynx and intestine; intestinal cells large, single-layered; rectum narrow, shorter than anal body width.
Female with prodelphic, outstretched ovary; vulva positioned close to anus; vagina about equal to anal body width; prominent sphincter surrounds terminal portion of vagina; rectum relatively thin-walled, shorter than anal body width, with well-cuticularised lining and prominent sphincter at base; post-vulva gland-cell not visible; oviparous; uterus contains 2–4 unembryonated, elliptical (3–4 times as long as wide) eggs; egg shell smooth; tail straight or moderately curved dorsally, with 3 caudal glands increasing in size posteriorly; terminal spinneret thin.
Male similar to female but posterior end straight; testis single, outstretched; spicules thin, regularly curved, with narrow, cephalate portion and slight ventral arch; gubernaculum short, lacking caudal process (caudal apophysis), envelopes posterior section of spicules; tail as long as that of female, with 3 caudal glands increasing in size posteriorly; genital supplements consists of single pre-anal, ventro-medial papilla, 2 separated pairs of post-cloacal papillae and single, ventral, subterminal seta (or seta-like papilla).
Measurements
Male (n = 3). Length 1.0 (0.9–1.1) mm; greatest width 37 (36–38); width at labial region (head diameter), 12; length of cephalic seta 3 (2–4); length of stoma 7, width 6; length of pharynx 190 (187–192); distance from head to anterior portion of amphid 18; amphid diameter 4; body width at amphid 18 (17–19); distance from head to anterior tip of gonad 320 (222–396); width at cloaca 28, at anus (anal body diameter) 25 (24–27); tail length 118 (116–122); distance from head to excretory pore 35 (31–40), to nerve-ring 96 (86–109); length of spicules 43 (41–44), greatest width 2.8 (2.5–3.0); length of gubernaculum 8, width 5 μm; length first caudal gland 17 (16–23), of second caudal gland 24 (21–26), of third caudal gland 25 (24–28); length ventral gland 35 (31–40), width 16 (17–18); distance from ventral gland to base of pharynx 84 (77–86); a = 25–29; b = 4.8–5.7; c = 8–9.
Female (n = 6). Length, 1.03 (0.93–1.12) mm; greatest width 37 (35–41); length cephalic setae 3 (2–4); length buccal cavity 7 (6–8), width 6 (5–7); distance from head to middle of amphid 20 (16–23); diameter of amphid 4.1 (3.5–4.5); distance from head to middle of nerve-ring 111 (107–115), to excretory pore 43 (30–55), to tip of gonad 306 (206–364), to base of pharynx 181 (177–187); length of tail 128 (118–136); body width at anus 50 (44–56); distance from vulva to tail tip 161 (152–168), to anus, 33 (32–36); length of intra-uterine eggs, 65 (59–76), width 20 (17–24); distance from tip of gonad to base of pharynx 190 (168–203); length of first caudal gland 16 (14–18), of second caudal gland 29 (27–33), of third caudal gland 35 (32–40); length ventral gland 40 (33–43), greatest width 18 (17–19); distance from middle of ventral gland to base of pharynx 80 (72–85); a = 27–28; b = 5.3–5.9; c = 8–9; V = 84–86%.
Diagnosis and remarks
The new species differs from H. socialis (Bütschli 1874), Andrássy, 2006, H. uniformis (Cobb, 1914) Andrássy, 2006, H. halophila Andrássy, 2006, H. continentalis Andrássy, 2006, H. hickeyi Zekely, Sørensen & Bright, 2006, H. cameroni (Steiner, 1958) Andrássy, 2006 and H. antarctica (Cobb, 1914) Andrássy, 2006, which all have a gubernaculum with a caudal process or apophysis. The new species also differs from H. ambiguoides (Bütschli, 1874) Andrássy, 2006, which has amphids located far from the anterior end (three labial widths from the head). The amphids are positioned within two labial widths from the head in H. parasitica n. sp. Also, in H. ambiguoides, the amphids are large, almost a third of the corresponding body width, whereas, in H. parasitica, they are less than a quarter of the corresponding body width. The new species differs from H. disjuncta (Bastian, 1865) Andrássy, 2006, which has spherical to oval eggs and a relatively longer female tail (2.5–3 times as long as the distance from the vulva to the anus). Also, in H. disjuncta, there are 10 short cephalic setae (versus only four of medium size in H. parasitica), the stoma contains no denticles, there is a posterior dilated portion to the stoma and the postcloacal genital papillae pair are adjacent rather than being separated as in H. parasitica. The new species differs from H. chitwoodi (Steiner, 1958) Andrássy, 2006, which has a tail length about equal to the distance from the vulva to the anus, and from H. glaciei (Blome & Riemann, 1999) Andrássy, 2006, which has a distance between the vulva and anus of 5 times the anal body width.
Biological observations
Populations of H. parasitica n. sp. were fairly common in the Aveiro estuary during the winter season, almost half of the examined amphipods being infected. Of 150 amphipods studied, 72 contained stages of H. parasitica in their body-cavities, indicating an infection rate of c.48%. Nematode populations ranged from 1–118 per host; however, the majority of amphipods contained 1–30 nematodes in their body-cavities. Since Talorchestia brito is a common shore amphipod along the European Atlantic coast, it is likely that the geographical and temporal distribution of H. parasitica extends beyond Portuguese waters.
At the present time, we have no information as to whether H. parasitica affects its amphipod host.
Discussion
Halomonhystera parasitica n. sp. is the first member of the Order Monhysterida parasitic in crustaceans. Monhysterids are mainly microbivorous (ingesting microbes such as diatoms, algae and bacteria), non-selective deposit feeders (Wieser, 1953); however, it is not known whether all of the assorted gut components are utilised as food items. Some species have been cultured on plates containing a single bacterial species (Overgaard Nielsen, 1949) or on bacteria growing in slime on the sides of seawater aquaria (Chitwood & Murphy, 1964).
