Abstract
Geometry optimizations have been performed on the M4(Pyr)2 (M = Ti-Ni, Pd and Pt, Pyr = C16H10) complexes by means of DFT method using BP86 and mPW1PW91 functionals combined to the TZP basis set. The M4 moiety encapsulated between two pyrene ligands tends to establish M-L bonding with various hapticies from η2 to η6. In accordance with the coordination modes, the pyrene behaves as neutral, dianionic, or tetraanionic ligand. For the Ti, V, and Fe, the low-spin (S = 0) and the high-spin (S = 1) structures are isoenergetic, while the Cr, Mn, and Co structures prefer the high-spin states. The Ni, Pd, and Pt structures are more favorable in low-spin state. The zigzag metallic chain is predicted to be more stable than that of the two-dimensional sheet for the Pd complexes. The spin state changes of the studied complexes in their ground states could be characterized in some cases by different molecular structure modifications (structural isomerisation, where structural modifications accompany the spin state modification like as bonds and angles), electronic configurations (low-spin or high-spin), or oxidation states with respect to the metal charges, in agreement with the metal nature. The optimized structures obtained by both BP86 and mPW1PW91 methods are consistent to each other, where the energetic parameters follow similar tendencies regarding the stability order between isomers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pyrene (C16H10 = Pyr) is a 16π-electron polyarene molecule composed of four benzene C6 rings. It can be compared to polycyclic aromatic hydrocarbons which have attracted considerable attention in materials science due to their shapes and other properties arising from their extended delocalized π-system [1–3]; they permit the introduction of one or more transition metals on the discrete aromatic rings [4].
Although, the coordination of polyarene to one, two, or three metal atoms has been widely explored theoretically and experimentally [5–12], the four or more metals encapsulated between two polycyclic aromatic hydrocarbons (PAHs) was scarcely investigated. Recently, Murahashi et al. have investigated the polypalladium sandwich complexes [13–16]. The main characteristic of these complexes is the direct metal-metal bonds, where the electronic communication between two metal centers can vary the particular chemical and physical properties of metallic complexes. This communication is dependent on the coordinating ligand and on the nature of the metal centers [17–19]. Spin contamination is a mix observable fact where certain spin state is combined with higher states. The admixture makes the spin of the originate state contaminated and will degrade the quality of the potential energy surface and spin densities. Also, problems are encountered for the compounds often involving energetically quasi-degenerate orbitals producing a static correlation effect [20, 21]. The broken-symmetry (BS) is one of the methods that involve the static correlation correction [22–25] by splitting α and β electrons into two different orbitals, i.e., different orbitals for different spins approach [26, 27]; thus, a singlet spin state with the strong static correlation is expressed as a singlet biradical. However, in those singlet biradicals of the BS methods emerges a serious problem called spin contamination error that higher spin states are implicated in the singlet wave function influencing, for example, the total energy, optimized geometry, and excited energy.
This paper reports the results of density functional theory (DFT) calculations of the electronic structure, geometric properties, and bonding analysis of M4(Pyr)2 (M = Ti-Ni, Pd, and Pt) neutral sandwich compounds, consisting of metal atoms flanked between two pyrene ligands depending on the spin state, molecular symmetry, and spin contamination effects. A primary object of this study was to predict the existence of M4(Pyr)2 (M = Ti-Ni, Pd, and Pt) complexes which are not available experimentally and identify the capability of the pyrene ligand to encapsulate a two-dimensional metal core.
This study was motivated by the developments on several fronts. Principally, the recent papers of Murahashi et al. [14, 28] reported the synthesis and properties of organometallic complexes with three, four, and five palladium atoms between aromatic hydrocarbons namely; [Pd3(C7H7)2], [Pd4(perylene)(cot)L2], and [Pd5(tetracene)2], respectively, which are characterized by X-ray diffraction.
The reliability of density functional theory (DFT) method using the non-local density approximation BP86 functional, which combines Becke’s 1988 exchange functional with Perdew’s 1986 gradient corrected correlation functional (see Computational details) is a valuable tool in determining the electronic structures, the geometrical parameters, the bonding analysis, and other properties from previous works of organometallic and inorganic systems [29–33]. This GGA functional is of a general use and known to provide accurate equilibrium ground-state geometries as well as the hybrid mPW1PW91 functional which provide consistent results to each other [34].
The atoms numbering adopted throughout the paper and the geometry optimization of free pyrene ligand are sketched in Scheme 1.
Furthermore, the canonical Lewis formulae for neutral, dianionic, and tetraanionic pyrene ligand are shown in Scheme 2.
Computational details
Density functional theory (DFT) calculations were carried out on the studied compounds using the Amsterdam Density Functional (ADF) program [35], developed by Baerends and co-workers [36–40]. Electron correlation was treated within the local density approximation (LDA) in the Vosko-Wilk-Nusair parametrization [41]. The first one is the BP86 method that combines Becke’s 1988 exchange functional (B) [42, 43] with Perdew’s 1986 gradient corrected correlation functional (P86) [44, 45]. The second one is the hybrid functional mPW1PW91, which is a combination of the modified Perdew–Wang exchange functional and Perdew–Wang’s 91 gradient-correlation functional [46]. The numerical integration procedure applied for the calculations was developed by te Velde et al. [40]. The atom electronic configurations were described by a triple-ζ Slater-type orbital (STO) basis set for H 1s, C 2s and 2p, N 2s and 2p augmented with a 3d single-ζ polarization for C and N atoms and with a 2p single-ζ polarization for H atoms. A triple-ζ STO basis set was used for the first row transition metals 3d and 4s, for Pd 4d and 5s and for Pt 5d and 6s augmented with a single-ζ 4p polarization function for the first row, a single-ζ 5p polarization function for Pd and a single-ζ 6p polarization function for Pt. A frozen-core approximation was used to treat the core shells up to 1s for C, N, 3p for the first row transition metals, 4p for Pd and 4f for Pt [36–40]. For the systems containing atoms in which Z is greater than 41, the scalar relativistic zero-order regular approximation (ZORA) was used (with the associated optimized valence basis set) [47]. Full geometry optimizations were carried out using the analytical gradient method implemented by Versluis and Ziegler [48]. Spin-unrestricted calculations were performed for all the open-shell systems. Frequency calculations [49, 50] were performed on all the studied compounds to check that the optimized structures are at local minima. Representation of the molecular orbitals and molecular structures were done using ADF-GUI [35] and MOLEKEL4.1 [51], respectively.
