Abstract
General principles of the analysis of intermetallic compounds with the program package TOPOS are considered. The nanocluster method is described in detail, which lies in the base of the TOPOS “Nanoclustering” procedure. The applications of the nanocluster method to intermetallic compounds as well as to porous materials are comprehensively overviewed. The perspectives of extending the nanocluster model to other classes of inorganic compounds are outlined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
“We are builders. We see that complicated structures can be described with simple building blocks, with units of simple structures. We are constructing the mathematics behind them.” (Andersson and Jacob [1]).
Introduction
The crystal chemistry of intermetallic compounds is one of the most difficult parts of materials science thanks to extraordinary complexity of their crystal structures. Many intermetallics have hundreds or thousands of atoms in large unit cells (up to 23,256 atoms in the cubic unit cell with a = 71.5 Å in an Al–Cu–Ta phase [2]). The coordination numbers of atoms are also very high (typically 10–16, up to 28 for interstitial Cs atoms in the inorganic clathrate Cs8Na16Ag5.9Ge131.1 [3]) and often are not well-determined because of different strength of interatomic contacts. Yet the forms of coordination polyhedra are diverse and cannot often be related to a definite type. As a result, modeling of intermetallic structures is usually a non-trivial task; moreover, some structures challenge crystal chemists for years having no conventional description so far.
A good example of such challenge is the so-called Samson’s monsters that are intermetallic phases NaCd2, β-Mg2Al3, and Cd3Cu4 obtained and described by Sten Samson in the 1960s [4–7]. Each of these phases contains more than 1,000 atoms in a cubic unit cell, which have various local environments. Besides Samson, other authors [8–12] proposed alternative models for the Samson’s monsters, using different building units, but none of these models was commonly accepted.
Recently [13], we proposed a method for description of any intermetallic compound following a universal algorithm that was implemented into the program package TOPOS. We called it nanocluster method because the crystal structure is represented as a set of non-interpenetrating clusters of nanosize. Using the nanocluster method, we described all the Samson’s monsters within a common scheme [13–15]. Further applications to other extremely complicated intermetallic structures [16–18] proved the universality of the method, however, had required its further development. Currently, the nanocluster method can also be applied to search for regularities in crystal architecture of large samples of intermetallic structures [19]. In this article, we consider the improved method in much more detail than before [13] to show its applicability to other classes of inorganic compounds.
The program package TOPOS: destination and philosophy
To continue the epigraph to this paper (all things should develop!), I would add at its end: “…and computer programs as the builders’ main tools”. The complexity of intermetallic structures especially requires computer methods, without which even a good mathematical background has no practical outcome. TOPOS was initially conceived to make the geometry and topology behind the crystal structure accessible for non-mathematicians. It has been developed since 1989 and its up-to-date version is available for free at http://www.topos.samsu.ru. The TOPOS philosophy is based on two principles:
-
(i)
Objectivity: the computer crystallochemical analysis should rest upon strict algorithms that require minimal participation of the user; moreover, the basic statements of the algorithms should have a clear physical background. Unlike traditional visual (even computer-aided) analysis, when the investigator follows the motto “I see it in that way”, most TOPOS procedures do not require drawing any pictures; they make conclusions themselves. The human preserves a key role: he has to correctly formulate the task and extract physical meaning from the results. For example, the objective analysis of Samson’s monsters should give all possible models within a particular set of options; the user will decide which one(s) should be preferred. At the end he can conclude “I have obtained all possible solutions and I choose this one by these reasons”.
-
(ii)
Completeness: to verify a new model one has to consider all available data on similar structures; the model should fit all of them; otherwise it should be improved or discarded. The model is almost useless if it accounts for just one structure. This was typical for intermetallic ‘monsters’: each of them was described by its own unique model.
These two principles are provided in TOPOS with a number of models and algorithms being essentially independent of chemical nature of the crystal structure. We consider below some of them to be especially useful for intermetallic structures.
