Abstract
Four Ln(III) coordination polymers, {[Ln2(1,3-bdc)3(H2O)4]·DMF·H2O} n (Ln = Sm 1, Eu 2) and [Ln2(mal)3(H2O)6] n (Ln = Sm 3, Eu 4) (1,3-H2bdc = isophthalate acid, H2mal = malonate acid), were hydrothermally synthesized and characterized by single-crystal X-ray diffraction, elemental analysis, IR spectra, UV–Vis–NIR absorption spectra, and fluorescence spectra. The structural analyses reveal that polymer 1 is a 3D coordination polymer. Its asymmetry unit contains two crystallographically independent Sm(III) ions, both are eight-coordinated. The 1,3-bdc2− anions show three different coordination modes. The structure of polymer 2 is isomorphous with that of 1. Polymer 3 is also a 3D coordination polymer, its asymmetry unit contains one Sm(III) ion, which is nine-coordinate. The mal2− anions have two different coordination modes. The structure of polymer 4 is isomorphous with that of 3. The luminescent study shows that polymers 1, 2, and 4 exhibit characteristic emission bands in the visible region, corresponding to the transitions of the Ln(III) ions. By comparison and analysis of luminescence, it is found that the incidence of the same ligand on the corresponding spectra of different Ln(III) ions is different, and the influence of different ligands on luminescence of the same Ln(III) ion is also very different.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ln(III) coordination polymers, due to their fascinating structure and unique physical property, always attract more and more attentions. The study for features and application exploration of such polymers are mainly focused on the luminescence, magnetics, molecular recognition and separation, medical diagnosis, cell imaging, etc. [1–10]. The luminescence of Ln(III) coordination polymers originates from the f–f transitions of Ln(III) ions. The intrinsic disadvantage of the f–f transitions is that the absorption coefficient is lower, thus resulting in weak emission [11, 12]. However, this weak absorbance can be overcome and improved effectively by coordinating suitable ligands with the antenna effect to the Ln(III) ion [13–17]. Typical ligands, such as β-diketonate, porphyrin, fluorescein, 8-quinoline as well as their derivatives, have been demonstrated the successful sensitization to the luminescence of Ln(III) ion. Apart from sensitizing the luminescence of Ln(III) ions, the different ligands are crucial to generation of the fascinating structures. As far as carboxylate ligands, the flexible carboxylate ligands (such as succinate, malonic, citric acids, etc.), while usually making a small contribution to sensitization of luminescence of Ln(III) ions (even quenching the luminescence), is more favorable for generation of the novel architectures and topologies because of their rich conformation capable of meeting the requirement for coordination geometry of different Ln(III) ions [18–20]. For example, the suc2− anion (H2suc = succinate acid) can feature cis- and trans-conformations to assembly with Ln(III) ions, generating various coordination frameworks from 1D to 3D [21, 22]; the mal2− anion (H2mal = malonic acid) can adopt chair, boat, and envelope three conformations to bridge Gd(III) ions to form a 2D layer structure; curiously, in another Gd-mal polymers, the mal2− ligands only show chair and boat two conformations, but it can bridge the Gd(III) ions into a 3D framework structure [23, 24]. Compared with the flexible ligand, under common circumstance, the rigid carboxylic ligand with high degrees of conjugation (such as benzene carboxylic acid, pyridine carboxylic acid as well as their derivatives, etc.), can efficiently sensitize the luminescence of Ln(III) ions after coordinating to the Ln(III) ions, usually presenting long luminescence lifetime and high luminescence intensity as well as good stability [8, 25, 26]. Therefore, ascertaining the luminescence, the relationship between luminescence and structure for Ln(III) coordination polymers has an important significance for in-depth investigation on various properties and exploiting their practical applications. In this study, we report four Ln(III) coordination polymers with 3D extended structure bridged by isophthalato and malonato ligands, respectively. Their structures were determined by single-crystal X-ray diffraction. The luminescent property of polymers 1–4 in visible region was analyzed and assigned. The influence of ligands on emission spectra of Ln(III) ions is discussed.
