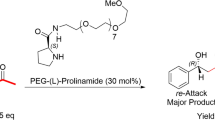
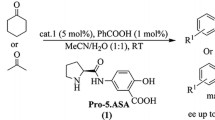
Abstract
We examined the activity of a series of L-hydroxyproline derivatives in enantioselective α-amination reaction between diethyl azodicarboxylate and propanal both in organic and aqueous media. In organic media most of the catalysts showed high activity and enantioselectivities comparable to that accessible with L-proline that is among the best catalysts in the reaction. The catalysts showed good activity under aqueous conditions as well; however, only low enantioselectivities were obtained in this case, primarily due to the racemisation of the product under the reaction conditions. Thus, the attempted achiral acid/base additive-driven stereocontrol was not feasible on a practical level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among L-proline-catalysed stereoselective reactions, α-amination (Fig. 1) has emerged as an important C–N bond formation reaction leading to valuable optically active amino acid and amino alcohol-type building blocks [1–4]. It was also among the first organocatalytic reactions where dual stereocontrol [5, 6] was systematically studied [7–15]. Initially, Jørgensen and co-workers observed that using α,α-diarylprolinol silyl ether-type catalysts, an inversion of the product enantioselectivity occurs compared to that obtained with L-proline catalysis [7]. Later, the groups of Blackmond and Armstrong reported stereocontrol in the reaction using L-proline in combination with an appropriate achiral base additive [9]. In the inversion cases the lack of the acidic proton in the catalyst was rationalized as a key factor for the altered stereochemistry (Fig. 2). In the case of Jørgensen’s catalyst the steric shielding by the bulky substituent on the pyrrolidine ring was responsible for promoting the si-face attack while in the L-Pro/DBU catalytic system of Blackmond and Armstrong the same shielding effect was provided by the carboxylate group of L-Pro originating from the deprotonation of the carboxylic acid by DBU.
Stereoselectivity in asymmetric α-amination reactions. In proline catalysis the reaction is directed to the (R)-product via H bonding with the carboxylic acid moiety of the catalyst (complex A). When no acidic proton is available in the catalyst, the (S)-product forms in excess directed by steric shielding (complexes B and C)
The concept of controlling enantioselectivity through controlling L-proline protonation state was extended to the field of aldol reactions as well, where several authors reported inversion of enantioselectivity in proline-based systems where deprotonation of the carboxylic acid moiety could occur [16–19].
Recently, we have shown that in aldol reactions of water-miscible acetone with aromatic aldehydes catalysed by hydroxyproline derivatives, product enantioselectivity could be controlled in the presence of a large amount of water, as an environmentally benign reaction media [20, 21]. The reactions occurred in a biphasic micellar system which is generated due to the presence of salting-out additives. The catalyst had the role of stabilizing the micellar system on the one hand and to catalyse the reaction on the other hand in which the protonation state of the amino acid catalyst was controlled by the nature of the salting-out additives. Addition of NH4Cl rendered the reaction medium slightly acidic, thus “classical” proline catalysis could occur within the acetoneous micelles leading to the (R)-product. On the other hand, in the presence of NaOAc the aqueous medium was basic where the enamine carboxylate played a key role in delivering the (S)-enantiomer in excess.
Based on our findings on the importance of catalyst structure in performing aldol reactions in aqueous environment, we wanted to investigate whether our observations could be extended to amination reactions as well. Furthermore, performing α-amination reactions in the presence of water is scarcely reported in the literature [22, 23], in contrast to recent efforts towards environmentally benign asymmetric synthesis [24, 25].
Materials and methods
Experimental methods
Commercial reagents (Aldrich, Alfa Aesar, Fluka) were purchased as reagent-grade and used without further purification. Solvents for extraction or column chromatography were of technical quality. Organic solutions were concentrated by rotary evaporation at 25–40 °C. Thin layer chromatography was carried out on SiO2-layered aluminium plates (60778-25EA, Fluka). Flash column chromatography was performed using SiO2–60 (230–400 mesh ASTM, 0.040–0.063 mm from Merck) at 25 °C. 1H NMR spectra were recorded on a Bruker AVANCE DRX 400 spectrometer. All spectra were recorded at 25 °C. The residual solvent peaks were used as the internal reference (CDCl3: δ H = 7.26 ppm).1H NMR spectra are reported as follows: chemical shift δ in parts per million relative to TMS (δ = 0 ppm), multiplicity, coupling constant (J in Hz), number of protons. The resonance multiplicity is described as s (singlet), d (doublet), t (triplet), q (quartet), combinations thereof, or m (multiplet). Broad signals are described with br. (broad). Quantitative analysis was carried out using gas chromatograph (GC) with a flame ionization detector (FID): Agilent Techn. 6890 N or YL6100 GC-FID and Cyclosil-B 30 m × 0.25 mm i.d. or HP CHIRAL 30 m × 0.25 mm i.d. chiral capillary column. Mass spectra (MS) were recorded on a Agilent 5973 GC-MSD.
