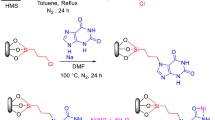
Abstract
The aim of present investigation is the synthesis of mesoporous catalyst based on hexagonal mesoporous silica. HMS support has exceptional properties such as easy synthesis, high surface area, large pore volume and wormhole pores. These distinctive characteristics beseem use of it as a support for synthesis of heterogeneous catalyst. With functionalization of HMS and immobilization of Zr-rhodanine complex into functionalized HMS, HMS/Pr-Rh-Zr was obtained. New synthesized catalyst (HMS/Pr-Rh-Zr) characterized by FT-IR, XRD, TGA, SEM, EDX, adsorption–desorption of nitrogen at 77 K and ICP techniques. These techniques confirmed that the heterogeneous catalyst of HMS/Pr-Rh-Zr was successfully synthesized. Also the catalytic activity of HMS/Pr-Rh-Zr is investigated for the synthesis of tetrahydrobenzo[b]pyran derivatives from reaction between aldehyde, malononitrile, dimedone in PEG at 80 °C and synthesis of 1,4-dihydropyrano[2,3-c]pyrazole derivatives from reaction between aldehyde, ethyl acetoacetate, malononitrile and hydrazine hydrate in H2O:EtOH at 35 °C. These results confirmed the HMS/Pr-Rh-Zr exhibited good catalytic performance. Furthermore, the synthesized heterogeneous catalyst could be recovered after the reaction and reused several times without any noticeable loss in activity. Also zirconium leaching of HMS/Pr-Rh-Zr was studied whereupon metal leaching of the catalyst was very low.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most challenging subject in the contemporary chemistry and chemical industry is the extension and advancement of eco-friendly technologies. In this light, the removal or reduction of wastes by using of eco-friendly solvents and reagents and efficiently reusable catalysts are significant parameters to achieve more sustainable approaches in agreement with green chemistry principles [1].
In porous solids the greater number of surface atoms can be lead to specific surface area whereupon trepan higher material’s reactivity consequently improved efficacy in relevant applications. The porous material classified into three groups such as microporous (less than 2 nm), mesoporous (between 2 and 50 nm) and macroporous (greater than 50 nm) [2]. Among various mesoporous materials, hexagonal mesoporous silica (HMS) are a promising group of porous materials with distinctive characteristics such as wormlike mesoporosity, large wall thickness [3], short channel, thermal stability, easily synthesis and functionalization [3, 4]. Owing to unique structural property of mesoporous materials like uniform pore size distribution, high surface area and large pore volume [3], these porous structures have been employed and studied in a wide variety of different fields from support for heterogeneous catalysts [3], adsorbents, drug delivery systems, biosensors [5], host for guest molecules and advanced engineered materials [6]. The post-synthetic grafting strategy demonstrates a simple route for immobilization of functional chelating ligands into the pores, to obtain the organo-functionalized mesoporous silicas that could be acted as catalyst [7].
Various research groups around the world are used multi-component reactions for the formation heterocyclic compounds [8]. Many uses of heterocyclic compounds such as benzopyran and pyrano[2,3-c]pyrazole have been recognized and are widely used in medicinal chemistry and biosciences. With a general look at drugs including antimicrobial [9, 10], anticancer [11, 12], anti-inflammatory [13, 14] and antioxidant [15, 16], it can be easily understood that these heterocyclic compounds display significant roles in medicinal.
In the continuation of our earlier work [4, 17,18,19,20] and based on the above descriptions, we sought to synthesized new catalyst-based HMS. For this light, the support of HMS was synthesized and functionalized with (3-chloropropyl)trimethoxysilane (CPTMS) to afford HMS/CPTMS. Then, reaction of HMS/CPTMS with rhodanine and ZrOCl2.8H2O leads to synthesis of HMS/Pr-Rh-Zr. The influences of new mesoporous catalyst were investigated for the synthesis of tetrahydrobenzo[b]pyran by one-pot multi-component reactions of aldehyde, malononitrile, dimedone in PEG at 80 °C and synthesis of 1,4-dihydropyrano[2,3-c]pyrazole from reaction between aldehyde, ethyl acetoacetate, malononitrile and hydrazine hydrate in H2O:EtOH at 35 °C.
Experimental
Materials and physical measurements
All reagents and solvents were purchased from Aldrich and Merck chemical companies. The FT-IR spectra were conducted as KBr pellets by FT-IR, VERTEX 70, Bruker, Germany, spectroscopy. The analysis of X-ray diffraction was performed using XRD, X’Pert PRO MPD, PANalytical, Netherland, Kα = 1.54. The instruments’ thermogravimetric analysis (TGA) applied was performed from room temperature to 800 °C by TGA, NETZSCH, Germany. The used gas for the thermogravimetric analysis was Argon. The content of Zr was determined by inductively coupled plasma optical emission spectrometry (ICP-OES, Arcos EOP, company of Spectro, Germany). Scanning electron microscopy (SEM) images were recorded on FE-SEM, TESCAN MIRA III, Czech. Elemental analysis was carried out on EDX, FE-SEM, TESCAN MIRA, SAMX, Czech. The nitrogen adsorption–desorption isotherm was performed with a BET, Micromeritics, Asap2020, USA at 77 K. The degassing temperature and treatment time in this analysis were 120 °C and 2 h, respectively. 1H-NMR spectra of the DMSO-d6 solutions were obtained at 300 MHz using TMS as an internal standard.
