Abstract
Quantum chemistry calculations were performed using density functional theory (DFT) to evaluate electronic and sensing properties in the presence and absence of gas molecules HF and H2O of pristine and dope carbon nanotubes (CNTs) zigzag (6, 0), and CNTs doped with gallium and zinc, which have a significant effect on improving the sensing properties. The results appear that the gas molecules (HF and H2O) show weak physisorption on ZnGa-doping CNT with adsorption energy (Ead) ranging from –0.95 to ‒0.21 eV, while a powerful chemisorption molecule on pristine CNT ranging from 0.05 to 0.4 eV. Where we note that the total energy of the cases above increased dramatically at add dopants and with adsorption of gas molecules with total energy (Etotal) ranging from –103 461 to –0.49651 eV. Through our results, we can recommend the use of ZnGa-doped CNT as a gas chemical sensor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
Carbon nanotubes (CNTs) since the discovery it has attracted great interest due to their distinctive chemical, physical, characteristics [1, 2]. Many researchers studied the properties and applications of this discovered and exceptional material [3]. They have wide range of application in nanoelectronics, nano-scaling biotechnology and biosensors [4–6] single-walled carbon nanotubes (SWCNTs) characterized by hollow structure and size large. It is considered a primary material for gas adsorption [7–10], biosensor and chemical sensor device [11, 12]. For example, experimentally, the possibility of CNTs has been verified for adsorption of gas molecules [13, 14], organic vapors [15, 16], biomolecules and different ions [17]. Due to its unique engineering structure of nanotubes, can make it as the electrochemical storage [18]. The researchers confirmed through their new studies that the physical properties of single-wall carbon nanotubes (SWNTs) are clearly affected when absorbed by adsorption of foreign atoms or molecules [19, 20]. For example, found that adsorption of O2, NO2, or NH3 on the surface of semiconducting SWCNTs Affects significantly and clearly the electrical resistance and thermoelectric power [21]. Researchers have suffered greatly from the problem of adsorption of gas molecules on the carbon nanotubes later considered this problem a useful feature of SWCNTs, where it enabled their use as gas sensors of pollutant gases, storage of fuels, and removal of hazardous pollutants from gas streams [22]. The sensing properties of CNTs can be improved to the detection of gases molecules by doping or when there are defects in the structure [23, 24]. The possibilities of using chemically doped CNTs as highly sensitive gas sensors are also under intensive investigation [25]. Recent studies have demonstrated that the use of pure nanotubes to detect gas molecules is ineffective for all gas molecules because it cannot be adsorbed completely on their surfaces. Therefore, the researchers focused their experimental and theoretical research on improving the electronic, structural and sensing properties of pristine tubes through by doping or functionalizing [26–29]. This is why in our work zinc and gallium elements were used to improve the sensing properties of carbon nanotube which limit the practical application of graphene based gas sensors. For improving the sensitivity of CNT towards gas molecules, the method of doping has proved to be very efficient. Doping of graphene with group III atoms such as boron (B), aluminum (Al), gallium (Ga), it was found that the sensing properties of the doped graphene were better than pure graphene [30, 31]. In our search fluoride gas (HF) was adsorbed on the surface of pristine and ZnGa doped CNT as it is extensively utilized in the petrochemical industry as a part of superacids, it is very toxic and affects human health [32]. And also H2O molecule was adsorbed Because it causes a serious environmental problem due to its large and increasing proportion in the atmosphere, as well as led to the elevation of earth temperature [33, 34]. In the current research, DFT calculations are performed to elucidate the relationship between the electronic structures of pristine and ZnGa-doped CNTs and the adsorption of (H2O and HF) molecules in order to reveal some clues for chemical sensor design. For this aim, at first step, all structures of nanotube/(H2O and HF) complex at different configurations have been optimized, and then, the electronic structure, properties, quantum parameters, adsorption energies, band gaps, HOMO and LUMO orbital parameters of all models of CNTs are investigated.
2 COMPUTATIONAL DETAILS
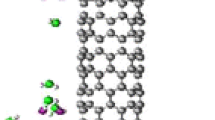
In this work, zigzag (6, 0) single-walled carbon nanotubes SWCNTs was designed using Avogadro program, it’s consisting of 60 atoms of carbon with 300 electrons (Fig. 1). In the first step, the structures were allowed to relax by all atomic geometrical parameters in the optimization at the DFT level of B3LYP exchange functional and 6-31G (d) standard basis set using the Gaussian 03 set of programs [35].
