Abstract
Two novel two-dimensional Ln coordination polymers (CPs) [Ln3 (μ3-OH)(IN)3(HIDA) (IDA)2]n (Ln = Eu, 1; Sm, 2; HIN = isonicotinic acid, and H2IDA = iminodiacetic acid) have been successfully synthesized under solvothermal conditions and characterized by IR, TG, and elemental analyses. The structures of 1 and 2 were determined by single-crystal X-ray structural analysis, which shows that LnIII ions interconnect through HIN and H2IDA molecules to generate a 1D chain, and the adjacent chains are joined together by the same form to form the 2D zonary plane of 1 and 2. Meanwhile, We also studied luminescence properties of 1 and 2. The luminescence lifetime and quantum yield of 1 are 1.32 ms and 25.30%, which are significantly longer and higher than the values obtained for reported Eu3+ coordination polymers in the solid state at room temperature.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a new class of porous materials, metal–organic frameworks(MOFs), a class of crystalline hybrid inorganic–organic materials formed by the connection of metal centers or clusters and organic linkers, have attracted tremendous attention for their wide applications in the areas of gas storage/separation [1–3], catalysis [4–6], magnetism [7, 8], drug delivery [9], sensors [10, 11], etc. Thus, a large number of MOFs have been designed and developed from various metal ions and organic ligands [12, 13]. Therefore, the luminescent MOFs have drawn special attention owing to their various applications in biomedical areas, optical displays, energy-harvesting device amplification, and fluorescent probes [14, 15]. The most interesting examples of luminescent MOFs are lanthanide (III) (LnIII) complexes because of their high quantum yield, large Stokes shift, and long emission lifetimes [16, 17].
To obtain lanthanide (III) (LnIII) complexes, a binding site and a powerful luminophore is required [18, 19]. Therefore, it is important to select appropriate ligands. On one hand, when constructing Ln-MOFs, organic ligands can not only serve as connective building blocks for the structure but can also function as antennae for the lanthanide ions [20–22]. In view of the previous work [23–26], carboxylate ligands are a good choice because Ln3+ ions are hard acceptors, making coordination with the hard carboxylate oxygen atoms more favorable, according to the “hard-soft acid-base” concept. Moreover, aromatic carboxylic groups are good luminescent chromophores, resulting in the intense luminescence of lanthanide. For example, isonicotinic acid (HIN)/iminodiacetic acid (H2IDA), containing carboxylate groups, have been used previously in lanthanide (III) (LnIII) complexes [27, 28]. Alternatively, amino nitrogen of H2IDA can also be considered as binding sites and be immobilized in luminescent MOFs.
For this purpose, we choose HIN and H2IDA reacting with lanthanide oxides in ethanol solution by self-assembly approach and obtained two trinuclear \(\left( {{\text{Ln}}_{3}^{\text{III}} } \right)\) complexes, [Ln3 (μ3-OH) (IN)3 (HIDA) (IDA)2]n (Ln = Eu, 1; Sm, 2). Meanwhile, We also studied luminescence properties of 1 and 2. The luminescence lifetime and quantum yield of 1 are 1.32 ms and 25.30%, which are significantly longer and higher than the values obtained for reported Eu3+ coordination polymers in the solid state at room temperature.
Experimental Section
Materials and Methods
All chemicals purchased were of reagent grade and were used without further purification. Powder X-ray diffraction (PXRD) were obtained on a Bruker D8X diffractometer equipped with monochromatized Cu Kα (λ = 1.5418 Å) radiation at room temperature. Data were collected in the range of 5°–50°, and the experimental XRD patterns are in agreement with the patterns simulated on the basis of the single-crystal structures. IR spectra were recorded on a Nicolet Impact 410 Fourier transform infrared spectrometer using KBr pellets in the 4000–500 cm−1 region. TG measurement was carried out on a Diamond thermogravimetric analyser in a flowing N2 atmosphere from 25 to 1000 °C with a heating rate of 10 °C min−1. The solid-state emission/excitation spectra were measured on an FP-6500 spectrofluorimeter equipped with a 450 W xenon lamp as the excitation source. Single-crystal structure determination was performed on a Bruker APEX2 CCD diffractometer at 296 K and a sealed tube X-ray source (Mo Kα radiation, λ = 0.71073 Å) operating at 50 kV and 30 mA.
