Abstract
A novel and simple organocatalyst derived from proline was designed, synthesized and tested for its catalytic potential in a direct asymmetric aldol reaction between 4-nitrobenzaldehyde and cychlohexanone in aqueous media. The relatively inexpensive and safe solvent, water stabilized the transition state, and increased reactivity and selectivity of catalyst. The diastereo- and enantio-selective products of the reaction were obtained in excellent yields.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Direct asymmetric aldol reactions are one of the most important reactions for C–C bond formation and have been widely used in the construction of natural and synthetic products [1, 2]. For the past three decades, biological catalysts, organometallic catalysts, and organocatalysts have been employed to catalyze these types of reactions [3–7]. Recently however, organocatalysts, in particular, have experienced explosive growth in their applications and extensive probes have been conducted to improve the efficiency and stereoselectivity of the organocatalysts ever since [8–17]. Inspired by nature’s aldolase enzymes—type I and type II- proline and its derivatives have been frequently reported as organocatalysts for a wide range of organic reactions such as Robinson annulations, Munnich reactions, and Michael additions [18–23]. A substantial part of this new work is founded in the enamine and iminium ion based catalysis. One of the drawbacks associated with proline catalysis is its poor solubility in common organic solvents. Most of the reactions catalyzed by proline or proline–embedded molecules are performed in polar solvents such as DMF and DMSO, which cause problematic issues such as low enantioselectivity. Previous studies have shown that a small amount of water can accelerate the reaction, while a large excess of water is detrimental to the reaction [24–29]. Catalytic asymmetric reactions that can be performed in water are of current interest, because water is a desirable solvent with respect to environmental concerns, safety and cost. However, although the use of water as a reaction solvent accelerates the yields and diastereoselectivity, it also often alters the catalyst’s activity by interrupting ionic interactions and hydrogen bonds which are critical for stabilizing the transition states of the reactions. Therefore, a special design was required for performing asymmetric reactions in water [23–34].
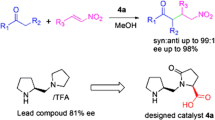
We envisage that the catalyst 1 which bears amide linkages and two hydrogen donating bonding groups –COOH and OH– would catalyze the aldol reaction via hydrogen bonding. Hydrogen bonding is expected to occur between the amide, COOH, and OH functionalities of the catalyst with the substrate remaining on one side, and nucleophilic attacks occur at the opposite side to obtain the highly favorable diastereo—and enantioselective formation of products [35–39]. Hence, in this work, we report herein, the design of a simple organocatalyst that is mediated by proline and is easy to synthesis via some short steps (Scheme 1).
2 Experimental
2.1 General
All chemicals were purchased and used without further purification. Analytical thin layer chromatography (TLC) was performed using Merck 60 F254 precoated silica gel plate (0.2 mm thickness). Flash chromatography was performed using Merck silica gel 60 (70–230 mesh). Fourier transforms infrared spectroscopy (FTIR); Perkin Elmer Spectrum 100 was used for identification of functional groups. NMR data were recorded on 500, 700 MHz for 1HNMR and 100, 127 MHz for 13C NMR (JEOL JNM ECA and Bruker) spectrometers. The relative and absolute configurations (dr) of the Aldol reactions were determined by comparison with 1H NMR spectroscopic analysis and chiral phase HPLC. Mass spectra (MS) were measured with a spectrometer (DIMS QP5050A SHIMADZU) and Agilent 1200 HPLC-Chip/MS Interface, coupled with Agilent 6520 Accurate-Mass Q-TOF LC/MS. Enantioselectivity were determined by HPLC (Waters 1525 Binary Pump and UV-Water 2489) analysis employing a Daicel ChiralCel OD-H, and ChiralPak AD-H columns (4.6 mm × 250 mm). ChemDraw Ultra 8. was used to draw chemical structures.