Typical experimental procedure for the aqueous α-amination reactions
Basic conditions
Sodium acetate (500 mg, 6.1 mmol) was dissolved in water (2 mL), then propionaldehyde (0.107 mL, 1.5 mmol), trans-4-hydroxy-l-proline (19.7 mg, 0.15 mmol) and diethyl azodicarboxylate (DEAD) (0.078 mL, 0.5 mmol) was added. The reaction mixture was stirred at 0 °C for 2 h then sat. NaClaq solution was added. The aqueous mixture was extracted with EtOAc and dried (MgSO4), then analysed by GC-FID (Table 1) and 1H NMR. 1H NMR (400 MHz, CDCl3) δ H = 1.27 (t, J = 7.1 Hz, 6 h), 1.38 (d, J = 7.3 Hz, 3 h), 4.12–4.28 (m, 4 h), 4.67 (br. s, 1 h), 7.10 (br. s, 1 h), and 9.66 (s, 1 h) ppm.
Acidic conditions
Ammonium chloride (100 mg, 1.9 mmol) was dissolved in water (1.7 mL), then propionaldehyde (0.107 mL, 1.5 mmol), trans-4-hydroxy-l-proline (19.7 mg, 0.15 mmol) and DEAD (0.078 mL, 0.5 mmol) was added. The reaction mixture was stirred at 0 °C for 2 h then sat. NaCl solution was added. The aqueous mixture was extracted with EtOAc and dried (MgSO4), then analysed by GC-FID (Table 1).
Typical experimental procedure for the α-amination reactions in DCM
Propionaldehyde (0.107 mL, 1.5 mmol) was dissolved in dichloromethane (2.5 mL), then O-(4-tert-butylbenzoyl)-trans-4-hydroxy-l-proline (29.1 mg 0.1 mmol) and DEAD (0.078 mL, 0.5 mmol) was added. The reaction mixture was stirred at 0 °C for 2 h then sat. NaCl solution was added. The aqueous mixture was extracted with EtOAc and dried (MgSO4), then analysed by GC-FID (Table 1).
Typical experimental procedure for the preparation of cyclic N-amino oxazolidinone derivative 4
L-proline (34.5 mg, 0.3 mmol) was suspended in DCM (4 mL) and cooled to 0 °C, then propanal (0.214 ml, 3 mmol) and DEAD (0.156 mL, 1 mmol) was added. The reaction mixture was stirred at 0 °C for 2 h followed by the addition of abs. ethanol (4 mL) and NaBH4 (100 mg, 2.64 mmol). The mixture was stirred at 0 °C for 40 min, then 1 M NaOH (10 mL) was added, and the stirring was continued for 2 h. The organic solvent was evaporated and the mixture was extracted with Et2O (2 × 10 mL) and dried (Na2SO4). The crude mixture was purified by flash column chromatography (SiO2, hexanes/EtOAc 1:1) to afford compound (R)-3-ethoxycarbonylamino-4-methyl-2-oxazolidinone (4) as a colourless oil (62 mg, 33 %). GC-MSD m/z (%) = 188 (1, [M]+), 143 (16), 116 (70), 100 (29), 57 (49), and 29 (100).
