Abstract
Adsorption of NH3 molecule on a carbon nanocone (CNC) was investigated using density functional theory in terms of energetic, structural, and electronic properties. It is mainly demonstrated that (i) the NH3 molecule preferentially tends to attach to the apex of the CNC through its N atom, releasing energy of 54.28 kcal/mol, (ii) the CNC may be a promising candidate in gas sensor devices in order to detect the NH3 molecule, and (iii) the field electron emission current may be enhanced from CNC surface upon the adsorption process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The environmental gas monitoring is recognized as an important field and much research has been focused on the development of suitable gas-sensitive materials for continuous monitoring and setting off alarms for hazardous chemical vapors present beyond specified levels [1–7]. It is known that the NH3 is a low boiling point compound and is volatile. Therefore, effective methods have been highly demanded to monitor and suppress the NH3 for atmospheric environmental measurements and controls [8, 9]. Since carbon nanotube (CNT) was discovered by Iijima [10], the properties and applications of this novel material have been extensively investigated [11–14]. CNTs have been recently emerged as a promising substitute for materials of different properties and various applications in hydrogen storage, gas sensors, textiles, and many more [15–17]. Besides CNTs, carbon nanocones (CNCs) have attracted increasing scientific and technological interests due to their special electronic and mechanical features [18]. Some geometrical defects can be introduced into a graphene network to form nonplanar structures. CNCs are hollow structures exclusively made of carbon having a conical shape and may be supposed as an intermediate structure between a graphene sheet and a nanotube [19]. Their walls can be described as a stacking of conical carbon layers with a graphite-like structure. The fabrication of CNCs has attracted attention because of better mechanical stability at the interface and a reasonably sharp tip structure. The mechanically stable CNC structures usually have lower density than CNTs which makes them appropriate for field emission due to the screening effect [20].
In nanostructure research, molecular interaction (e.g., CO, NO, CH4, and so forth) with the nanostructure surface is a subfield of considerable interest due to potential applications such as storage, chemical sensors, and electronic devices [21–23]. Their high surface area is beneficial to practical gas sensors. The fundamental sensing mechanism for these devices is the modulation of their conductivity as a result of the charge transfer between nanotube and adsorbates [1]. In this study, the interaction of NH3 with CNC will be theoretically investigated based on analysis of structure, energies, electronic properties, etc. The main purpose of this study was obtaining fundamental insights of adsorbed molecules influence on electronic properties of the nanocone, and how these effects can be applied to design more sensitive gas sensing devices.
Computational methods
Geometry optimizations and frequency calculations (to find a real local minimum structure) were performed on a CNC and different NH3/CNC complexes using X3LYP functional [24] with 6-31G (d) basis sets as implemented in GAMESS suite of the program [25]. Energy calculations and density of states (DOS) analysis were done applying the same functional with 6-311G (d,p) basis set. It has been shown that the X3LYP extended functional for DFT significantly improves the accuracy for van der Waals interactions, over the most popular and accurate method, Becke three-parameter hybrid functional combined with Lee–Yang–Parr correlation functional (B3LYP) [26]. The adsorption energy (E ad) of an NH3 molecule on the CNC is obtained using the following equation:
where E(CNC/NH3) is the energy of NH3/CNC complex, and E(NH3) and E(CNC) are referred to the energies of an isolated NH3 molecule and the pristine CNC, respectively. The negative value of E ad indicates the exothermic specificity of the adsorption.
Results and discussion
Geometric optimization
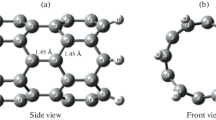
At first, the accuracy of the method used in this study has been tested initially to describe the properties of NH3 molecule in gas phase. The bond length of individual N–H and bond angle of free NH3 from our approach are 1.01 Å and 108°, which are in good consistency with the experimental values of 1.01 Å and 107° [27], respectively. In Fig. 1, we have shown top and side views of the optimized structure of the CNC. The proposed pristine CNC consists of 46 carbon atoms and the dangling bonds of CNC are saturated with hydrogen atoms. The length and height of the optimized pure CNC have been computed to be about 6.97 and 9.06 Å, respectively. Several types of C–C bond are observed in CNC with different lengths.