2.2 Synthesis of Catalyst l-Pro-5.ASA
N-Boc-L-Pro-OSu (2.1 g, 6.73 mmol) and 5-amino-2-hydroxybenzoic acid (2 g, 13.07 mmol) were dissolved in DMF (5 mL). By adding NMM (N-methylmorpholine) pH was adjusted at 6.0–6.5. The resulting mixture was stirred for overnight. The reaction progress was monitored by TLC. The reaction mixture was worked up with EtOAc and water (2 × 5 mL). Organic layer was then washed with saturated brine (10 mL). After that, organic layer was dried over anhydrouse Na2SO4 and evaporated by rotary under vacuum. The residue was oily form and was precipitated by cold dry Ether. The precipitate was then filtered off and washed by cold ether. (4.35 g, yield 95 %).
In the next step, the Boc-group was removed by using 10 % HCl/1,4 dioxane. The product which obtained in former step (4.35 g) was dissilved in AcOH (5 mL) and then 10 % HCl/1,4 dioxane (20 mL) was added to the solution mixture. Boc-group was removed after 1 h. The solvent was evaporated by rotary under vacuum. The oily product was precipitated by cold dry ether. The impure product was purified using preparative HPLC (xbridge RP-C18, 250 mm × 10 mm column). After purification the solvents were evaporated and at the end precipitated by dry ether, (2.26 g, yield 73 %).
3 Result and Discussion
As shown in Scheme 1, a novel, small organocatalyst of proline derivative 1 was prepared from commercially available l-proline and 5-aminosalicylic acid (5-ASA).
The catalytic activity of this new catalyst (5 mol%) was then evaluated in the direct asymmetric aldol reaction of 4-cholorobenzaldehyde (60 mg, 0.397 mmol) with cyclohexanone (77.93 mg, 0.794 mmol) in i-PrOH (1 mL). The pH was adjusted to between 5.0 and 5.5 by adding diluted N-methylmorpholine (NMM). Good yields (88 %), good diastereoselectivities (anti/syn, 91/9) and moderate enantioselectivities (70 % ee) were obtained (Table 1, entry 2). The ratio of catalyst was then optimized (Table 1, entries 1–3) and we found that the best ratio is 5 mol%. In addition, when the catalyst load was reduced from 5 to 2 %, reactivity decreased noticeably (Table 1, entry 1). As efficiency of organocatalysts in terms of reactivity and selectivity can be altered with different solvents [40–42], we probed the effect of different solvents on the aldol model reaction. The results are summarized in Table 1. Good yields and moderate stereoselectivities were obtained in polar solvents, both protic and aprotic, such as MeCN, DMF, NMP, CHCl3, i-PrOH and MeOH. However, more polar or aprotic solvents seemed to suppress the reactivity of the system (Table 1, entries 1–9). Interestingly, when water as a co-solvent was added to i-PrOH and MeCN, both yields and stereoselectivities were significantly increased (Table 1, entries 10, 11).
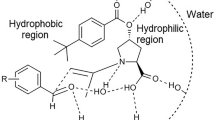
Water is able to stabilize the transition state by forming hydrogen bonds with the carboxylic, hydroxyl, and NH groups and inhibits the formation of intermolecular hydrogen bonds between the –OH and COOH groups.
The mechanism of organocatalysis with a pyrrolidine-based catalyst in the aldol reaction is well known to proceed via an enamine intermediate [43, 44]. Therefore, any factors, which could promote the enamine formation and hydrogen bonding between catalyst and substrate, will improve the reactivity and selectivity of the reaction. An appropriate acid could effectively enhance the activity of the catalysts [43]. The COOH group of 5-ASA has a pKa value of 3.0, and the –OH group 13.9. With this fact in mind, in order to find a proper acid additive to match our catalyst, we investigated the influence of acid additives on the reactivity (Table 2, entries 1–5). Boc-group was removed by using 10 % HCl/1,4-dioxane, thus, catalyst 1 is a hydrochloric salt of the catalyst and is strongly acidic. HCl. Pro-5.ASA (1) was observed in the aldol reaction of 4-cholorobenzaldehyde with cyclohexanone in H2O/MeCN over 24 h. Very poor yields and an almost racemic product were obtained (Table 2, entry 1). To further investigate, the HCl.Pro–5.ASA was desalted using an ion-exchange resin. The desalted catalyst was also tested in the model aldol reaction. Although, a significant enhancement in yields was observed, the selectivity of the catalyst remained poor (Table 2, entry 2). Adding benzoic acid and acetic acid gave similar good yields and enantioselectivities (Table 2, entries 3, 4). A stronger acid like TFA gave a moderate yield and good diastereo- and enantio-selectivity (Table 2, entry 5). Benzoic acid produced the optimal performance, where the yield and stereoselectivity were the most desirable.