Results and discussion
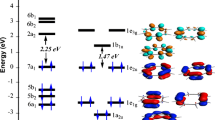
The bonding within the M4(Pyr)2 sandwich structures are described by interactions between the M4 pseudo-square sheet and the two separated pyrene ligands. In order to give a deeper insight into this bonding type, a simplified qualitative diagram of M4(Pyr)2 for d4 metal has been provided in Scheme 3.
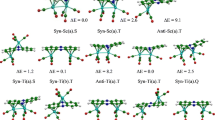
The 16 occupied π-type ligand combinations (right side of Scheme 3) interact with their corresponding metallic (bonding and antibonding) combinations (left side of Scheme 3) for the four Ti metals, 20 orbitals are of d-type and 16 of (s + p) type. The interactions between (s + p) metal orbitals with those of pyrene ligands within a framework of one-to-one interaction conduct to 16 Ti-C bonding orbitals (mainly ligands) and 16 Ti-C antibonding orbitals (mainly metals). The d-bock is slightly perturbed giving rise to 10 bonding and 10 antibonding d-d combinations. For the Ti (d4) metal, only eight among 20 d-d levels are occupied corresponding to four σ Ti-Ti and four π Ti-Ti bonds, thus describing a formal double bond order providing a small HOMO-LUMO gap. A decreasing of M-M bond order is expected by filling metallic antibonding levels. Accordingly, for the Ti4(Pyr)2 sandwich structures, singlet and triplet spin state isomers are found as energy minimum (Fig. 1). Going from Ti towards to the rich metals corresponds to occupation of antibonding MOs; thus, a progressive decreasing of M-M bonding should occur.
Titanium models
The Ti4(Pyr)2 singlet C s structure is obtained as lowest energy isomer by both BP86 and mPW1PW91 methods, in which the four Ti atoms form a pseudo-square sheet justified by comparable Ti-Ti bond distances of 2.708 and 2.715 (BP86) or 2.675 and 2.687 Å (mPW1PW91). Each Ti metal center is coordinated to both pyrene ligands in η6 manner. This kind of coordination enables each pyrene ligand to involve all its 16π-electron in interactions with Ti4 sheet. The obtained η6 coordination for each metal center to both pyrene ligands conducts us to consider that the 16π-electron is shared equitably by the four metals. This coordination mode is based on the short Ti-C bond distances, as highlighted in the Table S1 of Supplementary information. Furthermore, alternating open and close angles of 110 and 70° are computed for Ti-Ti-Ti bond angles (see Supplementary information), in accordance with the pseudo-square Ti4 plane. In order to give a deeper insight into the metal-metal bonding within the M4 sheet of the studied species besides that inferred from metal-metal bond distances and electron counting, the Wiberg Bond Indices (WBIs) were determined using Natural Bond Orbital (NBO) analysis [52] by means of NBO 6.0 program [53]. The calculated Ti-Ti bond distances mentioned above suggest a formal double bond and well corroborated by the obtained WBI values of 0.67 and 0.64 conducting to formal Ti-Ti bond orders of 2 and roviding the 16-MVE configuration for each Ti center. The small HOMO-LUMO gap of 0.24 (BP86) or 0.88 eV (mPW1PW91) matches well with the Ti4(Pyr)2 electron deficient structure. The 16 d-metallic electrons insuring the metal-metal bonds should correspond to 4σ and 4π Ti-Ti bonding orbitals as discussed earlier (Scheme 3), while the bonding and antibonding δ-type orbitals are vacant. It is interesting to mention that the bond lengths between the metals and the external carbon atoms ranging from 2.404 to 2.443 (BP86) or from 2.381 to 2.408 Å (mPW1PW91) are slightly shorter than those between Ti metals and the inner carbon ones, showing a relatively weak slippage of 4% towards the external edges (see Supplementary information). The singlet low-spin structure obtained by broken symmetry method “unrestricted calculations” does not correspond to an energy minimum structure characterized by a large imaginary frequency of −280 cm−1 and featuring a contaminated spin state <S2> = 0.74 of an ideal value of zero. The high-spin (S = 1) structure is obtained only 2.1 (BP86) or 1.5 kcal/mol (mPW1PW91) above the global minimum, a difference which is not signifi-cant at the considered level of theory. Weak Ti-Ti bond dis-tances lengthening are observed for the triplet state matching well with depopulation of the Ti-Ti HOMO bonding orbital and population of an antibonding LUMO one. The two un-paired electrons are localized on Ti(1) and Ti(3) atoms (Scheme S1) well associated with the spin density values of 0.76 and 0.78, where <S2> value of 2.06 and emphasizes the absence of spin contamination, thus, giving a reliable structure.
The pyrene ligands are not symmetrical corresponding to one neutral ligand and the other dianionic, in relation with the Ti4 2+ sheet interacting with dianionic (Pyr)2− considered as two-electron reduced form in previous works [54, 55]. The Ti-C bond lengths are in the range of 2.419–2.448 (BP86) or 2.384–2.409 Å (mPW1PW91), pointing out an η6,η6 coordination mode of each Ti metal to both pyrene ligands. It is interesting to note that the quintet (S = 2) and septet (S = 3) spin titanium structures are found very high in energy (see Supplementary information) displaying a strong relative instability with respect to the low-spin structures; thus, they are not discussed in this section. In the case of titanium structures, going from the low-spin to the high spin does not induce remarkable modifications concerning the geometrical parameters and oxidation states of metals as displayed in Table 1.
Vanadium models
For the V4(Pyr)2 sandwich structures, low-spin and high-spin isomers are found as genuine minima without any imaginary frequencies (Fig. S1).