Synthesis of [Eu3 (μ3-OH) (IN)3 (HIDA) (IDA)2]n (1)
A mixture of Eu2O3(0.1760 g, 0.5 mmol), HIN (0.1231 g, 1.0 mmol), H2IDA(0.1331 g, 1.0 mmol), ethanol (8 mL) was stirred for 12 h in the air until all reagents dissolve, then this solution was sealed in a 25 mL Teflon-lined autoclave and kept at 170 °C for seven days. Cooled to room temperature under natural cooling. Yellow block crystal were obtained (0.1555 g, yield of 37.88% based on Eu), which were washed with ethanol and dried at room temperature for 24 h. IR (cm−1): 1583 (vs), 1419 (s), 1312 (w), 1260 (w), 1120 (w), 1020 (w), 1005 (w), 646 (w). Elemental analysis calc: C, 29.23, H, 2.19, N, 6.82%; found: C, 29.35, H, 2.26, N, 6.78%.
Synthesis of [Sm3 (μ3-OH) (IN)3 (HIDA) (IDA)2]n (2)
Complex 2 as yellow block single crystals was prepared (0.1505 g, yield of 36.70% based on Sm) according to the same procedure as 1, except with Eu2O3 instead of Sm2O3 (0.1744 g, 0.5 mmol). IR (cm−1): 1607 (vs), 1417 (s), 1323 (w), 1255 (w), 1125 (w), 1007 (w), 917 (w), 611 (w). Elemental analysis calc: C, 29.35, H, 2.20, N, 6.85%; found: C, 29.41, H, 2.29, N, 6.74%.
X-ray Crystallography
Data for the compounds of 1 and 2 were collected by using a Bruker Apex 2 CCD single-crystal diffractometer with Mo Kα radiation (λ = 0.71073Å) at 296(2) using ω-2θ scan method. The single crystals of all compounds 1 and 2 were chosen and held on a thin glass fiber by epoxy glue in air for data collection. The SHELX software package (Bruker) was used to solve and refine the structures. Absorption corrections were applied empirically using the SADABS program. All the non-hydrogen atoms were refined anisotropically. The hydrogen atoms of organic molecule were placed in calculated positions, assigned isotropic thermal parameters, and allowed to ride on their parent atoms, while the H atoms of water and disordered organic molecules were not located. A total of two structures were solved by direct methods and refined on F 2 by full-matrix least-squares methods using the SHELX-2014/7 crystallographic software package. All non-H atoms were refined anisotropically. Further details of the X-ray structural analyses for compounds 1 and 2 are given in Table 1. The selected bond lengths for 1 and 2 are listed in Tables S1–S2, respectively. Crystallographic data for the structure reported in this paper has been deposited in the Cambridge Crystallographic Data Center with CCDC Numbers 1527504–1527505 for 1 and 2.
Results and Discussion
Synthesis
Solvothermal synthesis has recently been demonstrated to be a powerful method in the synthesis of solid-state rare-earth clusters. During a specific solvothermal synthesis, many factors can affect the nucleation and crystal growth of the final products, such as the type of initial reactants, starting concentrations of the reactants, pH values, solvents, reaction temperature and reaction time. In our case, the temperature has great influence on the yields of 1 and 2. We tried to change the reaction temperature to 80–170 °C, and the yields are the highest when the reaction temperature is 170 °C. Although ethanol is not included in the final products, but we cannot obtain the above 2 compounds without ethanol. Therefore, ethanol can be used as structure directing agent. The above compounds could be easily obtained under solvothermal synthesis and have good reproducibility.
Crystal Structures
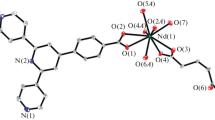
Single-crystal X-ray diffraction analyses revealed that compounds 1–2 are isostructural and their crystal system, space group, unit cell dimensions, related bond distances, and bond angles are only slightly different. Therefore, only the structure of 1 will be discussed in detail. Compound 1 crystallizes in the triclinic space group P − 1. The structure unit of 1 contains three crystallographically independent Eu atoms, one μ3-OH−, three IN−, one HIDA− and two IDA2− (Fig. 1). The Eu(III) center adopts a NO8 donor set completed by one N donor coming from the H2IDA molecule and eight oxygen atoms belonging to one μ3-OH−, one HIN molecules, and four different H2IDA molecules (Fig. S8). The Eu–O distances are from 2.391(5) to 2.511(5) Å and the O–Eu–O bond angles range from 68.27(18) ° to 147.57(16) °. The values fall into the reported normal range [29]. Scheme 1 shows the coordination modes of H2IDA and HIN ligands: the former adopts the 4.22101 and 5.22111 (using Harris notation [30]) coordination modes to coordinate with metal atoms (I: coordinating with four Eu atoms, II: coordinating with five Eu atoms), and the latter adopts the 2.11 and 2.21 coordination modes to coordinate two Eu atoms.