In order to determine minimum energy adsorption structures, NH3 molecule was initially placed at different positions above the cone surface with different orientations and then relax optimizations have been done. Simplifying, we have considered four most stable ones (Fig. 2) in which the NH3 molecule is as near as possible to the head of CNC including: (P) the NH3 molecule is bonded via nitrogen atom to a C1 atom in top of CNC, (Q) the NH3 molecule is bonded via hydrogen atom to C1 atom in top of CNC, (R) three hydrogen atoms of NH3 adsorbed on cone surface, and (S) nitrogen atom of NH3 adsorbed on cone surface. More detailed information including values of E ad and the charge transfer (Q T) are listed in Table 1.
As shown in Table 1, the E ad values corresponding to various adsorption configurations are in the range of −44.17 to −54.28 kcal/mol. The P configuration gives rise to an E ad of −54.28 kcal/mol, which is higher than that of Q (−46.46 kcal/mol), R (−44.17 kcal/mol), and S configurations (−46.87 kcal/mol). Meanwhile, a large charge transfer (0.736e) from NH3 molecule to the nanocone was predicted. The highest E ad of the P configuration may be rationalized by the fact that, in the CNC, the lowest unoccupied molecular orbital (LUMO) is mainly located on the apex of the CNC (Fig. 3). As a result, the HOMO of NH3, locating on the N atom, donates electrons preferably to the LUMO centered on the apex of the CNC. It has also been found that the CNC undergoes an obvious distortion upon NH3 adsorption via P configuration (Fig. 2), so that the C1–C2 and C1–C3 bonds of CNC in adsorption area were changed from 1.25 and 1.58 Å in pristine form to 1.35 and 1.47 Å, respectively. Also molecular angle of NH3 was increased from 108° in the free molecule to 111° in the adsorbed state. Three other possible stable configurations are Q, R, and S in which the NH3 molecule was adsorbed forming the H–C bond with gas–cone distances of 2.35, 3.00, and 2.77 Å, respectively (see Fig. 2Q–S). Based on the Mulliken population charge analysis, net charge transfers of 0.062e, 0.154e, and 0.023e from the molecule to the cone were occurred in the Q, R, and S, configurations, respectively. For these configurations, especially in the Q case, a locally structural deformation at the adsorption site can be observed, where the adsorbing C atom was pulled out of the surface.
Electronic properties
In the following, we have studied the influence of the NH3 adsorption on the electronic properties of the nanocone. The difference in energies between HOMO and LUMO, E g, was calculated via DOS plots. For the bare CNC (Fig. 1), it can be concluded that it is a semiconductor material with an E g of 1.37 eV. Referring to Fig. 4, it can be seen that the NH3/CNC complexes attain E g value ranging from 1.77 to 1.87 eV. It is well known that the E g (or band gap in bulk materials) is a major factor determining the electrical conductivity of a material and there is a classic relation between them as follows [28]
where σ is the electrical conductivity and k is the Boltzmann constant.
According to the equation, larger E g, at a given temperature, leads to smaller electrical conductivity. For example, in P configuration (as the most stable), the DOS near the conduction and valence levels have a distinct change compared to that of the pristine CNC and the E g increases from 1.37 to 1.87 eV upon the adsorption of NH3 molecule. The considerable change of about 0.50 eV in the E g value demonstrates the high sensitivity of the electronic properties of CNCs towards the presence of NH3 molecule. Therefore, the presences of the NH3 molecules may be detected by calculating the conductivity change of CNC before and after the adsorption process. The results suggest that the CNCs may be promising candidates for serving as effective sensors to detect the NH3.
We think that the proposed sensor may benefit from some advantages including high sensitivity: the E g of CNC is appreciably sensitive towards the presence of NH3 so that it increases by about 36.5 % upon the adsorption of NH3, pristine application: this nanocone can detect the NH3 molecule in its pristine type without manipulating its structure through doping, chemical functionalization, making defect, etc., short recovery time: the adsorption energy of NH3 molecule is not so large to hinder the recovery of CNCs and therefore the sensor will possess short recovery times since, the recovery time can be expressed as (3) based on the conventional transition state theory
where T is the temperature, k is the Boltzmann constant, and ν0 is the attempt frequency. According to this equation, more negative E ad values will prolong the recovery time in an exponential manner.