Differently to the Ti case, the V4(Pyr)2 singlet state “BS” structure obtained without symmetry constraints is found as energy minimum (Fig. 1), in which the four V atoms form a pseudo-square plane characterized by short V-V bond distances in the range 2.495–2.800 (BP86) or 2.456–2.773 Å (mPW1PW91), corresponding to alternating double (formal V-V bond order of 2) and triple (formal V-V bond order of 3) bonds and matching well with the WBI values of 1.09, 0.42, 1.08, and 0.43 (BP86), conducting to the 18-MVE configuration for each V center as neutral V(0), which share equitably the 32π-electron of both pyrene ligands. Each V metal center is coordinated to both pyrene ligands in an η6 manner giving rise to V4(η6,η6,η6,η6-Pyr)2 structure. This kind of coordination enables each pyrene ligand to involve all its 16π-electron in the interactions with V metals. It is worth noting that BS structure does not suffer from a spin contamination of higher spin states as displayed by the <S2> value of zero. Indeed, the triplet (S = 1), quintet (S = 2), and septet (S = 3) vanadium structures are found very high in energy (Table 1) characterized by long V-V and V-C bond distances compared to those of the singlet one obtained by broken symmetry method. Likewise, the restricted singlet structure (SA) characterized by small HOMO-LUMO gap of 0.30 (BP86) or 0.92 eV (mPW1PW91) is found 43.7 (BP86) or 45.9 kcal/mol (mPW1PW91) above the unrestricted “BS” structure. The spin eigenvalue for the specific state is S(S + 1), where S is the net spin state. The deviation from the specific eigenvalue is considered as spin contaminated. The spin density calculation (α − β) reveals that the primary spin density for the triplet structure is equally located on the V(2) and V(4) as V(0) atoms with a value of 1.06 much important than those calculated for Ti complex (0.75 and 0.77) (Scheme S1) which gives a reliability of the triplet spin state structure without spin contamination (<S2> = 2.07 relative to the ideal value of 2). In light of these results, one can observe the lengthening of V-V bond distances and formation of new V(2)-V(4) bond for the high spin structures (S = 2 and S = 3) as shown in Table 1. However, the V-C bond distances undergo negligible modifications as summarized in Table S1 of supplementary information, thus, keeping the same coordination mode for all vanadium structures.
Chromium models
Three optimized Cr4(Pyr)2 structures with singlet, triplet, and quintet spin states are found as ground states (Fig. S2 and Table 2).
The Cr4(Pyr)2 singlet structure corresponding to the high D 2h symmetry is calculated less stable by 13.9 (BP86) or 11.8 kcal/mol (mPW1PW91) than the triplet isomer obtained as global minimum (Fig. 2) inversely to Ti and V complexes. The relative instability of the low-spin structure is in accordance with its small HOMO-LUMO gap of 0.15 (BP86) or 0.77 eV (mPW1PW91), in which each Cr metal is coordinated to each pyrene ligand in an η6 manner. Based on the MOs localization, the Cr-Cr bond lengths of 2.692 (BP86) or 2.653 Å (mPW1PW91), the WBI value of 0.5446 (BP86), and the obtained η6,η6 coordination mode suggest the presence of Cr-Cr double bonds (formal Cr-Cr bond order of 2) in the Cr4 plane, wherein each Cr center is coordinated to each pyrene ligand through η6 fashion, thereby satisfying the 18-MVE configuration. Going from the singlet structure to the triplet one does not remarkably modifies the M-M bond distances, which are lengthened only by about 0.057 Å. Inversely, it extremely modifies the M-L distances by a lowering of the coordination mode from η6 to η4, where Cr-C bond distances lengthen from 2.692 to 2.749 (BP86) or 2.654 to 2.726 Å (mPW1PW91), in agreement with depopulation of the bonding HOMO and population of the antibonding (non-bonding) LUMO. Really, Cr(1) and Cr(3) metals preserve the same η6,η6 coordination, but the remaining Cr(2) and Cr(4) undergo a coordination decrease from η6,η6 to η4,η4 accompanied by Cr(2)-C(5), Cr(2)-C(5′), Cr(2)-C(10), Cr(2)-C(10′), Cr(4)-C(5), Cr(4)-C(5′), Cr(4)-C(10), and Cr(4)-C(10′) bond cleavages giving rise to two cationic Cr(I) centers. The Cr4 2+ plane is coordinated to unsymmetrical pyrene ligands, where one is deemed as neutral ligand, but the other is dianionic (Pyr)2−. This situation of Cr4(η6,η4,η6,η4-Pyr)2 structure gives two neutral Cr(0) and two cationic Cr(I) having 16- and 15-MVE configurations, respectively, responding to Cr-Cr single bonding. The 15-MVE configuration shows that the two unpaired electrons are located on the Cr(2) and Cr(4) centers, in accordance with their spin density of 1.11 (BP86) (Scheme S1) providing a weak spin contamination of 2.05. The quintet state structure lies only 4.9 kcal/mol above the global minimum keeping the same coordination mode than the triplet structure and indicates that the four unpaired electrons should be roughly localized on the separated Cr(2) and Cr(4) centers (Scheme S1). The quintet state seems to be unreliable structure due to the obtained large spin contamination based on the <S2> value of 7.32 compared to the expected value of 6, thus, showing a considerable admixture with higher spin states and rending the spin of the originate state impure. Likewise, the septet state structure lies 7.6 (BP86) or 2.5 kcal/mol (mPW1PW91) higher in energy than the lowest-energy triplet structure displaying long Cr-Cr bond distances of 2.905 Å. Noticing that the “BS” structure is not obtained as energy minimum authenticated by large imaginary frequency and strong spin contamination value <S2> = 1.63 for an expected value of zero. As results, the spin state changes lead either to Cr-Cr or Cr-C bond distance modifications in relation with bond order or the established coordination mode, respectively. Also, the metal charges undergo substantial variations as displayed in Table 2. Furthermore, the septet (S = 3) structure features significant Cr-Cr bond distances lengthening and decoordination of Cr-C bonds as shown in Table 2 and Table S3 of the supplementary information.
Manganese models
Different Mn4(Pyr)2 structures are obtained with various spin states (singlet, triplet, quintet, and septet) as displayed in Fig. S2 and Table 2.