Crystal structure as a periodic net
Chemistry begins when at least two atoms are connected together forming a stable associate. Hence the bonding in crystals is the main subject of crystal chemistry. If we rest upon standard crystallographic data (space group, unit cell dimensions, atom coordinates) the bonding should be described as a connectivity of crystal space that is expressed in terms of its topological properties. If atoms in a crystal are represented as points, the topology can be defined as a set of links (segments) connecting the points; in this case, the crystal space is modeled as an infinite periodic graph whose nodes and edges coincide with atoms and interatomic bonds, respectively. If the graph has no disconnected parts (that is typical for intermetallics), it is called net, so further we shall deal with periodic nets.
To construct a periodic net, TOPOS incorporates several methods based on Voronoi partition of the crystal space. Since the bonding in intermetallics is essentially non-directional, the simplest Voronoi approach can be used, when each face of the atomic Voronoi polyhedron is assumed corresponding to a bond (Fig. 1, left). As a result, we obtain the complete representation that can be used to generate other structure representations (see below). To estimate the relative bond strength, the solid angle of a Voronoi polyhedron face can be used (Fig. 1, right).
Being specified, the periodic net unambiguously determines the crystal structure topology as defined above. To compare topologies of different nets, TOPOS uses a combination of topological indices: coordination sequence, which contains the numbers of atoms in subsequent coordination shells of a given atom, as well as point and vertex symbols, which give the information about minimal cycles and rings in the net [20]. The nets with the same set of topological indices are assumed having the same topology and belonging to the same topological type. The sets of topological indices for more than 70,000 topological types are deposited to the TOPOS Topological Databases (TTD) collection, using which TOPOS can easily determine the topological type for a particular crystal structure. At present, the TTD collection contains more than 2,000 topological types of intermetallic compounds.
The concepts of structure representation and underlying net
As was mentioned above, the coordination numbers of atoms are not always well-determined in intermetallics. How can one describe the topology in this case? A possible solution lies within the scope of the structure representation concept. We call structure representation such description of the structure that leads to a particular net being referred to as the underlying net of this representation. If one considers all possible interatomic contacts, the corresponding underlying net will be the most complicated; such representation is called complete. One can then reduce the topology of the complete representation using two simplification procedures: (i) ignoring some atoms and/or bonds, and (ii) selecting groups of atoms (clusters) and contracting them into their centroids, but keeping the net connectivity (Fig. 2). Both these procedures are useful for intermetallics: the first one ignores weak interatomic contacts, while the second one relates to cluster models widely used in intermetallics structural chemistry. Reducing the topology results in partial representations that keep a general part of topological information but forget some inessential (for a particular task) details. For instance, the underlying net of a cluster representation describes the topology of cluster packing but ignores the information on the internal structure of the clusters; it is important for the nanocluster method.
Left Clusters in the periodic net of ZrZn22 (complete representation); the corresponding cluster representation [21]. Right The centers of two types of clusters (icosahedron and Friauf polyhedron) are shown as yellow and blue balls, respectively (Color figure online)
The nanocluster method: basic concepts
The complete representation of any structure is quite complicated; in intermetallics, the topology remains weird even if the weakest contacts are omitted (by default, TOPOS ignores all contacts with the Voronoi solid angles less than 1.5 % of 4π steradian). For example, one of the Samson’s monsters, β-Mg2Al3, has the following complete representation for 1,168 atoms in the cubic unit cell (Fig. 3, top). To perceive this structure, one has to simplify it in some way by separating structural units; this method is quite common for crystal chemistry. However, it is well developed only for the first coordination shell; the description of crystal structure as a set of coordination polyhedra is conventional. Nonetheless, for many intermetallics (including β-Mg2Al3) such description hardly elucidates the whole architecture. Therefore, larger local configurations are widely used to facilitate the understanding of such structures. Usually, these configurations are clusters of coordination polyhedra or multi-shell onion-like clusters, but there was no general method to separate them. As a result, β-Mg2Al3 has several alternative cluster models [6, 8–12]. Adopting the TOPOS philosophy, we should invent an unambiguous algorithm to go beyond the first coordination shell; such algorithm has been proposed in the nanocluster method [13].