Experimental
Materials and measurements
The salt of Ln(NO3)3·6H2O was prepared by dissolving the corresponding lanthanide oxide compound (purity is 99.99%) in the excess nitric acid, and then naturally crystallizing. Other starting materials and reagents were AR and used as purchased. The elemental analyses were performed on a PE-2400 elemental analyzer. The crystal structures were determined with Bruker Smart APEX-II CCD X-ray single-crystal diffractometer. The FT-IR spectra were recorded in 4,000–220 cm−1 scopes on a JASCO FT-480 spectrometer with pressed KBr pellets technique. The diffuse reflectance absorption spectra were obtained in 200–2,500 nm scopes on a JASCO V-570 UV–Vis–NIR spectrometer. The excitation and emission spectra in UV–Vis region were measured with a JASCO FP-6500 fluorescence spectrometer.
Syntheses of the polymers
{[Sm2(1,3-bdc)3(H2O)4]·DMF·H2O} n 1
Sm(NO3)3·6H2O (0.22 g, 0.5 mmol) was dissolved in aqueous solution (5.0 mL), to which a mixture of 1,3-H2bdc (0.06 g, 0.35 mmol) and water (5.0 mL) was added under stirring and heating (50 °C). Then, to the resulting clear solution, an appropriate amount of 1.0 mol L−1 NaOH solution was added dropwise to adjust the pH value to about 6. While, some white precipitate occurred, but they were dissolved following addition of DMF solution (5 mL). Then, the final solution was transferred into a Teflon bottle sealed in an autoclave, which was heated at 90 °C for 120 h. After cooling to room temperature and a standing period of 3 days, the reaction solution was filtered and the brown-yellow block crystals were obtained, washed out with mother liquid, and dried out in the air. The yield was 39% (0.19 g) based on Sm. Calc. for C27H29Sm2NO18 1 (Found): C, 33.91 (33.73); H, 3.06 (3.04); N, 1.46 (1.45)%. IR(KBr, cm−1): 3407s, 3037w, 2929w, 2855w, 1650m, 1608s, 1599s, 1541s, 1480m, 1448m, 1395vs, 1278w, 1167w, 1103m, 823w, 747s, 707m, 654m, 535m, 425m.
Polymer 2 was synthesized by the same procedure as 1 except for the replacement of Sm(NO3)3·6H2O with Eu(NO3)3·6H2O.
{[Eu2(1,3-bdc)3(H2O)4]·DMF·H2O} n 2
Eu(NO3)3·6H2O (0.22 g, 0.5 mmol) were used. The colorless block crystals were obtained. The yield was 42% (0.20 g) based on Eu. Calc. for C27H29Eu2NO18 2 (Found): C, 33.80 (33.62); H, 3.05 (3.03); N, 1.46 (1.45)%. IR(KBr, cm−1): 3433s, 3035w, 2929w, 2857w, 1647m, 1608s, 1600s, 1544s, 1477w, 1449w, 1396vs, 1280w, 1170w, 1103m, 819w, 750s, 703m, 659m, 532m, 425m.
[Sm2(mal)3(H2O)6]n 3
Sm(NO3)3·6H2O (0.22 g, 0.5 mmol) was dissolved in aqueous solution (5.0 mL), to which a mixture of H2mal (0.1 g, 1 mmol) and water (5.0 mL) was added under stirring and heating (50 °C), getting colorless solution. An appropriate amount of 1 mol L−1 NaOH solution was added dropwise to adjust the pH value to about 4. Then, the final solution was stirred for 10 min and transferred into a Teflon bottle sealed in an autoclave, which was then heated at 90 °C for 120 h. After cooling to room temperature and a standing period of 2 days, the reaction solution was filtered and the colorless block crystals were obtained, washed out with mother liquid, and dried out in the air. The yield was 40% (0.14 g) based on Sm. Calc. for C9H18Sm2O18 3 (Found): C, 15.12 (15.04); H, 2.54 (2.52)%. IR(KBr, cm−1): 3335vs, 2914w, 1699s, 1567vs, 1448s, 1382vs, 1277s, 1187m, 964s, 712m, 647m, 625m, 532w, 454m.