Synthesis of HMS/Pr-Rh-Zr
Synthesis of HMS
HMS was prepared according to previous report in the literature [4]. In a typical experiment, dodecylamine (5 g) was dissolved in 70% w/w ethanol aqueous solution. Then, tetraethyl orthosilicate (TEOS, 20.8 g) was added dropwise, stirred for 5 h at room temperature under vigorous stirring. The mixture was aged for 18 h at room temperature and filtered and dried at room temperature. Finally, the resultant solid was soxhelt extraction at 80 °C for 24 h. In order to the removing the template, synthesized support was calcined at 500 °C in air for 5 h. Finally, HMS was obtained.
Synthesis of HMS/CPTMS
Following a general procedure, HMS have been functionalized with 3‐chloropropyltrimethoxysilane, in toluene, as follows: HMS (0.5 g) in toluene and 3‐chloropropyltrimethoxysilane (1.5 mL) was added dropwise, refluxed for 24 h, under nitrogen atmosphere. Then, the resulting silica (HMS/CPTMS) was collected by filtration, then washed with toluene and dried at room temperature.
Synthesis of HMS/Pr-Rh
In this step, HMS/CPTMS (1 g) was dispersed in DMF for 30 min and reacted with rhodanine (2 mmol) at 100 °C for 28 h to afford HMS/Pr-Rh. After that, the resulting solid was filtered and washed with water and ethanol.
Synthesis of HMS/Pr-Rh-Zr
Finally, slightly catalyst (HMS/Pr-Rh-Zr) was synthesized by adding of 2.5 mmol ZrOCl2·8H2O to 1 g of HMS/Pr-Rh in CH3CN at room temperature for 24 h. The reaction mixture was filtered, washed several times with H2O and dried at room temperature to overnight.
General procedure for the synthesis of tetrahydrobenzo[b]pyran
0.05 g of HMS/Pr-Rh-Zr was added to a mixture of aldehyde (1 mmol), dimedone (1 mmol) and malononitrile (1 mmol) in PEG at 80 °C. Completion of the reaction was monitored by TLC. After the completion of the reaction, ethyl acetate was added and HMS/Pr-Rh-Zr was separated by filtration. Then, ethyl acetate and H2O were added and extracted. The organic layer was dried with anhydrous Na2SO4. After evaporation of solvent, desired compound was obtained then purified through recrystallization in EtOH.
General procedure for the synthesis of 1,4-dihydropyrano[2,3-c]pyrazole
A molar ratio mixture of aldehyde (1 mmol), hydrazine hydrate (1 mmol), ethyl acetoacetate (1 mmol) and malononitrile (1 mmol) in the presence HMS/Pr-Rh-Zr (0.01 g) as catalyst was reacted in H2O:EtOH (3:1 mL) at 35 °C. Completion of the reaction was continuously observed by TLC. After the consumption of the starting material, the catalyst was separated with filtration then hot EtOH was added. Finally, recrystallization with EtOH was applied to afford the pure products.
Characterization data of selected compounds
2–Amino–3–cyano–7,7–dimethyl–4-(2-nitrophenyl)–5–oxo-4H–5,6,7,8-tetrahydro benzopyran (Table 3, entry 5): 1H NMR (300 MHz, DMSO-d6): δ = 0.87 (s, 3H), 1.00 (s, 3H), 2.00 (d, J = 15 Hz, 1H), 2.19 (d, J = 18 Hz, 1H), 2.41–2.56 (m, 2H), 4.93 (s, 1H), 7.17 (s, 2H), 7.33–7.44 (m, 2H), 7.65 (t, J = 9 Hz, 1H), 7.80 (d, J = 9 Hz, 1H) ppm.
2–Amino–3–cyano–7,7–dimethyl–4-(4-methylphenyl)–5–oxo-4H–5,6,7,8-tetrahydro benzopyran (Table 3, entry 7): 1H NMR (300 MHz, DMSO-d6): δ = 0.94 (s, 3H), 1.02 (s, 3H), 2.07 (d, J = 15 Hz, 1H), 2.21–2.26 (m, 3H), 2.43–2.55 (m, 3H), 4.11 (s, 1H), 6.94 (s, 2H), 7.00 (d, J = 9 Hz, 2H), 7.07 (d, J = 9 Hz, 2H) ppm.
4,4′-(1,4-phenylene)bis(2-amino-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile) (Table 3, entry 12): 1H NMR (300 MHz, DMSO-d6): δ = 0.98 (s, 6H), 1.02 (s, 6H), 2.03–2.25 (m, 4H), 2.56 (s, 4H), 4.13 (s, 2H), 6.94 (s, 4H), 7.02 (d, J = 6 Hz, 4H) ppm.