The nanocluster method is based on several assumptions, which have clear physical meaning.
-
(i)
The structure is composed of multi-shell onion-like primary clusters; topologically each cluster is represented as a shell graph [22]. This means that the shells are being grown according to the net connectivity, not according to geometrical forms of nested polyhedra as in other cluster models. The shell graph imitates the growth of nanoparticles from a seed; the seed can be both a single atom and a simple non-centered cluster like tetrahedron, octahedron, or icosahedron (Fig. 3, middle). This makes the algorithm independent of geometrical distortions, to which the nested-polyhedron models are sensitive. Moreover, the growth of the shell graph is fully predetermined by the net topology. The resulting primary (nano)clusters usually have 2–3 shells (Fig. 3, middle) and the diameter of 1–2 nm that causes the name of the method.
-
(ii)
The centers of primary nanoclusters occupy the most symmetrical positions of the structure. This condition assumes that the prenucleation processes lead to appearance of high-symmetrical local regions (fundamental configurations [13]) that tend to be preserved in the crystal. The existence of such regions means that the short-range forces inside the regions are stronger than between them. The subsequent translational ordering in the bulky phase can distort fundamental configurations to some extent, but their topology remains the same if the distortions are continuous.
-
(iii)
The primary nanoclusters do not interpenetrate, i.e. have no common internal atoms, but can intersect each other, i.e. have common atoms in their outer shells. In practice, this condition restricts the maximal size of primary nanoclusters up to 2–3 shells (Fig. 3, middle). It follows from the concept of fundamental configuration; the interpenetration should lead to absorbing one nanocluster by another and to changing the sizes of the primary nanoclusters to obey this condition. In some cases, the primary nanoclusters can have no common atoms at all, but connect to each other with the external atoms like nanoclusters A in Fig. 3. The resulting set of primary nanoclusters form a packing; one can suppose that in such packing the nanoclusters possess a larger freedom.
-
(iv)
The primary nanoclusters include all atoms of the structure, as a rule. If there are atoms not belonging to the primary nanoclusters, another most symmetrical position should be chosen and one more sort of nanocluster is included into the model until the condition is obeyed. In some cases, small one-shell clusters or separate atoms can fill the gaps between primary nanoclusters, but the number of such spacers should be small. Note that other cluster models usually do not include this condition.
-
(v)
If several models fit conditions (i)–(iv), the model with minimal number of 1–3-shell nanoclusters is preferred. Very rare cases with larger nanoclusters require a special analysis. This parsimony condition implies that the nucleation centers should not be numerous; moreover, the size of the primary nanoclusters should not be too large since the atomic correlation in the melt sharply decreases with distance thanks to thermal motion.
These rules are implemented into TOPOS as the “Nanoclustering” procedure, which outputs all models that obey conditions (i)–(iv) and orders these models by the number of nanoclusters. The “Nanoclustering” procedure can work for a particular crystal structure as well as in a batch mode. Thus both TOPOS philosophy principles are satisfied: we obtain all possible solutions for all crystal structures under consideration according to a strict universal algorithm. The user can treat the different models to get a deeper insight into complicated structures according to the epigraph of this paper.
For instance, the results of the nanocluster analysis of β-Mg2Al3 are shown in Fig. 3. The position 8b is the most symmetrical occupied one in the space group \( Fd\bar{3}m \) so it should be the center of a nanocluster according to condition (ii). Only two shells can be grown around the center until the primary nanoclusters (A) get touched each other (condition (iii)). Not all atoms belong to these nanoclusters, so according to condition (iv) one more center should be chosen; the most symmetrical remaining occupied position is 16c. The resulting primary nanocluster B is also two-shell, and the set of two cluster sorts include all atoms of the structure. The underlying net of this cluster representation has well-known topology of the Laves phase MgCu2. Finally, the Samson monster β-Mg2Al3 can be described as a MgCu2-like net of two-shell nanoclusters A and B in the ratio 1:2 (Fig. 3, bottom) [14].