Polymer 4 was synthesized by the same procedure as 3 except for the replacement of Sm(NO3)3·6H2O with Eu(NO3)3·6H2O.
[Eu2(mal)3(H2O)6]n 4
Eu(NO3)3·6H2O (0.27 g, 0.6 mmol) were used. The colorless block crystals were obtained. The yield was 45% (0.19 g) based on Eu. Calc. for C9H18Eu2O18 4 (Found): C, 15.05 (14.97); H, 2.53 (2.51)%. IR(KBr, cm−1): 3342vs, 2912w, 1699s, 1570vs, 1448s, 1383vs, 1277s, 1186m, 964s, 710m, 647m, 622m, 532w, 457m.
Single-crystal structural determinations
X-ray single-crystal diffraction data for polymers 1–4 were collected on a Bruker Smart APEX-II CCD diffractometer at 293(2) K with Mo-Kα radiation (λ = 0.71073 Å) by ω scan mode. Empirical absorption corrections were applied to the data using the SADABS program. Structures were solved by the direct methods and refined by full-matrix least squares on F 2 using the SHELXTL version 5.1 [27]. All of the non-hydrogen atoms were refined anisotropically. The hydrogen atoms bound to carbon atoms were placed in calculated position and refined isotropically with a riding mode. Hydrogen atoms of coordinated water molecules were found via Fourier difference map, then restrained and refined isotropically. Hydrogen atoms of lattice water molecules did not find further, so they could not be introduced in the refinement, but were included in the structure factor calculation. All the structural calculations and drawings were generated with the SHELXL-97 crystallographic software package. The main crystallographic data and structural refinement parameters of polymers 1–4 are summarized in Table 1.
Results and discussion
Structural descriptions
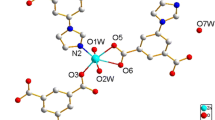
Structural analysis shows that polymer 1 is a 3D coordination polymer. Its asymmetric unit comprises two crystallographically independent Sm(III) ions (Sm1 and Sm2), three 1,3-bdc2− anions, four coordinated water molecules, one uncoordinated DMF molecule, and one lattice water molecule (Fig. 1). The Sm1(III) ion is coordinated by the eight O atoms from five different 1,3-bdc2− anions and two coordinated water molecules, forming a distorted square antiprism geometry (Fig. S1a in Supplementary material). The bond lengths of Sm1–O are in the range of 2.362(3)–2.560(3) Å (Table S1 in Supplementary material). The Sm2(III) ion is also eight-coordinate with six O atoms from five different 1,3-bdc2− anions and other two O atoms from two coordinated water molecules, forming a distorted square antiprism geometry (Fig. S1b in Supplementary material). The bond lengths of Sm2–O are in the range of 2.367(3)–2.538(3) Å (Table S1 in Supplementary material). The three crystallographically independent 1,3-bdc2− anions bond to Sm(III) ions in three different coordination modes: I-type, II-type, and III-type, respectively (Fig. 2). Each of carboxylate groups of I-type 1,3-bdc2− anion bridges two different Sm(III) ions (Sm1 and Sm2 ions) with a bridging bidentate mode in syn–anti configuration [28] (Fig. 2a). The separation of Sm1···Sm2 is 4.929 Å. Each carboxylate group of II-type 1,3-bdc2− anion chelates to one Sm(III) ion (Sm1 or Sm2) with chelating bidentate mode [28] (Fig. 2b). The two carboxylate groups of III-type 1,3-bdc2− anion bridge two equivalent Sm(III) ions(Sm1 or Sm2 ions) with a bridging bidentate mode in syn–anti configuration[28], respectively(Fig. 2c). The separations of Sm1C···Sm1B and Sm2···Sm2D are 4.689 and 4.705 Å, respectively.