Where we doping carbon nanotubes with zinc and gallium atoms with the doping ratio reached 1.6% as shown in Fig. 2. The adsorption energy (Eads) of (HF and H2O) molecules on the pristine and ZnGa-doped CNT was calculated as follows:
where E(pristine system) and E(doping system) represent total energies of the gas molecules on the CNT and ZnGa–CNT, respectively, ECNT and EZnGa–CNT represent the energies of the isolated CNT and ZnGa–CNT and Egas is the energy of the isolated gas molecule. The diversity of relative energy of the highest occupied (HOMO) and the lowest unoccupied molecular orbital (LUMO) of free ZnGa–CNT and adsorbed molecule on ZnGa–CNT demonstrated the mechanism of interaction [36].
3 RESULTS AND DISCUSSION
The optimum bond length for (C–C) is about 1.45 Å for pristine carbon nanotube (PCNTs) as shown in Fig. 1a, which is in agreement with previous results [37]. Figure 2 shows the obtained optimized structures of Zn–CNT and Ga–CNT and it retains the planar geometry of pristine CNT as seen in Fig. 2. The dopant atom-carbon distance is found to be d(Zn–CNT) = 1.86 Å in Zn–CNT and d(Ga–CNT) = 1.86 Å in Ga–CNT (see Fig. 2).
Table 1 explains the electronic properties of PCNTs and the effect of Zn and Ga doping to CNT, where (HOMO) and (LUMO) energies and also the DOS analysis values were used for theirs.
Based on the results shown in Table 2, the energy gap (Eg) of carbon nanotube at the doping with Zn and Ga is gradually reduced and thus leads to an improvement in the electronic and sensing properties of carbon nanotubes.
We also studied the adsorption properties of PCNT and ZnGa-doped CNT to H2O and HF. Figures 3, 4 and Table 2 show the most stable adsorption structures and corresponding data for one gas molecule adsorption on ZnGa–CNT. Two molecules have been being placed on sites on nanotubes (parallel to NT), it is found that all the gas molecules tend to adsorb near the dopant (Zn and Ga atoms) site due to its high adsorption activity. H2O and HF interact with ZnGa-doped CNT with physisorption, due to the polyvalent properties of the zink and gallium atoms. Where we note that the total energy (Etotal) of the PCNTs and ZnGa-doped CNT and with the presence of above gas molecules increased dramatically ranging from ‒103 461 to –0.49651 eV, this is proof that the impurities used in our research were the best option to increase the sensing of nanotubes.
The chemisorption between gas molecules and the CNTs surface in the adsorption process causes breakage of gas molecules structure, thus reducing the usability of the gas sensor because it impedes the absorption of the gas molecule. The adsorption distance between the gas molecules and the pristine and the doping CNT are 1.5 Å. It can be noticed from Table 2, the Eg for undoped and doped CNT with the presence of gas molecules are larger than those without the above gases. Thus, PCNT and ZnGa-doped CNT, in particular, can be used in manufacturing sensors for the detection of the molecules (H2O and HF).
The electronic density of states (DOS) describes the energy-level distribution of electrons and it is important for experimental measurements. The difference in DOS between the bare ZnGa–CNT and the complex of ZnGa–CNT with the studied gases has features that extend through the entire range of energies; those close to the Fermi level are the most relevant for our discussion. It is known that the Fermi level is a thermodynamic quantity represents the chemical potential for electrons. DOS plots of the isolated ZnGa–CNT and its complexes with guest molecules are presented in Figs. 5–7.
These plots demonstrate that ZnGa–CNT composites are conductors and that their Fermi level lies in the middle of the gap. The energy value of Fermi levels for three complexes is between 3.89 to 4.41 eV and the interaction of the gaseous molecule has a significant effect on its variation. Overall, the Fermi levels shift significantly to valance bond for the H2O, however, HF shows greater shifting behavior than H2O. HOMO, LUMO energy levels and the energy gap are additional parameters may be extracted from the DOS plot.