The nanocluster model provides structural building blocks and the methods of their assembling for intermetallics. Hence, it gives two main ways of classification by searching for all intermetallics: (i) containing the same sorts of nanoclusters, and (ii) having the same methods of cluster assembling (the same underlying nets). Both of these tasks can be solved in TOPOS.
The first way shows how stable a particular configuration is in different intermetallic systems. Indeed, if it occurs in many structure types as well as in crystal structures of different chemical composition, this means that such configuration is essentially independent of the structure peculiarities, can be stable in melt or even in vacuum, and thus can be called fundamental. To find fundamental configurations in crystals, TOPOS realizes a unique algorithm of searching for a finite graph of any complexity in any infinite periodic net. For example, nanocluster B was found in 44 structure types, which include 2,366 intermetallic compounds [14], so this configuration can be considered fundamental. It is important, that the user can search for any configurations, including those obtained by simulation methods. This is a possible way to check the correctness of theoretical modeling schemes.
The second way leads to a better understanding of methods of crystal formation. Quite different structures can be assembled according to the same scheme. For instance, three intermetallic structure types, ZrZn2, ZrZn22, and NaCd2 are built similarly as they have the same underlying nets of the MgCu2 type but decorated with different nanoclusters [14].
The nanocluster model: some results
All results for intermetallic structures obtained with the TOPOS “Nanoclustering” procedure can be arranged in two groups.
Determining primary nanoclusters and the methods of their assembling
In this case, the complete decomposition of the intermetallic structure was performed; the underlying net and the method of assembling nanoclusters were discussed in detail. Most of the structures investigated are cubic; among them there are all Samson’s monsters (Table 1). These, yet not numerous, results allow us to draw some general conclusions.
-
(i)
The number of topological sorts of nanoclusters is not large; they occur in different structure types of intermetallics. To designate nanoclusters, we use the following rules of short notation: (a) the polyhedral core is specified by letters T, O, C, I, Z for tetrahedron, octahedron, cube, icosahedron, and a Frank-Kasper polyhedron, respectively; the number of the polyhedron vertices is given after the letter; (b) the core polyhedron is assumed centered by default, for empty polyhedra the prefix “o” is applied; (c) the numbers of atoms in subsequent shells are separated by the “@” divider; (d) for nanoclusters consisting of n polyhedra the symbol K n is applied, the total number of atoms in the nanocluster is given at the end; for instance, K6i -50 denotes a 50-atom nanocluster composed of six condensed icosahedra.
-
(ii)
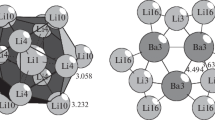
Different primary nanoclusters can have the same polyhedral or even poly-shell internal configurations and differ only by external shells. In particular, the I12@32 configuration corresponding to the Bergman cluster can exist both as a separate primary nanocluster in Mg2Zn11, K6Na15Tl18H, or Tm3In7Co9, and as a part of the three-shell nanocluster I12@32@92 in Li19Na8Ba15.
-
(iii)
Differences in the composition and structure of some intermetallics can be interpreted as minor differences in the composition and structure of primary nanoclusters and/or spacers. For instance, the transition from β- to β′-Mg2Al3 can be considered as a result of “splitting” the nanocluster Z16@44 into two nanoclusters Z15@41 and Z16@43 [14]. Three intermetallics: Mg2Zn11 as well as relating K6Na15Tl18H and Tm3In7Co9 differ only by octahedral spacers: Mg2Zn11 contains only empty octahedra oO6, K6Na15Tl18H includes also centered octahedra O6, while octahedral spacers in Tm3In7Co9 are all centered [18].