In the crystal of 1, first, [Sm(H2O)2]3+ cations are bridged by 1,3-bdc2− anions with I-type and III-type coordination modes, forming three different binuclear units of [Sm2(1,3-bdc)2(H2O)4], named A, B, and C, respectively (Fig. S2 in Supplementary material). These binuclear units exhibit an ABCBABCB sequence along the a axis, forming a 1D chain (Fig. 3, Fig. S3 in Supplementary material). Second, the remaining carboxylate groups of intra-chain I-type 1,3-bdc2− anions together with II-type 1,3-bdc2− anions all coordinate to the Sm(III) ions from the adjacent chains along the b axis, building the 2D layers in the ab plane as shown in Fig. 4. Finally, these layers are further extended along the c axis through the III-type 1,3-bdc2− anions with coordinate of Sm(III) ions from adjacent layers, generating the 3D network (Fig. S4 in Supplementary material). Uncoordinated DMF molecule and lattice water molecule adhere to the framework of polymer 1 through hydrogen bonds (Fig. S5 in Supplementary material), which contributes to the stabilization of crystal structure.
The structure of polymer 2 is isomorphous with that of 1, with the Eu(III) ion taking the place of the Sm(III) ion. The bond lengths of Eu1–O and Eu2–O are 2.351(4)–2.544(3) and 2.350(3)–2.528(3) Å, respectively (Table S1 in Supplementary material).
Polymer 3 is also a 3D coordination polymer. Its asymmetric unit comprises one Sm(III) ion, one and one-second mal2− anions, and three coordinated water molecules (Fig. 5). The Sm(III) ion is nine-coordinate with six O atoms from four different mal2− anions and three O atoms from three coordinated water molecules, forming a distorted monocapped square antiprism geometry (Fig. S6 in Supplementary material). The bond lengths of Sm–O are in the range of 2.319(5)–2.611(4) Å (Table S1 in Supplementary material). The mal2− anions bond to Sm(III) ions in two different coordination modes, which are described as μ2-mal2− and μ4-mal2−, respectively (Fig. S7 in Supplementary material).
In the crystal of 3, first of all, [Sm(H2O)3]3+ cations are bridged by μ2-mal2− anions, forming a 1D chain along b axis (Fig. 6). Second, the 1D chains are further linked by one carboxylate group of μ4-mal2− anion in turn along c axis, resulting in formation of the 2D coordination layer in the bc plane as shown in Fig. 7. Finally, the left carboxylate groups of intra-layer μ4-mal2− ligands coordinate to the Sm(III) ions from neighboring layers, thus the 2D layers are linked in turn along a axis, building a 3D network (Fig. S8 in Supplementary material).
The structure of polymer 4 is isomorphous with that of 3, with the Eu(III) ion taking the place of the Sm(III) ion. The bond lengths of Eu–O are 2.307(4)–2.605(4) Å (Table S1 in Supplementary material). These distances are comparable to the corresponding values of the reported Eu–mal complexes [29]. Although the structure of polymer 4 is similar to that reported in the literatures [29, 30], the synthesis method, the crystal system and space group as well as property in this article are different from those reported. In this paper, we mainly focus on the luminescent properties of the four polymers, and the influence of structures of polymers on the luminescent properties.
Photophysical properties
UV–Vis–NIR absorption spectra
The UV–Vis–NIR absorption spectra of polymers 1–4 are shown in Fig. S9 in Supplementary material. The absorption spectra of the polymers 1 and 3 are composed the absorption bands of ligand and the f–f transition absorption bands of Sm(III) ions (Fig. S9a, c in Supplementary material). The absorption bands of ligand mainly lie in the ultraviolet region, while the f–f transition absorption bands of Sm(III) ion appear in the visible and near infrared region. The UV–Vis–NIR absorption spectra of polymers 2 and 4 are very similar (Fig. S9b, d in Supplementary material), composed of the absorption bands of ligand and the ligand-to-metal charge transition absorption bands (LMCT). They do not show the characteristic absorption bands of Eu(III) ions. The assignments of absorption spectra of four polymers and the corresponding ligands are listed in Table 2.
Fluorescence spectra
At room temperature, luminescence properties of the four polymers are investigated in the solid state.