4 CONCLUSIONS
In this work, we studied the effect of the adsorption of gas molecules (H2O and HF) on the surface of PCNTs and ZnGa-doped CNT by using the density functional theory (DFT) calculations, as well as structural and electronic properties including bond lengths, bond angles, energy gaps, molecular orbital energies, adsorption energy and total energy. Our results suggest that the ZnGa-doped CNT are more favorable than PCNT models for gases adsorption due to large adsorption energy (Ead) at doping. Thus can be used to design nanostructure as chemical sensors, and PCNTs and ZnGa-doped CNT could be used to build sensors for the detection to purify the air of pollutants.
REFERENCES
S. Iijima, Nature (London, U.K.) 354 (6348), 56 (1991).
C. Liu et al., Science (Washington, DC, U. S.) 286 (5442), 1127 (1999).
E. Zurek and J. Autschbach, J. Am. Chem. Soc. 126, 13079 (2004).
O. Zhou et al., Acc. Chem. Res. 35, 1045 (2002).
Z. Yao et al., Nature (London, U.K.) 402 (6759), 273 (1999).
Y. Kong et al., MRS Online Proc. Library Archive 773 (2003).
D. S. Rawat, M. M. Calbi, and A. D. Migone, J. Phys. Chem. C 111, 12980 (2007).
J. Zhao et al., Nanotechnology 13, 195 (2002).
Y. S. Choi et al., J. Am. Chem. Soc. 126, 9433 (2004).
O. Byl et al., J. Am. Chem. Soc. 125, 5889 (2003).
A. Upadhyay et al., Int. J. Electrochem. Sci. 6, 3466 (2011).
G. E. Froudakis et al., Phys. Rev. B 68, 115435 (2003).
J. Kong et al., Science (Washington, DC, U. S.) 287 (5453), 622 (2000).
X. Feng et al., J. Am. Chem. Soc. 127, 10533 (2005).
J. Li et al., Nano Lett. 3, 929 (2003).
S. Agnihotri et al., Carbon 44, 2376 (2006).
Z. Liu et al., Nano Res. 2, 85 (2009).
M. Hirscher et al., Appl. Phys. A 72, 129 (2001).
J. Cheng et al., Carbon 42, 2019 (2004).
S. Peng et al., Chem. Phys. Lett. 387, 271 (2004).
S. Dag, O. Gülseren, and S. Ciraci, Chem. Phys. Lett. 380, 1 (2003).
R. Q. Long and R. T. Yang, J. Am. Chem. Soc. 123, 2058 (2001).
S. Peng and K. Cho, Nano Lett. 3, 513 (2003).
S. B. Fagan et al., Nano Lett. 4, 1285 (2004).
P. G. Collins et al., Science (Washington, DC, U. S.) 287 (5459), 1801 (2000).
W. An et al., J. Phys. Chem. C 111, 14105 (2007).
R. Wu et al., J. Phys. Chem. C 112, 15985 (2008).
J. Beheshtian et al., Struct. Chem. 23, 653 (2012).
W. An and C. H. Turner, Chem. Phys. Lett. 482, 274 (2009).
S. Sharma and A. Verma, Phys. B (Amsterdam, Neth.) 427, 12 (2013).
Y. Mao, J. Yuan, and J. Zhong, J. Phys.: Condens. Matter 20, 115209 (2008).
Y. Sun et al., Solid State Commun. 150, 1906 (2010).
Z. Chen et al., Front. Environ. Sci. Eng. 7, 326 (2013).
X. Xu et al., Microporous Mesoporous Mater. 62, 29 (2003).
M. J. Frisch et al., Gaussian 03 (Gaussian Inc., Pittsburgh PA, 2003).
H. R. Jappor and S. A. M. Khudair, Sensor Lett. 15, 432 (2017).
A. M. Khudhair, F. N. Ajeel, and M. H. Mohammed, Int. J. Nano Dimens. 8, 82 (2017).
ACKNOWLEDGMENTS
The authors would like to thank the Iraqi ministry of higher education and scientific research for its care of scientific researchers through the Iraqi virtual science library (IVSL) and to my wife.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hassan, A.j. DFT Study of HF and H2O Adsorption on Zn and Ga-Doped Single-Walled Carbon Nanotube. Russ. J. Phys. Chem. 94, 1636–1642 (2020). https://doi.org/10.1134/S0036024420080105
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420080105