The high-spin septet structure is computed as the global minimum (Fig. 2) contrary to those of Ti, V, and Cr complexes where the singlet and triplet structures are the most stables ones. The Mn(Pyr)2 septet (S = 3) structure corresponds to a localization of the six unpaired electrons on both metallic centers namely Mn(2) and Mn(4) as elucidated by the spin density value of 3.05 on each metal center considered as neutral Mn(0) (Scheme S2).
The average Mn-Mn bond length of 2.895 (BP86) or 2.856 Å (mPW1PW91) could correspond to a single bond order based on the obtained average WBI value of 0.180 is comparable to that observed in the binuclear Mn2(CO)10 complex with a direct Mn-Mn bond of 2.903 Å [56], for which our theoretical calculations at the same level of theory attribute a comparable WBI value of 0.152. The Mn-C bond distances in the range 2.061–2.149 (BP86) or 2.001–2.112 Å (mPW1PW91) suggest a perfect η3,η3 coordination mode for Mn(1) and Mn(3), whereas, the Mn-C bond distances ranging from 2.084 to 2.374 (BP86) or 2.015–2.335 Å (mPW1PW91) provide an η4,η4 coordination mode for Mn(2) and Mn(4) atoms comparable to those of previous works [5, 6, 11]. These findings attribute a 16-MVE configuration for Mn(1) and Mn(3) as cationic centers and the 17-MVE configuration to Mn(2) and Mn(4) as neutral ones; thus, the obtained structure does not display a spin contamination (<S2> = 12.09 relative to the ideal value of 12) corresponding to a localization of the spin density only on Mn(2) and Mn(4) comparable to those found for Chromium (S = 3) complex (Scheme S2). The singlet (SA and BS) C s structures lie 57.4 and 50.5 kcal/mol (BP86) above the high-spin (S = 3) one found as global minimum, where the former structure displays a very small HOMO-LUMO gap of 0.20 (BP86) or 0.75 eV (mPW1PW91). For the triplet and quintet structures are found less stable than the septet one by 10.0 and 15.9 (BP86) or 7.3 and 9.9 kcal/mol (mPW1PW91), respectively, wherein the bond distances of triplet and quintet structures are comparable to those obtained for the trimetallic manganese complexes exhibiting the same tendencies regarding the WBI values [57]. The high spin state structure (S = 3) corresponds to the longest Mn-Mn bond distances of 2.905 and 2.885 and to the enhancement of the metal oxidation states of +1.7, but keeping the same bond order of 1, which is comparable to those of S = 1 and S = 2 structures. It is noteworthy for the quintet structure the formation of a supplementary M(2)-M(4) bond distance of 2.947 Å.
Iron models
Full geometry optimizations of Fe4(Pyr)2 species gave rise to structures of various spin states of D 2h symmetry as gathered in Table 3 and the lowest energy structure is shown in Fig. 3.
Although the Fe4(Pyr)2 low-spin D 2h structure exhibits a small HOMO-LUMO gap of 0.50 (BP86) or 0.88 eV (mPW1PW91), it was found as the global minimum structure and predicted to be more stable than that obtained by broken symmetry method by only 2.8 (BP86) or 3.5 kcal/mol (mPW1PW91), a value which is not significant at the considered level of theory. The D 2h symmetry yields iron centers identical two-two, where two among four are described by η3-allylic coordination mode giving rise to dicationic M(1) and M(2) as Fe2+ centers and leads to a puckered C6 ring, in junction with their slippage towards the external carbon atoms, while the two M(2) and M(4) centers are described by an η4 coordination mode. Accordingly, the Fe4 4+ plane is sandwiched between two symmetrical pyrene (Pyr)2− ligands, considered in previous works as two-electron reduced form [54, 55]. For the Fe4(η3,η4,η3,η4-Pyr)2 singlet structure, the Fe(1) and Fe(3) atoms are bound to the pyrene ligand in an η3 manner through short Fe(1)-C bond distances of 2.085 (BP86) or 2.002 Å (mPW1PW91), displaying a slippage towards carbon atoms, in agreement with distorted C6 rings, whereas, the Fe(2)-C of 2.184 (BP86) or 2.150 Å (mPW1PW91) (Supplementary information) correspond to C6 rings coordinated in an η4 fashion are slightly long.
The obtained Fe-Fe bond distances of 2.896 (BP86) or 2.843 Å (mPW1PW91), although they are long, they could be considered as single ones. This hypothesis might be based on the calculated WBI values of 0.184 suggesting a single Fe-Fe bond (formal Fe-Fe bond order equal to 1) and which are comparable to those find in previous work [57]. Based on the previous discussion, the Fe(1) and Fe(3) as dicationic Fe2+ centers are deficient as 16-MVE metals, while the neutral Fe(2) and Fe(4) as neutral centers obeying the 18-MVE rule. The calculated angles within the Fe4 plane of 63 and 117° (see Supplementary information) show open and close angles alternation in adequation of pseudo-square shape. The Fe4(η3,η4,η3,η4-Pyr)2 triplet structure lies 2.8 (BP86) or 2.2 kcal/mol (mPW1PW91) above the global minimum, suggesting interconversion in solution, which shows slight shortening of Fe-Fe bond distances from 2.896 to 2.862 (BP86) or 2.843 to 2.811 Å (mPW1PW91), but significant lengthening of Fe-C bond distances from 2.085 and 2.184 Å to 2.100 and 2.228 Å (BP86), respectively, due to population of an antibonding orbital. Similarly, the quintet and septet structures lie 7.3 and 14.7 (BP86) or 5.2 and 11.4 kcal/mol (mPW1PW91) above the lowest energy singlet one, respectively. All the three high spin structures present spin contaminations <S2> of 2.66, 6.71, and 12.44 (BP86) for ideal values of 2 (S = 1), 6 (S = 2), and 12 (S = 3), respectively. Thereby, these high spin structures seem to be unreliable structures due to the obtained large spin contamination showing an admixture with higher spin states and rending the spin of the originate state impure. Furthermore, these high spin structures undergo notable structural modifications and important oxidation state variations as noted in Table 3.