-
(iv)
Underlying nets which characterize the methods of assembling can be the same for intermetallics with quite different chemical composition (NaCd2 and Mg2Al3) and structure of nanoclusters (ZrZn22 and Cd3Cu4).
Searching for particular fundamental configurations
As was mentioned above, existence of the same configuration in different intermetallics can indicate the configuration stability. The TOPOS ability to search for a given configuration through the whole database was used as follows.
-
(i)
Searching for a particular configuration that exists as a primary nanocluster in the crystal structure of an intermetallic compound. Such kind of search was performed for nanoclusters Z16@44, I12@50, K4i -46, K6i -50, K8i -86 [13, 14, 23] and showed that all the nanoclusters occur in many intermetallics.
-
(ii)
Searching for a particular multi-shell nanocluster that in not necessary primary but exists as a local configuration in an intermetallic compound. Thus in [19] a comprehensive study of occurrence of so-called Frank-Kasper nanoclusters (i.e. the nanoclusters that are based on a Frank-Kasper I12, Z14, Z15, or Z16 polyhedral core) was undertaken. As a result, several new fundamental configurations were found that represent two-shell nanoclusters being stable in many intermetallics. In particular, the analogs of well-known Bergman and Mackay clusters were revealed that are based on the Friauf (Z16) polyhedron.
-
(iii)
Searching for a model nanocluster that was generated by a simulation method. In [13], it was shown that many model clusters from the Cambridge Cluster Database (http://www-wales.ch.cam.ac.uk/CCD.html) exist as local configurations in intermetallics. Such kind of search could be an important proof of the cluster stability; this proof would rest upon experimental data and would be independent of the simulation method and the force field.
-
(iv)
Searching for hybrid configurations that are composed of several nanoclusters thanks to their intergrowth. In [13], such configurations were called cluster–centaurs; they represent the process of transformation of different kinds of nanoclusters to each other (Fig. 4). By some reason, this process can be “frozen” at some stage that leads to conservation of the cluster-centaurs in the crystalline phase.
The nanocluster model: beyond intermetallics
The nanocluster model is naturally applicable to intermetallics, but it could be extended to any crystal structures that are assembled of finite polyatomic structural units. In [24], a special modification of the model was elaborated for zeolites and other porous materials. The only difference concerns the first statement: instead of nanoclusters, the zeolite framework is considered to be built of minimal cages (natural tiles) that form the single natural tiling whose skeleton coincides with the framework (Fig. 5). The natural tiles are always empty and can be unambiguously determined with the algorithm described in [25]. The net of the centers of natural tiles (dual net) coincides with the underlying net in the nanocluster model and hence shows the method of assembling the zeolite framework from cages. It was shown [24] that all known 194 types of the zeolite frameworks are built with 308 combinatorially distinct types of natural tiles that are embedded into TOPOS as an electronic library. The “Nanoclustering” procedure was extended with the natural tiling approach and can be applied to determine natural tiles and dual nets for porous structures of any complexity. It was used to analyze in detail the methods of assembling the framework of one of the most complex zeolite structures, paulingite (PAU) [26].
Concluding remarks
In this article, we considered merely one way of application of the program package TOPOS. Nonetheless, in our opinion, the nanocluster approach characterizes well the basic TOPOS philosophy. All algorithms mentioned above are strict and work perfectly for any intermetallic compound, moreover, they can easily be improved to explore the crystal structure of other nature. All assumptions of the mathematical models have rather clear physical meaning. The “Nanoclustering” procedure was tested with all known intermetallics and zeolite frameworks. The next step could be a successive consideration of intermetallic structures that will yield comprehensive information on structural units and their assembling motifs to be useful for further theoretical investigations. This information could be stored as a searchable database of nanocluster types and underlying net topologies (like the library of the combinatorial types of natural tiles) that could be used both for analysis of new intermetallic structures and for understanding the assembling processes. As a result, the transformations of a set of nanoclusters into a crystalline phase will be elucidated that will help to build a bridge between nano- and macrostate.