The crystallographically established coordination modes of H2IDA and HIN in complex 1 and the Harris notation [30] which describes these modes
In order to understand the structure of compound 1 clearly, we can view [Eu3] as a unit. According to different arrangements in space, this unit can be divided into unit A (Fig. 2a) and unit B (Fig. 2b). Then, along the c-axis, 1D chain (Fig. 2c) is formed by units A connected to unit B through HIN and H2IDA molecules. The adjacent chains are joined together by the same form to form the 2D zonary plane of 1 (Fig. 2d).
Powder X-ray Diffraction
The measured and simulated powder X-ray diffraction (PXRD) patterns of 1 and 2 are shown in Fig. S1-S2. The measured XRD patterns are in agreement with the calculated patterns, which indicate the phase purity of compounds 1 and 2. The difference in reflection intensities during collection of the experimental XRD data are probably due to the preferred orientation effect of the powder.
IR Spectra
The IR spectra of 1 and 2 are presented in Fig. S3–S4. Because 1 and 2 are isomorphic, and only the IR spectra of 1 is discussed in detail. Bands originating from carboxylate anion vibrations are: (1) asymmetric stretch νasym (COO−) located at 1596 cm−1; (2) symmetric stretch νsym (COO−) at 1397 cm−1. The peaks in the range of 610–750 cm−1 for 1 correspond to the ν (Eu–O) vibrations. The features are in agreement with the reported compounds [31].
Thermal Analysis
The thermogravimetric (TG) analysis experiments were performed under N2 atmosphere in the range of 25–1000 °C. Thermal analysis shows that the 1 and 2 are analogous in Fig. S5–S6. Take 1 as an example. As shown in Fig. S5, there is no obvious weight loss from room temperature to about 350 °C. Complex 1 exhibits an apparent weight loss of 54.50% from 350 to 735 °C, matching with the calculated value of releasing HIN and H2IDA ligands molecules (calcd 55.81%). Further heating above 735 °C gives rise to the collapse of the framework. The final residual weight was 42.51% corresponding to the Eu2O3 (calc. 42.88%).
Luminescence Property
The luminescent behavior of Ln-based compounds has attracted increasing interest due to the high color purity and technological applications in light-emitting diodes, lasers, plasma displays, and sensory probes and optical amplifiers, etc. [32–34]. Because the good shielding of the 4f electrons by the outer 5s and 5p electrons leads to well-defined absorption and emission bands [35], Ln ions still keep their atomic properties upon complex formation. As a result, the luminescence properties of 1 and 2 in the solid-state have been measured at room temperature. As shown in Fig. 3, under 396 nm excitation wavelength solid emission spectrum of compound 1 demonstrates a characteristic transition of Eu3+: 5D0 → 7F0 (580 nm), 5D0 → 7F1 (596 nm), 5D0 → 7F2 (620 nm), 5D0 → 7F3 (657 nm), and 5D0 → 7F4 (705 nm). The existence of 5D0 → 7F0 (580 nm) symmetry-forbidden transition emission spectrum illustrates Eu3+ is in the presence of a non-centrosymmetric coordination environment. As we know, the magnetic dipole transition 5D0 → 7F1 is insensitive and hardly varies with the coordination environment, whereas the electric dipole transition 5D0 → 7F2 is a hypersensitive transition, whose intensity is very sensitive and apt to be influenced by the host environment [36, 37]. From the figure, we can clearly see that 5D0 → 7F2 (the electric dipole transition emission peak) is significantly stronger than the 5D0 → 7F1, which also indicates that Eu3+ has the lower symmetric coordination environment. The dynamics of the Eu3+ PL (photoluminescence) was measured at room temperature (Fig. 4). The luminescence decay was successfully fitted by ways of a single exponential law (square correlation factor R2 > 0.99). The luminescence lifetime and quantum yield of 1 are 1.32 ms and 25.30%, which are significantly longer and higher than the values obtained for reported Eu3+ coordination polymers in the solid state at room temperature [38, 39]. The major reason for which may have two factors: (a) there are not coordination waters and OH groups in 1, which cannot quench emission and shorten luminescence lifetime through non-radiative relaxation processes [40]; (b) in 1, each EuIII cation is coordinated by a HIN ligand and four H2IDA ligands that more effectively sensitize the EuIII emission.