Cobalt models
Three structures are identified as energy minimum for the Co4(Pyr)2 models (one singlet, one triplet, and one quintet) (fig. S3 and Table 3).
The high-spin (S = 2) structure is found as the global minimum (Fig. 3) and calculated relatively more stable than that of triplet and singlet isomers by 5.1 and 7.2 (BP86) or 6.3 and 8.1 kcal/mol (mPW1PW91), respectively. Although the “BS” structure lies only 1.9 (BP86) or 2.3 kcal/mol (mPW1PW91) above the lowest energy structure, it is characterized by a large spin contamination of 1.70 for an expected value of zero, confirming the suffering of the broken symmetry method from a spin contamination of higher spin states as discussed by Kitagawa et al. [58, 59]. For the global minimum D 2h structure, the Co-Co bond distances of 2.994 (BP86) or 2.958 Å (mPW1PW91) are long; thus, they could not be considered as single one with respect to the weak WBI of 0.091 corresponding to a zero as formal Co-Co bond order. The calculated Co-C bond distances are in the range 2.078–2.187 (BP86) or 1.997–2.146 Å (mPW1PW91), which are relatively short pinpointing strong interactions between the Co4 4+ plane and the dianionic pyrene (Pyr)2−, where the Co(1) and Co(3) exhibit an η3,η3 coordination mode considered as dicationic Co(II), thus, acquiring the 17-MVE configuration, while the two others Co(2) and Co(4) are neutral metals bound to the ligand in an η2,η2 fashion giving Co(1) and Co(2) atoms the 15-MVE configuration. The four unpaired electrons are primary localized on the four metallic centers as elucidated by the almost equally distribution of the spin density (α − β) values of 1.18 for Co(1) and Co(3) and of 0.90 for Co(2) and Co(4) (scheme S3) with respect to spin contamination <S2> value of 6.05 for an expected value of 6.0, suggesting a reliable structure. It is worth noting the difference of the spin density distribution obtained of Fe and Co complexes as shown in Schemes S2 and S3.
The low-spin (S = 0) structure displays a small HOMO-LUMO gap of 0.15 (BP86 or 0.76 eV (mPW1PW91), wherein each cobalt center is described by an η3,η3 coordination mode giving rise to four dicationic Co(II) metals bound to both tetraanionic pyrene ligands (pyr)4−, which has been highlighted as four-electron reduced form (Lewis Scheme) [60]. The Co4 8+ plane features only two direct Co-Co bonds corresponding to WBIs of 0.228 ( 2.843 Å longer than that encountered in Co2(CO)8 of 2.554 Å) instead of four, while the two others Co….Co distances of 3.360 Å allow positive interactions. In light of these results, it can be concluded that for the cobalt species, the pyrene ligand adopt various Lewis structures as neutral, dianionic, or tetraanionic according to the spin state and the oxidation state of the cobalt centers. The triplet structure lies 5.1 (BP86) or 6.3 kcal/mol (mPW1PW91), seems to be questionable owing to the very high spin contamination <S2> = 3.20 against an ideal value of 2, indicating that the corresponding originate spin state is severely contaminated by higher spin states, while the septet structure lies 5.7 (BP86) or 5.0 kcal/mol (mPW1PW91) above the lowest energy quintet structure exhibiting somewhat a spin contamination <S2> = 12.25 against an expected value of 12. From the Table 3, it has been observed that the high spin structure (S = 3) shows important Co….Co repulsions and Co-C decoordination in accordance with the cobalt negative charges of −0.33 and −0.36, against cobalt positive charges and short Co-Co contacts of S = 2 and S = 1 spin state structures.
Nickel, palladium, and platinum models
Going from cobalt to nickel, Pd and Pt as d10 metals, it turned out that the singlet spin state (SA and BS structures) isomers are obtained as global minimum for the M4(Pyr)2 (M = Ni, Pd, and Pt) models, which are the most rich complexes of the studied series. As known, the additional electrons should occupy higher energy MOs, particularly those of antibonding character; this would lead to M-M or M-L bonding ruptures for Ni, Pd, and Pt complexes. Indeed, it is what has happened in these cases resulting in coordination lowering. For the Ni4(Pyr)2 D 2h structure (Fig. 3 and Table 4), the nickel atoms are identical two-two, where Ni(1) and Ni(3) separated atoms are in an η3-allylic coordinated to both symmetrical (Pyr)2− dianionic ligands conducting to two Ni(II) d8 metal centers attaining the 16-MVE configuration as observed in previous works [61, 62], while the two remaining nickel atoms exhibit only η2 coordination mode giving rise to deficient Ni(0) d10 metal centers with 14-MVE configuration. A small, but a significant HOMO-LUMO gap of 0.65 (BP86) or 1.05 eV (mPW1PW91) is calculated for the global minimum Ni4(η3,η2,η3,η2-Pyr)2 singlet D 2h structure in which both pyrene ligands are symmetrical through the Ni4 pseudo-square plane. The calculated Ni-Ni distances of 3.852 (BP86) or 3.801 Å (mPW1PW91) are long; thus, they do not predict direct bonds, in accordance with neglected WBI values of 0.058 matching with formal Ni-Ni bond order of zero. The obtained triplet isomer is found to be less stable by 6.3 (BP86) or 5.8 kcal/mol (mPW1PW91) than the global minimum and keeps the same coordination modes with weak geometrical parameter modifications. The obtained singlet Ni4 chain structure is found only 4.6 (BP86) or 4.4 kcal/mol (mPW1PW91) above the global minimum of the monolayer form, in which the Ni1-Ni2-Ni3 and Ni2-Ni3-Ni4 angles of 165 and 162°, respectively, deviate slightly from the linearity (see Supplementary information), and the calculated Ni-Ni bond distances of 2.361, 2.350, and 2.330 Å are comparable to Ni3 [63], Ni5 [64], Ni4, and Ni7 [65] chain systems characterized previously by X-Ray diffraction in sandwich shape. However, its homolog of triplet spin lies much high in energy. As results, the spin state changes do not affect the different nickel sandwich structures with the same coordination modes and comparable bond distances.