Computational methods
Initial three-dimensional structures of C1 and C7 were built up by means of MOE program [26]. In order to identify their global minimum (GM) structure, the sampling of the conformation space was carried out with a stochastic conformational search protocol, using the MMFF94x force field [27] and an improved (GB/VI) Generalized Born (GB) implicit solvent [28] for surrounding media. The static dielectric constant was set to 78.36 and 8.93 for water and DCM, respectively. In this protocol, 10,000 initial structures were generated for each molecule and optimized. After optimization, the new conformer is judged to be equal with one of the previously obtained individual conformers if the optimal heavy atom root mean square superposition distance is less than the tolerance limit (0.25 Å). After failure of the consecutive 1000 trials for new conformer, the procedure is terminated. These conformational searches were conducted using MOE program [26]. In order to obtain molecular properties for the global minimum structure, each force field GM geometries were re-optimized by density functional B3LYP/6-31G(d) level of theory [29, 30] applying a tight convergence criterion as implemented in Gaussian09 program package [31]. Here, the integral equation formalism of the polarizable continuum model (PCM) with the radii and non-electrostatic terms of Truhlar and co-workers (SMD) [32] was used to mimic the surrounding environment. The normal mode analysis was carried out on structures optimized at the B3LYP/6-31G(d) level of theory to ensure the structure corresponds to energy minimum.
Results and discussion
The 4-hyroxyproline-derived catalysts that were used in this study are shown in Fig. 3 and were synthesized according to a previously described procedure [33].
As a first step we evaluated the performance of our catalysts compared to L-proline that is still among the best catalysts for asymmetric α-aminations, by performing the reaction under conditions that are previously optimized by other authors [1–4]. In CH2Cl2 at 0 °C with one exception, all of the acyloxyprolines delivered comparable or even higher enantioselectivities than that obtained with L-proline. In terms of conversions only catalyst C5 performed weaker than L-Pro, likely due to solubility reasons. It has to be noted that we found this catalyst inefficient in asymmetric aldol reactions as well both under aqueous conditions and in acetone as solvent [21]. In line with previous reports, after prolonged reaction times a drop in enantioselectivity was observed, as a result of product racemisation (Table 2, entry 9 vs 10) [2, 34]. Racemisation occurred not only under the reaction conditions but also in the isolated sample of product; however, it took longer time (about 1 week for an ee decrease from 94 % (R) to 14 % (R)). To prevent racemisation of the stereochemically sensitive amination product, we converted compound 3 to its cyclic N-amino oxazolidinone derivative 4 (Fig. 4). This compound was isolated showing the same ee values as measured for compound 3 if the reduction step was performed at sub-zero temperatures. Performing the reduction step at ambient conditions resulted in the decrease of ee in the cyclic product. Furthermore, in the amination reaction we also observed slight dependence of the enantioselectivity on the catalyst loading; higher amount giving somewhat higher values without changes in conversion and selectivity (Table 2, entry 10 vs. 11). Using D-proline instead of L-proline the product with opposite enantioselectivity was detected. Interestingly, L-hydroxyproline was completely inactive in the reaction under these conditions as no product could be detected in this case (Table 2, entry 8). In fact, there are only two examples in the literature where hydroxyproline was used as catalyst—in ionic liquids and under microwave irradiation—in α-amination reactions, in both cases giving low or moderate yields and moderate enantioselectivities [35, 36].
After establishing the structure-activity relationship within the catalyst series in organic media, we turned our attention to basic aqueous environment (NaOAc additive) to test whether the sense of enantioselectivitiy could be altered from (R) to (S) similarly to aldol systems. From the results (Table 3) it is clear that the protonation state of the catalyst is influencing the stereochemistry as in the presence of aqueous NaOAc solution the (S) amination product formed in excess in several cases (Table 3, entries 5–10), however, with poor enantioslectivities. Temperature had an effect on both the ee and selectivity values. Using catalyst C1 at 25 °C moderate selectivity was obtained and low ee (Table 3, entry 1) without inversion compared to the value in organic media. The addition of poly(propylene glycol) (PPG-425) under the same conditions helped to improve the selectivity, while the ee remained low but inverted (Table 3, entry 2). The effect of PPG-425 in improving selectivity is in line with our earlier findings in aldol reactions [20]. The selectivity value obtained at 0 °C (without PPG-425 additive) exceeded the room temperature value (Table 3, entry 3) while the ee remained the same as obtained with PPG additive. In most of the cases conversion of the starting material (DEAD) was close to completion as indicated by the disappearance of its characteristic orange colour. In the case of lower selectivities, we cannot exclude that the competitive hydroacylation reaction route was also operating between propanal and DEAD reactants [37]. This is also supported by the observation that some conversion was obtained when no amine catalyst was used in the NaOAc/H2O system; however, the formation of the desired product was not detected.