As shown in Fig. 5, complex 2 displays the characteristic emissions of the Sm(III) ion at 565, 596, 646, and 709 nm, which correspond to the 4G5/2 → 6HJ (J = 5/2, 7/2, 9/2, 11/2) transitions. The dynamics of the Sm3+ PL was measured at room temperature (Fig. S7). The luminescence decay was successfully fitted by ways of a single exponential law (square correlation factor R2 > 0.99). From the decay curve of 4G5/2 → 6H9/2 from Sm3+ in 2 under 400 nm excitation, the decay lifetime was determined to be 35.5 μs. The quantum yield of 2 is 1.20%, which is higher than the values obtained for reported Sm3+ coordination polymers in the solid state at room temperature [41].
Conclusions
In summary, two novel two-dimensional lanthanide (III) coordination polymers constructed from isonicotinic acid and iminodiacetic acid have been synthesized. Of particular interest, 1 and 2 show good luminescence properties. Further systematic studies for the design and synthesis of such crystalline materials based on the HIN and H2IDA ligands are currently under way.
References
K. Liu, B. Y. Li, Y. Li, X. Li, F. Yang, G. Zing, Y. Peng, Z. J. Zhang, G. H. Li, Z. Shi, S. H. Feng, and D. T. Song (2014). Chem. Commun. 50, 5031.
Y. Liu, S. F. Wu, G. Wang, G. P. Yu, J. G. Guan, C. Y. Pan, and Z. G. Wang (2014). J. Mater. Chem. A. 2, 7795.
H. M. Yin, J. Q. Wang, Z. Xie, J. H. Yang, J. Bai, J. M. Lu, Y. Zhang, D. H. Yin, and J. Y. S. Lin (2014). Chem. Commun. 50, 3699.
O. Kozachuk, I. Luz, F. X. L. Xamena, H. Noei, M. Kauer, H. B. Albada, E. D. Bloch, B. Marler, Y. M. Wang, M. Muhler, and R. A. Fischer (2014). Angew. Chem. Int. Ed. 53, 1.
K. Manna, T. Zhang, and W. B. Lin (2014). J. Am. Chem. Soc. 136, 6566.
M. Yoon, R. Srirambalaji, and K. Kim (2011). Chem. Rev. 112, 1196.
Z. Q. Xu, W. Meng, H. J. Li, H. W. Hou, and Y. T. Fan (2014). Inorg. Chem. 53, 3260.
P. Dechambenoit and J. R. Long (2011). Chem. Soc. Rev. 40, 3249.
S. Rojas, E. Quartapelle Procopio, F. J. Carmona, M. A. Romero, J. A. R. Navarro, and E. Barea (2014). J. Mater. Chem. B. 2, 2473.
D. X. Ma, B. Y. Li, X. J. Zhou, Q. Zhou, K. Liu, G. Zeng, G. H. Li, Z. Shi, and S. H. Feng (2013). Chem. Commun. 49, 8964.
M. Zhang, G. Feng, Z. G. Song, Y. P. Zhou, H. Y. Chao, D. Q. Yuan, T. T. Y. Tan, Z. G. Guo, Z. G. Hu, B. Z. Tang, B. Liu, and D. Zhao (2014). J. Am. Chem. Soc. 136, 7241.