Tetranickel chain sandwich complexes have been characterized and theoretically studied previously [66, 67]. Also, polypalladium sandwich are widely investigated, where tetrapalladium Pd4 chain is encapsulated between two monocyclic C9H9 and C8H8 ligands [13] or Pd4 pseudo-square plane is flanked by perylene (PAH) and monocyclic C9H9 ligand [14]. In this study, we were interested in determining the bonding and electronic structure of the hypothetical Pd4(Pyr)2 models (Fig. 4 and Table 5). However, to our knowledge, polyplatinium sandwich is not known.
The full optimization geometries show that the singlet structure is found as global minimum displaying a large HOMO-LUMO gap of 1.10 (BP86) or 1.95 eV (mPW1PW91) and more stable than the triplet structure by 17.0 (BP86) or 10.9 kcal/mol (mPW1PW91). The Pd(1) and Pd(3) are connected to both pyrene ligands in η3,η3 manner through an average Pd-C bond distance of 2.490 (BP86) or 2.458 Å (mPW1PW91) leading to Pd2+ cations. However, The Pd(2) and Pd(4) are bound to both pyrene ligands through an η2 coordination mode with short Pd-C bond distances which are comparable to previous theoretical work [68]. The lowering of the D 2h symmetry to the C s one gives two unsymmetrical pyrene ligands and stabilization of the latter structure by 5.9 (BP86) or 7.1 kcal/mol (mPW1PW91), but keeps the same coordination mode as that obtained for the D 2h one. The Pd4(η3,η2,η3,η2-Pyr)2 singlet C s structure features large HOMO-LUMO gap of 1.23 (BP86) or 2.05 eV (mPW1PW91) and short Pd(1)-C(2) and Pd(1)-C(2′) bond distances of 2.241 and 2.225 Å rather than 2.251 Å obtained for the D 2h structure (BP86) (Supplementary information). The long Pd···Pd distances of 4.192 Å indicate the absence of direct palladium-palladium bonds.
It was revealed that the geometry optimizations of Pd4 structures without symmetry constraints gave a pseudo-linear chain more stable than those of Pd4 sheet plane ones whatever the spin state (Fig. 4 and Table 5). Indeed, the linear chain structure of singlet state exhibits large HOMO-LUMO gap of 1.48 (BP86) or 2.20 eV (mPW1PW91) is found to be the global minimum lying 26.8 (BP86) or 19.3 kcal/mol (mPW1PW91) below the triplet structure of the same shape and 18.6 and 35.6 (BP86) or 13.2 and 24.1 kcal/mol (mPW1PW91) below the Pd4 monolayer plane of singlet and triplet structures, respectively. Each Pd atom is bound to each pyrene ligand through η2 coordination mode leading to short Pd-C bond distances ranging from 2.325 to 2.414 (BP86) or 2.291 to 2.379 Å (mPW1PW91); thus, the Pd4 chain is coordinated to eight carbon atoms array at the edge positions of the pyrene ligand showing a likeness with that encountered in perylene sandwich complexes [16] and in [Pd4(DPOT)2]2+ [67, 69]. The stabilization of the Pd4 chain is due essentially to Pd-Pd interactions with 2.742, 2.659, and 2.787 (BP86) or 2.709, 2.612, and 2.748 Å (mPW1PW91) bond distances occurred for this type of structure despite its electronic deficiency. The obtained Pd-Pd bond distances, the WBIs values of 0.101, 0.114, and 0.109 (BP86), and the orbital localisation with Pd-Pd σ-bonding are consistent with a single bond (scheme S4) giving rise to a 14-MVE configuration for Pd(1) and Pd(4) as cationic Pd(I) centers and a 16-MVE configuration for Pd(2) and Pd(3) as neutral Pd(0) metals.
This situation gives unsymmetrical pyrene ligands with one dianionic (Pyr)2− and the another neural (Pyr) with respect to interaction between Pd4 2+ and (Pyr)2 2−. The Pd(1)-Pd(2)-Pd(3) and Pd(2)-Pd(3)-Pd(4) angles of 138° and 144° deviate considerably from the linearity corresponding to a Pd4 zigzag chain, in agreement with the Pd atoms coordinated to the external carbon edges of the pyrene ligands.
Different tendencies are observed for Pt4 structures, where the Pt4 chain shape and the Pt4 sheet are isoenergetic for the same spin state with an energy splitting does not exceed 1.3 (BP86) or 2.0 kcal/mol (mPW1PW91) (Fig. 4 and Table 5), insignificant value at the considered level of theory.
For the Pt4(Pyr)2 chain isomer which exhibits large HOMO-LUMO gap of 1.56 (BP86) or 2.30 eV (mPW1PW91), the Pt-Pt-Pt angles of 101 and 150° are different to those obtained for Pd4 of 144 and 138° (BP86) (Supplementary information) and the Pt(1)-Pt(2), Pt(2)-Pt(3), and Pt(3)-Pt(4) bond distances are of 2.921, 2.832, and 2.750 (BP86) or 2.880, 2.797, and 2.711 Å (mPW1PW91).
It is important to notice that for the triplet Ni, Pd, and Pt structures, the two unpaired electrons are not localized exclusively on the metal centers, but also on the pyrene ligands inversely to that obtained for the earlier Ti, V, Cr, Mn, Fe, and Cr metals. Indeed, we show in this study the comparable behavior of the Pd4 pseudo-square sheet than that encountered for the Ni4 one. Interestingly, for the Ni, Pd, and Pt elements, the HOMO-LUMO gaps increase from Ni to Pt. The Pd-Pd distances of 4.390 Å are out of the range for bond interactions. Noteworthy, the nickel, palladium, and platinum complexes display the same coordination mode and similar electronic structures. It was deemed important to put emphasis on the spin density calculations to verify that the ground states of the palladium sandwiches were not magnetic. The BS method gives unreliable Pd and Pt structures due to high spin contamination, confirming the suffering of this method from a spin contamination of higher spin states as encountered in polynuclear metal complexes [41].