With the optimal conditions in hand, we tested the efficacy of the catalyst using different aromatic aldehydes and the results are summarized in Table 3. Aromatic aldehydes with an electron-withdrawing group on the benzene ring gave high yields and enantioselectivities (up to 91) (Table 3, entries 1–9). However, benzaldehyde also produced the product in good yields and enantioselectivities (Table 3, entry 8). When an electron-donating group was on the aromatic ring, the products were obtained in poor yields and slightly lower enantioselectivity (Table 3, entry 10).

The reactions of an acyclic ketone (acetone) with aromatic aldehydes were also examined (Table 3, entries 11, 12). The reaction of acetone with aromatic aldehydes bearing electron-withdrawing groups led to excellent yields (up to 97 %) and moderate enantioselectivities (up to 65 %). Electron-withdrawing groups in the ortho position exhibited slightly lower yields and enantioselectivities compared to the para position, as the latter position caused steric hindrances between the NO2 group and the catalyst due to close proximities.
According to the stereochemistry of the products in the above direct aldol reactions catalyzed by Pro-5.ASA, we propose that our catalyst could catalyze the direct asymmetric aldol reactions between ketones and aldehydes via the plausible chiral-like transition state (Scheme 2). The aldehydes could be activated by hydrogen bonding with the NH, COOH and –OH groups of the catalyst in such a manner that the C–C bond formation takes place from its favorite face. The presence of water and Bronsted acid helped to stabilize the transition state [45]. The reason why the addition of water enhanced enantioselectivity is that, water may disrupt the intramolecular forces between the COOH and –OH groups which in turn, enabled the carboxylic group to form hydrogen bonds with the aldehydes.
In conclusion, we have synthesized a very simple and small proline-based organocatalyst and tested its efficacy in a direct asymmetric aldol reaction successfully. High yields (up to 97 %), enantioselectivities (up to 91 %) and diastereoselectivities (up to 99/1) were obtained using this catalyst system. Water as an eco-friendly solvent was very compatible with our catalyst and enabled the achievement of high yields, and also high stereoselectivity. We also deduce that having more functional groups (NH, COOH, –OH groups) on the catalyst, allowed the formation of hydrogen bonds with aldehydes which could, in turn, stabilize the transition state and as a result, increase the reactivity and selectivity.
References
Zhong L, Gao Q, Gao J, Xiao J, Li C (2007) J Catal 250(2):360–364
Machajewski TD, Wong CH (2000) Angew Chem Int Ed 39(8):1352–1375
Kofoed J, Nielsen J, Reymond J-L (2003) Bioorg Med Chem Lett 13(15):2445–2447
Dean SM, Greenberg WA, Wong CH (2007) Adv Synth Catal 349(8–9):1308–1320
Alcaide B, Almendros P (2003) Angew Chem Int Ed 42(8):858–860
Ito Y, Sawamura M, Hayashi T (1986) J Am Chem Soc 108(20):6405–6406
Trost BM, Ito H, Silcoff ER (2001) J Am Chem Soc 123(14):3367–3368
List B, Lerner RA, Barbas CF (2000) J Am Chem Soc 122(10):2395–2396
Qiao Y, Chen Q, Lin S, Ni B, Headley A (2013) J Org Chem 2013(78):2693–2697