With λEx = 311 nm, polymer 1 exhibits the characteristic emission bands of Sm(III) ion at 490, 546, and 589 nm (Fig. 8a), which can be ascribed to the 4G2/5 → 6H J (J = 5/2, 7/2, 9/2) transition of the Sm(III) ion, respectively. The emission band from the transition (4G2/5 → 6H7/2) is the strongest, which is similar to that in other Sm(III) complexes [31–33]. Compared with the theoretical emission bands of the Sm(III) ion [34] and the emission bands of other Sm(III) complexes reported [31–33], the emission bands of Sm(III) ion in polymer 1 present obviously blue-shift.
Polymer 2 reveals the characteristic emission bands of the Eu(III) ion upon excitation at 395 nm. As shown in Fig. 8b, the emission bands are attributed to 5D0 → 7F J (J = 0, 1, 2, 3, 4) transition, i.e., 579.5 nm (5D0 → 7F0), 589 and 592.5 nm (5D0 → 7F1), 613.5 and 619 nm (5D0 → 7F2), 652 nm (5D0 → 7F3), and 697 nm (5D0 → 7F4) [35, 36]. The emission band (5D0 → 7F0) is strictly forbidden in a field of symmetry, thus, the presence of which in polymer 2 reveals that the Eu(III) ion features a low-symmetry coordination environment [37, 38], in agreement with the result of X-ray single-crystal structural analysis. This is also reflected by the fact that the intensity ratio (5D0 → 7F2/5D0 → 7F1) in the solid state is high up to about 6.89, much higher than the value (0.67) for a centro-symmetric Eu(III) complex [37, 39]. The most intense transition is 5D0 → 7F2, which implies a red emission light of 2. The spectrum of polymer 2 is similar to those obtained for related Eu(III)–aromatic-carboxylate complexes [31, 38].
With λEx = 300 nm, the fluorescence spectra of polymer 3 is obtained (Fig. 8c). Only the characteristic emission bands of ligand appear in the range of 321–440 nm, while the emission bands of Sm(III) ion are not observed.
Upon excitation at 395 nm, polymer 4 shows the characteristic emission bands of Eu(III) ion (Fig. 8d). The bands at 579, 590, 613, 651, and 695 nm are attributed to the f–f transition of 5D0 → 7F J (J = 0, 1, 2, 3, 4), respectively. The most intense transition is also 5D0 → 7F2. The presence of only one sharp peak in the region of the 5D0 → 7F0 transition suggests the existence of a single chemical environment around the Eu(III) ion [40], which is in good agreement with the X-ray crystal structure of 4. The emission spectrum of 4 is similar to that of Eu(III)–mal complex obtained at room temperature [29, 41].
The comparison and discussion of the luminescent properties of four polymers
Comparing and analyzing the emission spectra of polymers 1–4, it can be found that
-
(1)
The characteristic emission bands of the Ln(III) ions are observed in polymers 1 (Sm), 2 (Eu), and 4 (Eu), while only the emission bands of ligand exist in polymer 3 (Sm). This indicates that the luminescence of Ln(III) ions in polymers 1, 2, and 4 get efficient sensitization from ligands.
-
(2)
The same ligand shows different sensitization effects on luminescence of the different Ln(III) ions. For example, in polymers 1 (Sm) and 2 (Eu) bridged by 1,3-bdc2− anion, although the 1,3-bdc2− ligand efficiently sensitized the emissions of Sm(III) and Eu(III) ions with evidenced by quenching of the emissions of ligand (Fig. S10a in Supplementary material), the extent of the sensitization is different. In the same determination condition, even if the Ex. slit is (3, 3 nm) for polymer 1 and (3, 1 nm) for polymer 2, the emission intensity of polymer 2 is still much higher than that of polymer 1. Similarly, for polymers 3 (Sm) and 4 (Eu), the mal2− ligand only play the role of sensitization on the Eu(III) ions in polymer 4. This may be due to that the emissive energy levels of different Ln(III) ions are different. This also was presented in other Ln(III) complexes reported [31].