It is presumed that developing stages (reproducing adults and juveniles) of H. parasitica in the body-cavity of Talorchestia brito obtained nourishment from the haemolymph of the amphipod. The gut contents of most specimens of H. parasitica removed from the body-cavity contained no particulate matter (bacteria, diatoms or fungal remains) but only a viscous deposit, which is interpreted as host haemolymph.
It is curious why additional species of the Monhysteridae have not adapted a parasitic life-style in aquatic invertebrates. Certainly, it would only be a slight physiological adjustment to move from a brackish or marine habitat to the haemocoel of invertebrate hosts living in the same environment. Perhaps the requirement for particulate food is a deterrent. While there appears to be no “resting” stage in the life-cycle of H. parasitica, (or in any other monhysterid for that matter), which could serve as the infective stage, transmission to the amphipod could result from the ingestion of nematode eggs released into the environment after the host died. These eggs could hatch in the amphipod gut and the juveniles could penetrate the gut-wall and enter the haemocoel. The denticles at the base of the stoma in H. parasitica may be useful for this purpose. Since nematode stages were also found under the body-plates of T. brito, it is possible that penetration into the haemocoel occurs through the intersegmental membranes.
References
Allen, S. A. (1933). Parasites and commensals of North Carolina crayfish. Journal of the Elisha Mitchell Scientific Society, 49, 119–121.
Baylis, H. A. (1915). Two new species of Monhystera (Nematoda) inhabiting the gill-chambers of land-crabs. Annals and Magazine of Natural History, 16, 414–421.
Bellan-Santini, D., Karaman, G., Krapp-Schickel, G., Ledoyer, M., & Ruffo, S. (1993). Gammaridea (Melphidippidae to Talitridae), Ingolfiellidea, Caprellidea. In: Ruffo, S. (Ed.) The Amphipoda of the Mediterranean. No. 13 (Part 3). Memories de l'Institute Océanographique, Fondation Albert I, Monaco, 813 pp.
Chitwood, B. G. (1935). Nematodes parasitic in, and associated with, Crustacea, and descriptions of some new species and a new variety. Proceedings of the Heminthological Society of Washington, 2, 93–96.
Chitwood, B. G., & Murphy, D. G. (1964). Observations on two marine Monhysterids—their classification, cultivation, and behavior. Transactions of the American Microscopical Society, 83, 311–329.
Coomans, A., & Eyualem-Abebe. (2006). Order Monhysterida. In Eyualem-Abebe, I. Andrássy, & W. Traunspurger (Eds.), Freshwater nematodes: Ecology and taxonomy. Cambridge: CABI Publishing, pp. 574–603.
Fonseca, G., & Decraemer, W. (2008). State of the art of the free-living marine Monhysteridae (Nematoda). Journal of the Marine Biological Association of the United Kingdom, 88, 1371–1390.
Harrington, D., Anderson, R. C., Bird, G. M., & Mai, W. F. (1967). Occurrence of Monhystrella plectoides (Nematoda: Monhysteridae) within the white oogonia of Chara seylanica. Canadian Journal of Botany, 45, 973–974.
Lincoln, R. J. (1979). British marine Amphipoda: Gammaridea. London: British Museum (Natural History), 658 pp.
Lorenzen, S. (1986). Odontobius (Nematoda, Monhysteridae) from the baleen plates of baleen whales and its relationship to Gammarinema living on crustaceans. Zoologica Scripta, 15, 101–106.
Marques, J. C. (1989). Amphipoda (Crustacea) bentónicos da costa portuguesa: estudo taxonómico, ecológico e biogeográfico. PhD Thesis in Applied Ecology, Faculdade de Ciências e Tecnologia—Universidade de Coimbra, Museu e Laboratório Zoológico, Coimbra, Portugal, 394 pp.
Meyers, T. R., Elston, R. A., & Georgi, M. E. (1985). A monhysterid nematode parasitizing captive American oysters (Crassostrea virginica). Journal of Invertebrate Pathology, 46, 205–208.
Osche, G. (1955). Über die Vergesellschaftung von Nematoden und Crustaceen, mit einer Beschreibung von Matthesonema tylosa n. g., n. sp. (Nematoda) aus dem Kiemenraum einer Assel. Zoologischer Anzeiger, 155, 253–262.
Overgaard Nielsen, C. (1949). Studies on the soil microfauna. II. The soil inhabiting nematodes. Natura Jutlandica, 2, 1–131.
Schneider, W. (1932). Nematoden aus der Kiemenhöhle des Flusskrebses. Archiv für Hydrobiologie, 24, 629–636.
Steiner, G. (1958).Monhystera cameroni n. sp.—a nematode commensal of various crustaceans of the Magdalen Islands and Bay of Chaleur (Gulf of St. Lawrence). Canadian Journal of Zoology, 36, 269–278.
Wieser, W. (1953). Die Beziehung zwischen Muhdhöhlengestalt, Ernährungsweise und Vorkommen bei freilebenden marinen Nematoden. Arkiv för Zoologi, 4, 439–484.
Acknowledgement
Thanks are extended to the Pluriannual Program (CIIMAR) for supplying funds for the present study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poinar, G., Duarte, D. & Santos, M.J. Halomonhystera parasitica n. sp. (Nematoda: Monhysteridae), a parasite of Talorchestia brito (Crustacea: Talitridae) in Portugal. Syst Parasitol 75, 53–58 (2010). https://doi.org/10.1007/s11230-009-9210-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-009-9210-x