It is worth noting that the high spin (S = 2 and S = 3) of Pd and Pt structures lie very high in energy than those of low-spin (S = 0). For example, the Pd and Pt quintet structures are found 81.4 and 64.8 kcal/mol (BP86) above the singlet ones (see Supplementary information). It is worth mentioning that the singlet S = 0 for Pd and Pt complexes are more stable than those of high spin state in accordance with the large HOMO-LUMO gaps, in accordance with the considerable M...M distance shortening for the D 2h structures and the M-M bond elongations from 2.742, 2.659, and 2.787 to 2.951, 2.835, and 2.872, respectively, for the zig-zag chain Pd structure. Furthermore, the passage from the low-spin to high-spin is accompanied with an increase of the metal charges as given in Table 5. Conversely to the Ni structures, the change of the spin state does not lead to important modifications as highlighted by the geometrical parameters and natural metal charges (Table 4).
Conclusion
This paper reports the results of the electronic and the molecular structure of M4(Pyr)2 sandwich compounds for the first row transition metal besides Pd and Pt atoms by means of BP86 and mPW1PW91 methods. The optimized structures obtained by both BP86 and mPW1PW91 methods are consistent to each other and with the same stability orders between isomers. The M-M and M-C bond distances obtained by BP86 are somewhat slight short than those obtained by mPW1PW91. The closed-shell structures obtained by BP86 exhibit small HOMO-LUMO gaps compared to those provided by mPW1PW91. The reported results point out the richness of the coordination of the pyrene ligand and its flexibility behaving as neutral, dianionic, or tetraanionic ligand with respect to the electronic demand of the metals following the nature of the metal and the spin state. Thus, depending on the electron count and the nature of the metal, η2-η2, η2-η4, η3-η3, η4-η4, η4-η6, and η6-η6 coordination modes can be adopted by the pyrene ligand. The electron count increasing leads to progressive partial decoordination from Ti to Ni, Pd and Pt. Various closed-shell and open-shell electronic configurations from 14 to 18 metal valence electrons are established for the studied transition metal complexes. The discussion has assigned formal M-M bond orders based on the bond distances and MO localizations. In order to provide additional insight regarding the metal-metal bonding in these species, the Wiberg bond indices were determined by the BP86 method using natural bond orbital analysis. This is consistent with the presence of double, single, or the absence of M-M bonding, in agreement with the predicted formal metal-metal bond orders, which diminish from Ti to Ni, Pd and Pt for the square-planar structures. For the palladium structures, it is interesting to note the preference of the chain shape than the monolayer sheet, while these both forms are isoenergetic for Ni and Pt structures. The Ti, V, Cr, Mn, Fe, and Co metals prefer to form pseudo-square plane than the chain arrangement which is not identified as energy minimum. It is worth noting that all singlet structures exhibit small HOM-LUMO gaps except those of the d10 metals consistent with a diamagnetic behavior; however, the Mn, Fe, and Co complexes tend to adopt an open-shell configuration, thus predicting a paramagnetic behavior. The reliable and unreliable structures are distinguishable by spin contamination values, where broken symmetry method shows its suffering in certain cases of high spin states. The obtained results should stimulate further experimental investigations of the pyrene ligand π-bonded polymetallic complexes; thereby, this work has opened a route to a new class of extended sandwich structures of various transition metals and should be helpful for their design.
Acknowldgements
The authors are grateful to the Algerian MESRS (Ministère de l’Enseignement Supérieur et de la Recherche Scientifique) and DGRSDT (Direction Générale de la Recherche Scientifique et du Développement Technologique) for the financial support.
References
Debad JD, Morris JC, Lynch V, Magnus P, Bard AJ (1996) J Am Chem Soc 118:2374
Harvey RG (1997) Polycyclic aromatic hydrocarbons. Wiley VCH, New York
Ohashi K, Kubo T, Masui T, Yamamoto K, Nakasuji K, Takui T, Kai Y, Murata I J Am Chem Soc
Shibasaki T, Komine N, Hirano M, Komiya S (2006) Organometallics 25:523
Bendjaballah S, Kahlal S, Costuas K, Be Villon E, Saillard J-Y (2006) Chem-Eur J 12:2048
Korichi H, Zouchoune F, Zendaoui S-M, Zouchoune B, Saillard J-Y (2010) Organometallics 29:1693
Farah S, Ababsa S, Benhamada N, Zouchoune B (2010) Polyhedron 29:2722
Bouchakri N, Benmachiche A, Zouchoune B (2011) Polyhedron 30:2644
Benmachiche A, Zendaoui SM, Bouaoud SE, Zouchoune B (2012) Electronic structure and coordination chemistry of phenanthridine ligand in first-row transition metal complexes: A DFT study. Int J Quant Chem 113(7):985-996
Chekkal F, Zendaoui SM, Zouchoune B, Saillard J-Y (2013) New J Chem 37:2293
Merzoug M, Zouchoune B (2014) J Organomet Chem 770:69
Zendaoui SM, Zouchoune B (2013) Polyhedron 51:123
Murahashi T, Inoue R, Isui K, Ogoshi S (2009) J Am Chem Soc 131:9888
T. Murahashi, N. Kato, T. Uemura and H. Kurosawa, Angew. Chem.Int, Ed., 46, 3509 (2007).
Tatsumi T, Shirato K, Murahashi T, Ogoshi S, Kurosawa H (2006) Angew Chem 118:5931
Murahashi T, Uemura T, Kurosawa H (2003) J Am Chem Soc 125:8436
Ceccon A, Santi S, Orian L, Bisello A (2004) Coord Chem Rev 248:683
Manriquez JM, Ward MD, Reiff WM, Calabrese JC, Jones NL, Carroll PJ, Bunel EE, Miller JS (1995) J Am Chem Soc 117:6182
Esponda E, Adams C, Burgos F, Chavez I, Manriquez JM, Delpech F, Castel A, Gornitzka H, Rivière-Baudet M, Rivière P (2006) J Organomet Chem 691:3011
Noodleman L, Han W-G (2006) J Biol Inorg Chem 11:674
Cremer D (2001) Mol Phys 99:1899
Löwdin PO (1955) Phys Rev 97:1509
Lykos P, Pratt GW (1963) Rev Mod Phys 35:496
Yamaguchi K (1990) Self-consistent field: theory and applications. In: Carbo R, Klobukowski M (eds) . Elsevier, Amsterdam, p. 727
Yamaguchi K, Kawakami T, Takano Y, Kitagawa Y, Yamashita Y, Fujita H (2002) Int J Quantum Chem 90:370
Hehre WJ, Random L, von Schleyer PR, Pople JA (1986) Ab initio molecular orbital theory. New York, Wiley
Szabo A, Ostlund NS (1996) Modern quantum chemistry. New York, Dover Publications, Inc.