-
(3)
The incidence of the structure of ligands and the coordination environment of Ln(III) ions obviously affect the luminescence of Ln(III) ions. The conjugated extent of the ligand is better, the luminescence is stronger for corresponding ligand Ln(III) complexes. For example, the 1,3-bdc2− ligand has rigid cyclo-conjugated structure, while the mal2− ligand has not. Therefore, the emission intensity of polymer 2 is much stronger than that of polymer 4 under the same determination condition (the same λEx and Ex. slit) (Fig. 9b). For polymers 1 and 3, the emission bands of Sm(III) ion have not been observed in polymer 3 (Fig. 9a). This may be due to that the emissive energy levels of 1,3-bdc2− ligand with π-conjugated structure are richer than that of mal2− ligand, so they may match well with the emissive energy levels of Ln(III) ion (Fig. S11 in Supplementary material). In addition, reducing the number of coordinated water molecules around Ln(III) ions can also be helpful for their luminescence, which is due to decreasing the non-radiative energy loss [37]. For example, there are two coordinated water molecules around Ln(III) ion for polymers 1 and 2, while there are three ones around Ln(III) ion for polymers 3 and 4. Therefore, the luminescence intensity of polymers 1 and 2 is stronger than that of polymers 3 and 4, respectively.
Conclusions
Four Ln(III) coordination polymers [Ln = Sm (1 and 3); Eu (2 and 4)] were hydrothermally synthesized and structurally characterized by single-crystal X-ray diffraction. Polymers 1 and 2 are isostructural, which possess 3D structure bridged by 1,3-bdc2− anion. Polymers 3 and 4 are also isostructural with 3D structure bridged by mal2− anion. The luminescence study shows that polymers 1, 2, and 4 exhibit characteristic emission bands of corresponding Ln(III) ions benefiting from the sensitization of ligands; while polymer 3 mainly presents the emissions of ligand. By the comparative analysis of luminescence, it is found that the structure of ligand, species, and coordination environment of central metal ions all obviously affect the luminescence of Ln(III) ions.
Supplementary data
CCDC—848006 (for 1), 848007 (for 2), 848008 (for 3), and 848009 (for 4) contains the supplementary crystallographic data for this article. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB21EZ, UK: Fax: + 44 1223 336033; or deposit@ccdc.cam.ac.uk).
References
Wang XJ, Cen ZM, Ni QL, Jiang XF, Lian HC, Gui LC, Zuo HH, Wang ZY (2010) Cryst Growth Des 10:2960–2968
Kitagawa S, Kitaura R, Noro S-I (2004) Angew Chem Int Ed 43:2334–2375
Pan L, Adams KM, Hernandez HE, Wang XT, Zheng C, Hattori Y, Kaneko K (2003) J Am Chem Soc 125:3062–3067
Radecka-Paryzek W, Kubicki M, Luks E (2010) Struct Chem 21:299–304
Wang P, Ma JP, Dong YB, Huang RQ (2007) J Am Chem Soc 129:10620–10621
Cañadillas-Delgado L, Fabelo O, Pasán J, Delgado FS, Lloret F, Julve M, Ruiz-Pérez C (2010) Dalton Trans 39:7286–7293
Dutra JDL, Gimenez IF, Costa NB Jr, Freire RO (2011) J Photochem Photobiol A 217:389–394
Sun YG, Rong ST, Yu W, Wu YL, Ding F, Gao EJ, Zhang WZ, Verpoort F (2009) Z Anorg Allg Chem 635:2585–2591
Wang HH, He P, Yan HG, Gong ML (2011) Sens Actuators B 156:6–11
Neves M, Gano L, Pereira N, Costa MC, Costa MR, Chandia M, Rosodo M, Fausto R (2002) Nucl Med Biol 29:329–338
Binnemans K (2009) Chem Rev 109:4283–4374