Looking at the results in comparison to that obtained in the aldol reactions between acetone and 2-nitrobenzaldehyde (Table 3), it can be noticed that the conversions in the amination reaction were higher in every case compared to the aldol reaction performed with the same catalysts. Regarding the ees the results show that catalysts C1 and C2 (Table 3, entries 3 and 4) that performed better in aldol reactions delivered poorer values in the amination reaction, while with catalysts C4–C9 that were weaker asymmetric catalysts in aldol reactions, comparably higher ees were obtained in the amination reactions.
These findings could be explained considering that in aqueous aldol reactions only acetone, a water miscible ketone was a liquid, while the aromatic aldehydes were all solid materials [20, 21]. As a first step of the aqueous aldol reaction, acetoneous micelles had to be formed due to the presence of salting-out additives and the stabilization effect of the hydroxyproline-based catalysts in which the solid aldehydes could dissolve. In this way both the catalyst and the two reactants were present in the confined space of the micelle. Different aggregation properties of catalysts bearing different acyl substituents might also play a role (Fig. 5). In case of the catalyst was not efficient in stabilizing the two-phase system, no reaction occurred (as in the case of C5 or C7, Table 3 entries 7 and 9). Contrary to the aldol system, under amination conditions both the propanal and the DEAD reagents are liquids (with limited solubility in water) providing a larger organic volume to dissolve the catalyst; hence, micelle formation may not be prerequisite for all components to be in proximity for reaction. In fact, the better solubility of L-Pro C8 and L-hydroxyproline C9 in water compared to the organic phase explains the low conversions obtained by these catalyst under aqueous conditions.
We also performed the amination reaction under acidic aqueous conditions using NH4Cl as additive to show that both enantiomers of the product can be accessed in water as reaction medium using a single chiral catalyst. The reaction was carried out using L-hydroxyproline C8 as we obtained high selectivity and comparably higher ees with this catalyst under basic (NaOAc additive) conditions (Table 3, entry 10). Although good enantioselectivity (83 % (R)) was obtained with inversion of ee compared to the basic conditions, the selectivity was low with pronounced amount of diverse side-product formation. It is noted, that a previous attempt on developing asymmetric α-amination reactions in the presence of water and Brønsted acids as additives resulted in low to moderate yields and enantioselectivities after long reaction times [23].
As the product aldehyde 3 is prone to racemisation in the presence of a base, we performed control experiments to find out the effect of the basic aqueous conditions on the obtained low enantioselectivities (Fig. 6). We performed the amination reaction in DCM under L-Pro catalysis and submitted the enantioenriched (ee 85 % R) product to basic aqueous conditions. Upon stirring compound 3 at 0 °C for 2 h the ee was found to decrease to 60 % (R). The same control was performed using aqueous NH4Cl solution, which did not cause any decrease in the enantioselectivity value.
From the results it is clear that the basicity of the NaOAc is responsible for the low enantioselectivities obtained under aqueous basic conditions. Although the described methodology could potentially be used to favour the amination reaction over the hydroacylation reaction that has been described to operate under aqueous conditions [37], the observed racemisation diminishing its usefulness in aqueous asymmetric synthesis of products of type 3.
In summary we have showed that α-amination reactions can be performed under both organic and aqueous conditions using L-hydroxyproline-based amine catalysts. Attempts to control the enantioselectivity of the product by controlling the acid/base properties of the aqueous media, however, turned out to be unfeasible on a practical level as poor ee values or racemic mixtures were obtained under basic conditions due to the racemisation of the product.