References
Andersson S, Jacob M (1997) The mathematics of structures. R. Oldenburg Verlag, Munich
Weber T, Dshemuchadse J, Kobas M, Conrad M, Harbrecht B, Steurer W (2009) Acta Crystallogr B 65:308
Beekman M, Wong-Ng W, Kaduk JA, Shapiro A, Nolas GS (2007) J Solid State Chem 180:1076
Samson S (1962) Nature 195:259
Samson S (1964) Acta Crystallogr 17:491
Samson S (1965) Acta Crystallogr 19:401
Samson S (1967) Acta Crystallogr 23:586
Yang Q-B, Andersson S, Stenberg L (1987) Acta Crystallogr B 43:14
Bergman G (1996) Acta Crystallogr B 52:54
Fredrickson DC, Lee S, Hoffmann R (2007) Angew Chem Int Ed 46:1958
Feuerbacher M, Thomas C, Makongo JPA, Hoffmann S, Carrillo-Cabrera W, Cardoso R, Grin Y, Kreiner G, Joubert J-M, Schenk T, Gastaldi J, Nguyen-Thi H, Mangelinck-Noël N, Billia B, Donnadieu P, Czyrska-Filemonowicz A, Zielinska-Lipiec A, Dubiel B, Weber T, Schaub P, Krauss G, Gramlich V, Christensen J, Lidin S, Fredrickson D, Mihalkovic M, Sikora W, Malinowski J, Brühne S, Proffen T, Assmus W, de Boissieu M, Bley F, Chemin J-L, Schreuer J, Steurer W (2007) Z Kristallogr 222:259
Dshemuchadse J, Jung DY, Steurer W (2011) Acta Crystallogr B 67:269
Shevchenko VYa, Blatov VA, Ilyushin GD (2009) Struct Chem 20:975
Blatov VA, Ilyushin GD, Proserpio DM (2010) Inorg Chem 49:1811
Blatov VA, Ilyushin GD (2010) Cryst Rep 55:1100
Ilyushin GD, Blatov VA (2010) Russ J Inorg Chem 55:1909
Ilyushin GD, Blatov VA (2010) Cryst Rep 55:1093
Blatov VA, Ilyushin GD (2011) Russ J Inorg Chem 56:729
Blatov VA, Ilyushin GD, Proserpio DM (2011) Inorg Chem 50:5714
Blatov VA, O’Keeffe M, Proserpio DM (2010) CrystEngComm 12:44
Blatov VA, Ilyushin GD (2009) Acta Crystallogr B 65:300
Blatov VA, Proserpio DM (2009) Acta Crystallogr A 65:202
Shevchenko VYa, Blatov VA, Ilyushin GD (2009) Glass Phys Chem 35:1
Blatov VA, Ilyushin GD, Proserpio DM (2010) J Phys Chem C 114:10160
Blatov VA, Delgado-Friedrichs O, O’Keeffe M, Proserpio DM (2007) Acta Crystallogr A 63:418
Ilyushin GD, Blatov VA (2011) Cryst Rep 56:75
Acknowledgments
This work was supported by Russian Foundation for Basic Research (Grant No. 09-02-01269). The author thanks Prof. V. Ya. Shevchenko, Dr. G. D. Ilyushin, and Prof. D. M. Proserpio for fruitful discussions in all aspects of the nanocluster method.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is dedicated to Sten Andersson on the occasion of his 80th birthday.
Rights and permissions
About this article
Cite this article
Blatov, V.A. Nanocluster analysis of intermetallic structures with the program package TOPOS. Struct Chem 23, 955–963 (2012). https://doi.org/10.1007/s11224-012-0013-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-012-0013-3