Scheffler U, Mahrwald R (2013) Chemistry—A. Eur J 19(43):14346–14396
Guillena G (2013) Organocatalyzed aldol reactions. Modern methods in stereoselective aldol reactions. Wiley-VCH Verlag GmbH & Co.KGaA, Germany, pp 155–268
Fesko K, Gruber-Khadjawi M (2013) Chem Cat Chem 5(6):1248–1272
Gruttadauria M, Giacalone F, Noto R (2011) Recyclable organocatalysts in asymmetric reactions. Catalytic methods in asymmetric synthesis. Wiley, NewYork, pp 83–175
Denmark SE, Beutner GL (2008) Angew Chem Int Ed 47(9):1560–1638
Sohtome Y, Nagasawa K (2014) Org Biomol Chem 12(11):1681–1685
Liu F (2013) Chirality 25(11):675–683
Trost BM, Brindle CS (2010) Chem Soc Rev 39(5):1600–1632
Notz W, Tanaka F, Barbas CF (2004) Acc Chem Res 37(8):580–591
Seayad J, List B (2005) Org Biomol Chem 3(5):719–724
Zheng Z, Perkins BL, Ni B (2010) J Am Chem Soc 132:50–51
Hayashi Y, Gotoh H, Hayashi T, Shoji M (2005) Angew Chem Int Ed 44(27):4212–4215
Mase N, Watanabe K, Yoda H, Takabe K, Tanaka F, Barbas CF (2006) J Am Chem Soc 128(15):4966–4967
Bui T, Barbas CF (2000) Tetrahedron Lett 41(36):6951–6954
Nyberg AI, Usano A, Pihko PM (2004) Synlett 2004(11):1891–1896
Hayashi Y (2006) Angew Chem Int Ed 45(48):8103–8104
Blackmond DG, Armstrong A, Coombe V, Wells A (2007) Angew Chem Int Ed 46(21):3798–3800
Gruttadauria M, Giacalone F, Noto R (2009) Adv Synth Catal 351(1–2):33–57
Córdova A, Notz W, Barbas CF III (2002) Chem Commun 24:3024–3025
Mase N, Nakai Y, Ohara N et al (2006) J Am Chem Soc 128(3):734–735
Mlynarski J, Bas S (2014) Chem Soc Rev 43(2):577–587
Hayashi Y, Sumiya T, Takahashi J, Gotoh H, Urushima T, Shoji M (2006) Angew Chem 118(6):972–975
Zotova N, Franzke A, Armstrong A, Blackmond DG (2007) J Am Chem Soc 129(49):15100–15101
Akagawa K, Sakamoto S, Kudo K (2005) Tetrahedron Lett 46(47):8185–8187
Psarra A, Kokotos CG, Moutevelis-Minakakis P (2014) Tetrahedron 70(3):608–615
Bayat S, Tejo BA, Salleh AB, Abdmalek E, Normi YM, Rahman MBA (2013) Chirality 25(11):726–734
Moyano A, Rios R (2011) Chem Rev 111(8):4703–4832
Hu S, Li J, Xiang J, Pan J, Luo S, Cheng J-P (2010) J Am Chem Soc 132(20):7216–7228
Yang H, Mahapatra S (2010) Cheong PH-Y, Carter RG. J Org Chem 75(21):7279–7290
Fotaras S, Kokotos CG, Kokotos G (2012) Org Biomol Chem 10(29):5613–5619
Raj M, Singh VK (2009) Chem Commun 44:6687–6703
Freund M, Schenker S, Tsogoeva SB (2009) Org Biomol Chem 7(20):4279–4284
Luo S-P, Li Z-B, Wang L-P, Guo Y, Xia A-B, Xu D-Q (2009) Org Biomol Chem 7(21):4539–4546
Clemente FR, Houk K (2004) Angew Chem 116(43):5890–5892
Pihko PM, Majander I, Erkkilä A (2009) Enamine catalysis. Asymmetric organocatalysis. Springer, Berlin, pp 145–200
Wang J, Lao J, Du Q, Nie S, Hu Z, Yan M (2012) Chirality 24(3):232–238
Acknowledgments
We gratefully acknowledge the Ministry of Higher Education (MOHE), Malaysia-for financial support through the Grant No. 5524292.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bayat, S., Salleh, A.B., Malek, E.A. et al. Design of a Simple Organocatalysts for Asymmetric Direct Aldol Reactions in Aqueous Medium. Catal Lett 145, 1750–1755 (2015). https://doi.org/10.1007/s10562-015-1583-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-015-1583-7