References
List B (2002) J Am Chem Soc 124:5656–5657
Bøgevig A, Juhl K, Kumaragurubaran N, Zhuang W, Jørgensen KA (2002) Angew Chem Int Ed 41:1790–1793
Kumaragurubaran N, Juhl K, Zhuang W, Bøgevig A, Jørgensen KA (2002) J Am Chem Soc 124:6254–6255
Duthaler RO (2003) Angew Chem Int Ed 42:975–978
Bartók M (2010) Chem Rev 110:1663–1705
Escorihuela J, Burguete MI, Luis SV (2013) Chem Soc Rev 42:5595–5617
Franzén J, Marigo M, Fielenbach D, Wabnitz TC (2005) Kjærsgaard, Jørgensen KA. J Am Chem Soc 127:18296–18304
Dinér P, Kjærsgaard A, Lie MA, Jørgensen KA (2008) Chem Eur J 14:122–127
Blackmond DG, Moran A, Hughes M, Armstrong A (2010) J Am Chem Soc 132:7598–7599
Kanzian T, Lakhdar S, Mayr H (2010) Angew Chem Int Ed 49:9526–9529
Hein JE, Armstrong A, Blackmond DG (2011) Org Lett 13:4300–4303
Hein JE, Burés J, Y-H L, Hughes M, Houk KN, Armstrong A, Blackmond DG (2011) Org Lett 13:5644–5647
Sharma AK, Sunoj RB (2011) Chem Commun 47:5759–5761
Schmid MB, Zeitler K, Gschwind RM (2012) Chem Eur J 18:3362–3370
Fu A, Tian C, Li H, Li P, Chu T, Wang Z, Liu J (2015) Chem Phys 455:65–72
Darbre T, Machuqueiro M (2003) Chem Commun 1090–1091
Zhong L, Xiao J, Li C (2006) J Catal 243:442–445
Breslow R, Ramalingam V, Appayee C (2013) Orig Life Evol Biosph 43:323–329
Szőllősi G, Fekete M, Gurka AA, Bartók M (2014) Catal Lett 144:478–486
Gurka AA, Szőri K, Szőllősi G, Bartók M, London G (2015) Tetrahedron Lett 56:7201–7205
Gurka AA, Szőri K, Bartók M, London G (2016) Tetrahedron Asymm 27:936–942
Hayashi Y, Aratake S, Imai Y, Hibino K, Chen Q-Y, Yamaguchi J, Uchimaru T (2008) Chem Asian J 3:225–232
Msutu A, Hunter R (2014) Tetrahedron Lett 55:2295–2298
Mlynarski J, Baś S (2014) Chem Soc Rev 43:577–587
Bartók M, Dombi G (2014) Curr Green Chem 1:191–201
Chemical Computing Group Inc. (2004) Molecular Operating Environment (MOE). Sci. Comput. Instrum. Chemical Computing Group Inc., 1010 Sherbooke St. West, Suite #910, Montreal.
Halgren TA (2000) J Comput Chem 17:520–552
Labute P (2008) J Comput Chem 29:1693–1698
Devlin FJ, Finley JW, Stephens PJ, Frisch MJ (1995) J Phys Chem 99:16883–16902
Becke A, Becke A (1993) J Chem Phys 98:5648–5652
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Baron V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd J, Brothers EN, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian09, Revision C.01. Wallinford, CT.
Marenich AV, Cramer CJ, Truhlar DG (2009) J Phys Chem B 113:6378–6396
For the synthesis of the catalysts, see refs 20 and 21.
Ötvös SB, Szloszar A, Mandity IM, Fülöp F (2015) Adv Synth Catal 357:3671–3680
Kotrusz P, Alemayehu S, Toma Š, Schmalz H-G, Adler, A (2005) Eur J Org Chem 4904–4911
Baumann T, Bächle M, Hartmann C, Bräse S (2008) Eur J Org Chem 2207–2212
Zhang Q, Parker E, Headley AD, Ni B (2010) Synlett 2453–2456
Acknowledgments
Financial support from the National Research, Development and Innovation Office, Hungary (OTKA Grants K 109278 and PD 115436), is gratefully acknowledged. Z. Szécsényi (Institute of Pharmaceutical Chemistry, University of Szeged) is acknowledged for technical support. M. S. was supported by a Magyary Zoltán fellowship within the framework of TÁMOP 4.2.4. A/2-11-1-2012-0001 (A2-MZPD-12-0139) and he is currently János Bolyai Research Scholar of the Hungarian Academy of Sciences (BO/00113/15/7). G. L. acknowledges the János Bolyai Research Scholarship from the Hungarian Academy of Sciences. We thank the anonymous reviewers for their insightful comments, which improved the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gurka, A.A., Szőri, K., Szőri, M. et al. Application of hydroxyproline derivatives in enantioselective α-amination reactions in organic and aqueous environments: a structure-activity relationship study. Struct Chem 28, 415–421 (2017). https://doi.org/10.1007/s11224-016-0873-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-016-0873-z