There has been great interest in field emission properties of nanocones recently [29]. As shown in Table 1, the E FL is significantly shifted to higher energies when the NH3 is adsorbed on the CNC via its N atom (in configurations P and S). For instance, in P configuration, the E FL is increased from −4.82 eV in pristine CNC to −4.00 eV in the NH3/CNC complex. However, these phenomena lead to a decrement in the work function which is important in field emission applications. The canonical assumption for Fermi level is that in a molecule (at T = 0 K) it lies approximately in the middle of the E g. In fact, what lies in the middle of the E g is the chemical potential, and since the chemical potential of a free gas of electrons is equal to its Fermi level as traditionally defined, herein, the Fermi level of the considered systems is at the center of the E g. The Fermi level change of a semiconductor upon the NH3 alters its field emission currents. The work function can be found using the standard procedure by calculating the potential energy difference between the vacuum level and Fermi level, which is the minimum energy required for one electron to be removed from the Fermi level to the vacuum. The decrement in the work function indicates that the field emission properties of the CNC are improved upon the NH3 adsorption. Furthermore, this results in reduced potential barrier of the electron emission for the nanocone, facilitating the electron emission from the CNC surface. All of the above calculations suggest that the NH3 adsorption has an advantageous effect on the field emission properties of CNC and also the CNC may function as a work function-based sensor for NH3 detection.
Conclusions
The interaction between a CNC and NH3 molecule has been explored by DFT calculations in terms of energetic, geometric, and electronic properties. The results indicate that NH3 molecule prefers to be adsorbed on the apex of CNC with appreciable adsorption energy and charge transfer, which would lead to the significant changes of CNC conductance. Thus, the CNC may be a promising candidate for NH3 detection. Furthermore, the NH3 adsorption may reduce the work function of the CNC, thereby, enhancing the field electron emission current from its head.
References
Beheshtian J, Peyghan AA, Bagheri Z (2012) Sens Actuators B Chem 171–172:846
Ayers GP, Gillett RW, Granek H, Deserves C, Cox RA (1997) Geophys Res Lett 24:401
Beheshtian J, Peyghan AA, Bagheri Z (2012) Comput Mater Sci 62:71
Dingle P, Franklin P (2002) Indoor Built Environ 11:111
Baei MT, Peyghan AA, Bagheri Z (2012) Chin Chem Lett 23:965
Beheshtian J, Baei MT, Bagheri Z, Peyghan AA (2012) Microelectron J 43:452
Ahmadi Peyghan A, Omidvar A, Hadipour NL, Bagheri Z, Kamfiroozi M (2012) Phys E 44:1357–1360
Jin Z, Su Y, Duan Y (2001) Sens Actuators B Chem 72:75
Guo P, Pan H (2006) Sens Actuators B Chem 114:762
Iijima S (1991) Nature 354:56
Dinadayalane TC, Kaczmarek A, Lukaszewicz J, Leszczynski J (2007) J Phys Chem C 111:7376
Dinadayalene TC, Murray JS, Concha MC, Politzer P, Leszczynski J (2010) J Chem Theory Comput 6:1351
Peralta-Inga Z, Boyd S, Murray JS, O’Connor CJ, Politzer P (2003) Struct Chem 14:431
Malcioğlu OB, Taşci E, Erkoç Ş (2005) Phys E 28:296
Contreas ML, Avila D, Alvarez J, Rozas R (2010) Struct Chem 21:573
Tetasang S, Keawwangchai S, Wanno B, Ruangpornvistui V (2012) Struct Chem 23:7
Talla JA (2012) Phys B 407:966
Iijima S, Ichihashi T, Ando Y (1992) Nature 356:776
Krishnan A, Dujardin E, Treacy MMJ, Hugdahl J, Lynum S, Ebbesen TW (1997) Nature 388:451
Ge M, Sattler K (1994) Chem Phys Lett 220:192
Beheshtian J, Bagheri Z, Kamifiroozi M, Ahmadi A (2012) Struct Chem 23:653
Hamadanian M, Khoshnevis B, Kalantari Fotooh G (2011) Struct Chem 22:1205
Lithoxoos GP, Labropoulos A, Peristeras LD, Kanellopoulos N, Samios J, Economou IG (2010) J Supercrit Fluid 55:510
Zhang Q, Muller RP, Goddard WA (2005) J Chem Phys 122:014105
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347
Xu X, Goddard WA (2004) Proc Natl Acad Sci 101:2673
Olmsted J, Williams GM (1997) Chemistry: the molecular science. WCB, Iowa
Li S (2006) Semiconductor physical electronics, 2nd edn. Springer, Berlin
Yu SS, Zheng WT (2010) Nanoscale 2:1069
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baei, M.T., Peyghan, A.A. & Bagheri, Z. Carbon nanocone as an ammonia sensor: DFT studies. Struct Chem 24, 1099–1103 (2013). https://doi.org/10.1007/s11224-012-0139-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-012-0139-3