Xi P, Gu XH, Chen CF, He YX, Huang XA (2007) Spectrochim Acta A 66:667–671
Viswanathan S, de Bettencourt-Dias A (2006) Inorg Chem 45:10138–10146
Klink SI, Hebbink GA, Grave L, Oude Alink PGB, van Veggel FCJM (2002) J Phys Chem A 106:3681–3689
Hu M, Wang QL, Xu GF, Deng GR, Yang GM, Yu M, Zhang YH (2007) Inorg Chim Acta 360:1684–1690
Wu MF, Wang MS, Guo SP, Zheng FK, Chen HF, Jiang XM, Liu GN, Guo GC, Huang JS (2011) Cryst Growth Des 11:372–381
Dolbecq A, Dumas E, Mayer CR, Mialane P (2010) Chem Rev 110:6009–6048
Zhang LZ, Gu W, Li B, Liu X, Liao DZ (2007) Inorg Chem 46:622–624
Wu AQ, Zheng FK, Liu X, Guo GC, Cai LZ, Dong ZC, Takano Y, Huang JS (2006) Inorg Chem Commun 9:347–350
Amghouz Z, Roces L, García-Granda S, García JR, Souhail B, Mafra L, Shi F-N, Rocha J (2009) J Solid State Chem 182:3365–3373
Bernini MC, Garro JC, Brusau EV, Narda GE, Varetti EL (2008) J Mol Struct 888:113–123
Manna SC, Zangrando E, Bencini A, Benelli C, Chaudhuri NR (2006) Inorg Chem 45:9114–9122
Hernández-Molina M, Ruiz-Pérez C, López T, Lloret F, Julve M (2003) Inorg Chem 42:5456–5458
Cañadillas-Delgado L, Pasán J, Fabelo O, Hernández-Molina M, Lloret F, Julve M, Ruiz-Pérez C (2006) Inorg Chem 45:10585–10594
Gawryszewska P, Ciunik Z (2009) J Photochem Photobiol A 202:1–9
Lu WG, Jiang L, Lu TB (2010) Cryst Growth Des 10:4310–4318
Sheldrick GM (1997) SHELXS97 and SHELXL97. University of Göttingen, Göttingen
Deacon GB, Phillips RJ (1980) Coord Chem Rev 33:227–250
Hernández-Molina M, Lorenzo-Luis P, Ruiz-Pérez C, López T, Martín IR, Anderson KM, Orpen AG, Bocanegra EH, Lloret F, Julve M (2002) J Chem Soc Dalton Trans 2:3462–3470
Zhang CZ, Mao HY, Wang YL, Zhang HY, Tao JC (2007) J Phys Chem Solids 68:236–242
Huang YG, Wu BL, Yuan DQ, Xu YQ, Jiang FL, Hong MC (2007) Inorg Chem 46:1171–1176
Wang GL, Tian YM, Cao DX, Yu YS, Sun WB (2011) Z Anorg Allg Chem 637:583–588
An BL, Gong ML, Li MX, Zhang JM (2004) J Mol Struct 687:1–6
Gao JZ, Yang W, Kang JW (1995) Spectroscopic properties of the lanthanide complexes in aqueous solution. University of Electronic Science and Technology of China Press, Chengdu, pp 89–95
Zhuravlev KP, Tsaryuk VI, Pekareva IS, Sokolnicki J, Klemenkova ZS (2011) J Photochem Photobiol A 219:139–147
Subhan MA, Hasegawa Y, Suzuki T, Kaizaki S, Shozo Y (2009) Inorg Chim Acta 362:136–142
Liu TF, Zhang WJ, Sun WH, Cao R (2011) Inorg Chem 50:5242–5248
Daiguebonne C, Kerbellec N, Guillou O, Bünzli JC, Gumy F, Catala L, Mallah T, Audebr N, Gérault Y, Bernot K, Calvez G (2008) Inorg Chem 47:3700–3708
Klink SI, Grave L, Reinhoudt DN, van Veggel FCJM, Werts MHV, Geurts FAJ, Hofstraat JW (2000) J Phys Chem A 104:5457–5468
Biju S, Reddy MLP, Cowley AH, Vasudevan KV (2009) Cryst Growth Des 9:3562–3569
Martín IR, Lahoz F, Lavín V, Hernández-Molina M (2000) Opt Mater 25:223–229
Acknowledgment
This study was supported by the National Natural Science Foundation of China (No. 20571037) and the innovational team project of Liaoning Province Educational Office (No. 2007T092).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jin, J., Wang, X., Li, Y. et al. Synthesis, structure, and photophysical property of series of Ln(III) coordination polymers with different carboxylato ligands (Ln = Sm, Eu). Struct Chem 23, 1523–1531 (2012). https://doi.org/10.1007/s11224-012-9957-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-012-9957-6