Murahashi T, Fujimoto M, Okao M, Hashimoto Y, Uemura T, Tatsumi Y, Nakao Y, Ikeda A, Sakaki S, Kurosawa H (2006) Science 313:1104
Zouchoune F, Zendaoui S-M, Bouchakri N, Djedouani A, Zouchoune B (2010) J Mol Struct 945:78
Farah S, Korichi H, Zendaoui SM, Saillard JY, Zouchoune B (2009) Inorg Chim Acta 362:3541
Peng A, Zhang X, Li QS, King RB, Scharfer III HF (2013) New J Chem 37:775
Wang H, King RB, Schaefer III HF (2008) Eur J Inorg:3698
Fan Q, Feng H, Sun W, Li H, Xie Y, King RB, Scharfer III HF (2013) New J Chem 37:1545
Peng A, Zhang X, Li QS, King RB, Schaefer III HF (2013) New J Chem 37(775)
ADF2014.01, Theoretical Chemistry, Vrije Universiteit: Amsterdam, The Netherlands, SCM
Baerends EJ, Ellis DE, Ros P (1973) Chem Phys 2:41
te Velde G, Baerends EJ (1992) J Comput Phys 99:84
Fonseca Guerra C, Snijders JG, te Velde G, Baerends EJ (1998) Theo Chim Acc 99:391
Bickelhaupt FM, Baerends EJ (2000) Rev Comput Chem 15:1
te Velde G, Bickelhaupt FM, Fonseca Guerra C, van Gisbergen SJA, Baerends EJ, Snijders JG, Ziegler T (2001) J Comput Chem 22:931
Vosko SD, Wilk L, Nusair M (1990) Can J Chem 58:1200
Becke AD (1986) J Chem Phys 84:4524
Becke AD (1988) Phys Rev A 38:3098
Perdew JP (1986) Phys Rev B 33:8822
Perdew JP (1986) Phys Rev B 34:7406
Adamo C, Barone V (1998) J Chem Phys 108:664
van Lenthe E, Ehlers AW, Bearends EJ (1999) J Chem Phys 110:8943
Versluis L, Ziegler T (1988) J Chem Phys 88:322
Fan L, Ziegler T (1992) J Chem Phys 96:9005
Fan L, Ziegler T (1992) J Phys Chem 96:6937
P. Flükiger, H. P. Lüthi, S. Portmann, J. Weber, MOLEKEL, Version 4.3.win32 Swiss Center for Scientific Computing (CSCS), Switzerland, 2000–2001. http://www.cscs.ch/molekel/.
Weinhold F, Landis CR (2005) Valency and bonding: a natural bond order donor–acceptor perspective. Cambridge University Press, U. K.
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F (2001) Natural Bond Orbitals “Analysis Programs” Theoretical Chemistry Institute. University of Wisconsin, Madison, WI
B. Eliasson, T. Lejon, U. Edlund (1984) J Chem Soc Chem Commun 591
Miillen K (1978) Helv Chim Acta 61:2307
Dahl LF, Rundle RE (1963) Acta Cryst 16:419
Wang H, Sun Z, Xie Y, King RB, Schaefer III HF (2011) Inorg Chem 50:9256
Kitagawa Y, Saito T, Ito M, Shoji M, Koizumi K, Yamanaka S, Kawakami T, Okumura M, Yamaguchi K (2007) Chem Phys Lett 442:445
Kitagawa Y, Saito T, Nakanishi Y, Kataoka Y, Matsui T, Kawakami T, Okumura M, Yamaguchi K (2009) J Phys Chem A 113:15041
Minsky A, Klein J, Rabinovitz M (1981) J Am Chem Soc 103:4586
Farah S, Bouchakri N, Zendaoui SM, Saillard JY, Zouchoune B (2010) J Mol Struct 953:143
A. Saiad, B. Zouchoune. Can. J. Chem., 93, 1096 (2015).
S. Aduldecha, B. Hathaway (1991) J Chem Soc Dalton Trans 993
S.-J. Shieh, C.-C. Chou, G.-H. Lee, C.-C. Wang, S.-M. Peng (1997) Angew Chem 109: 57; Angew. Chem. Int. Ed. Engl 36:56.
Peng S-M, Wang C-C, Jang Y-L, Chen Y-H, Li F-Y, Mou C-Y, Leung M-K (2000) J Magn Magn Mater 209:80
López X, Huang M-Y, Huang GC, Peng SM, Li FY, Bénard M, Rohmer M-M (2006) Inorg Chem 45:9075
Bera JK, Dunbar KR (2002) Angew Chem Int Ed 41:4453
Philpott MR, Kawazoe Y (2007) Chem Phys 337:55
Murahashi T, Mochizuki E, Kai Y, Kurosawa H (1999) J Am Chem Soc 121:10 660
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
Bond distances (Å), bond angles (°), and the total bonding energies (eV) for the optimized geometries of the computed compounds in their different spin states and various symmetries are given in Tables S1–S6. BP86-optimized structures are given in Figs. S1–S3, values and plots of spin densities are given in Schemes S1–S3, and MO diagram of Pd4(Pyr)2 is given in Scheme S4. (DOCX 2595 kb)
Rights and permissions
About this article
Cite this article
Fadli, S., Zouchoune, B. Coordination chemistry and bonding analysis of tetranuclear transition metal pyrene sandwich complexes. Struct Chem 28, 985–997 (2017). https://doi.org/10.1007/s11224-016-0905-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-016-0905-8