M. Li, D. Li, M. O’Keeffe, and O. M. Yaghi (2014). Chem. Rev. 114, 1343.
S. L. James (2003). Chem. Soc. Rev. 32, 276.
D. Sarma, M. Prabu, S. Biju, M. L. P. Reddy, and S. Natarajan (2010). Eur. J. Inorg. Chem. 24, 3813.
J. Rocha, L. D. Carlos, F. A. A. Paz, and D. Ananias (2011). Chem. Soc. Rev. 40, 926.
L. D. Carlos, R. A. S. Ferreira, V. D. Bermudez, and S. J. L. Ribeiro (2009). Adv. Mater. 21, 509.
M. D. Allendorf, C. A. Bauer, R. K. Bhakta, and R. J. T. Houk (2009). Chem. Soc. Rev. 38, 1330.
S. Liu, Z. Xiang, Z. Hu, X. Zheng, and D. Cao (2011). J. Mater. Chem. 21, 66493.
Y. Q. Xiao, Y. J. Cui, Q. Zheng, S. C. Xiang, G. D. Qian, and B. L. Chen (2010). Chem. Commun. 46, 5503.
H. B. Zhang, L. J. Zhou, J. Wei, Z. H. Li, P. Lin, and S. W. Du (2012). J. Mater. Chem. 22, 21210.
H. H. Li, W. Shi, N. Xu, Z. J. Zhang, Z. Niu, T. Han, and P. Cheng (2012). Cryst. Growth Des. 12, 2602.
H. Wang, S. J. Liu, D. Tian, J. M. Jia, and T. L. Hu (2012). Cryst. Growth Des. 12, 3263.
J. M. Zhou, W. Shi, N. Xu, and P. Cheng (2013). Inorg. Chem. 52, 8082.
B. Zhao, X. Y. Chen, Z. Chen, W. Shi, P. Cheng, S. P. Yan, and D. Z. Liao (2009). Chem. Commun. 21, 3113.
M. L. P. Reddy and S. Sivakumar (2013). Dalton Trans. 42, 2663.
A. R. Ramya, D. Sharma, S. Natarajan, and M. L. P. Reddy (2012). Inorg. Chem. 51, 8818.
X. J. Gu and D. F. Xue (2007). Inorg. Chem. 46, 5349.
J. B. Peng, Q. C. Zhang, X. J. Kong, Y. Z. Zheng, Y. P. Ren, L. S. Long, R. B. Huang, L. S. Zheng, and Z. Zheng (2012). J. Am. Chem. Soc. 134, 3314.
J. W. Zhao, H. L. Li, Y. Z. Li, C. Y. Li, Z. L. Wang, and L. J. Chen (2014). Cryst. Growth Des. 14, 5495.
R. A. Coxall, S. G. Harris, D. K. Henderson, S. Parsons, P. A. Tasker, and R. E. P. Winpenny (2000). J. Chem. Soc. Dalton Trans. 14, 2349.
L. J. Chen, F. Zhang, X. Ma, J. Luo, and J. W. Zhao (2015). Dalton Trans. 44, 12598.
F. S. Richardson (1982). Chem. Rev. 82, 541.
S. J. A. Pope, B. J. Coe, S. Faulkner, E. V. Bichenkova, X. Yu, and K. T. Douglas (2004). J. Am. Chem. Soc. 126, 9490.
J. C. G. Buünzli and C. Piguet (2005). Chem. Soc. Rev. 34, 1048.
G. J. Sopasis, M. Orfanoudaki, P. Zarmpas, A. Philippidis, M. Siczek, T. Lis, J. R. O’Brien, and C. J. Milios (2012). Inorg. Chem. 51, 1170.
G. R. Choppin and D. R. Peterman (1998). Coord. Chem. Rev. 174, 283.
P. T. Ma, R. Wan, Y. N. Si, F. Hu, Y. Y. Wang, J. Y. Niu, and J. P. Wang (2015). Dalton Trans. 44, 11514.
X. F. Li, Y. B. Huang, and R. Cao (2012). Dalton Trans. 41, 6195.
X. Ma, X. Li, Y. E. Cha, and L. P. Jin (2012). Cryst. Growth Des. 12, 5227.
D. T. Lill, A. Bettencourt-Dias, and C. L. Cahill (2007). Inorg. Chem. 46, 3960.
W. S. Lo, J. H. Zhang, W. T. Wong, and G. L. Law (2015). Inorg. Chem. 54, 3725.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, W., Zhang, CJ., Wang, H. et al. Two Novel Two-Dimensional Lanthanide (III) Coordination Polymers Constructed from Isonicotinic Acid and Iminodiacetic Acid: Synthesis, Structure, and Luminescence Properties. J Clust Sci 28, 2005–2015 (2017). https://doi.org/10.1007/s10876-017-1194-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-017-1194-0