6-Amino-4-(4-chlorophenyl)-3-methyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (Table 5, entry 1): 1H NMR (300 MHz, DMSO-d6): δ = 1.78 (s, 3H), 4.62 (s, 1H), 6.92 (s, 2H), 7.19 (d, J = 9 Hz, 2H), 7.36 (d, J = 9 Hz, 2H), 12.13 (s, 1H) ppm.
6-Amino-3-methyl-4-(3-nitrophenyl)- 1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (Table 5, entry 3): 1H NMR (300 MHz, DMSO-d6): δ = 1.80 (s, 3H), 4.87 (s, 1H), 7.04 (s, 2H), 7.61–7.68 (m, 2H), 8.01–8.13 (m, 2H), 12.20 (s, 1H) ppm.
4,4′-(1,4-Phenylene)bis(6-amino-3-methyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile) (Table 5, entry 12): 1H NMR (300 MHz, DMSO-d6): δ = 1.73 (s, 6H), 4.56 (s, 2H), 6.84 (s, 4H), 7.10 (d, J = 3 Hz, 4H), 12.07 (s, 2H) ppm.
Results and discussion
Preparation of HMS/Pr-Rh-Zr
The synthetic route for the HMS/Pr-Rh-Zr is shown in Scheme 1. First, the silylating agent of (3-chloropropyl)trimethoxysilane (CPTMS) reacted with the hydroxyl groups of HMS to obtain HMS/CPTMS. Then, rhodanine was applied to afford HMS/Pr-Rh. Finally, ZrOCl2·8H2O was applied to obtain the HMS/Pr-Rh-Zr.
After fabrication of catalyst, for its characterization, different techniques such as FT-IR spectroscopy, N2 adsorption–desorption, inductively coupled plasma (ICP-OES), X‐ray diffraction (XRD), thermogravimetric analysis (TGA), scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) were applied.
Characterization of HMS/Pr-Rh-Zr
For confirmation of functional groups of the synthesized compounds, the FT-IR spectroscopy was applied. In Fig. 1, the FT-IR spectroscopy of HMS (a), HMS/CPTMS (b), HMS/Pr-Rh (c) and HMS/Pr-Rh-Zr (d) indicated. FT-IR spectra of HMS (a) demonstrated the peaks at 3435 cm−1 contributed to silanol group and the peaks at 804 cm−1 and 1073 cm−1 are contributed to Si–O–Si symmetric and asymmetric stretching vibration, respectively [4]. In spectrum of HMS/Pr (b), the C–H stretching vibrations are appearing in the 2928 cm−1. As shown in spectrum of HMS/Pr-Rh (c), the peak at 3429 cm−1 can be ascribed to the O–H stretching vibration, also stretching vibration of C=N appeared at 1659 cm−1. In additional, the C–H stretching vibrations observed in 2926–2975 cm−1. In the spectrum of HMS/Pr-Rh-Zr (d), due to the coordination with the zirconium, the bond of C=N, shifts from 1659 cm−1 to 1636 cm−1 and the O–H stretching vibration shifts from 3429 cm−1 to 3435 cm−1.
Low angle X-ray diffraction (XRD) patterns of HMS and HMS/Pr-Rh-Zr are demonstrated in Fig. 2. These patterns exhibit one sharp reflection at 2θ angles about of 2. The location of peak in XRD pattern of HMS/Pr-Rh-Zr was consistent with the standard diffraction pattern of HMS that reported in the previous literature [21]. As it can be seen, the decrease in intensity of the characteristic diffraction peak contributed to HMS/Pr-Rh-Zr in comparison with HMS was due to immobilization of organic moieties on the pore wall of HMS. Also, this result indicated that immobilization of organic moieties on the pore wall of HMS for synthesis of catalyst does not change the phase of HMS Scheme 2.
The thermogravimetric analysis (TGA) of HMS and HMS/Pr-Rh-Zr is indicated in Fig. 3. The thermal behavior of HMS/Pr-Rh-Zr was evaluated that demonstrated the mass loss peaks: the weight loss below 100 °C contributed to volatilization of the physically adsorbed water and organic solvent, the weight loss about 34% at 100–600 °C attributed to decomposition of organic fragments immobilized in the surface of pores and at above 600 °C related to densification of the silica matrix [7]. Also, in order to determine Zr content loaded in modified HMS in synthesized catalyst, ICP-AES analysis was applied whereupon 0.16 mmol g−1 resulted.
The morphology, sizes and the particle sizes distribution of the synthesized catalyst were performed by a scanning electron microscopy (SEM). These images are shown in Fig. 4. As shown in images, the prepared catalyst has regular and ordered structure with particle sizes of less than 40 nm.
EDX spectrum carried out on HMS/Pr-Rh-Zr demonstrated that synthesized catalyst has constitutive elements including: Si, O, N, C, S and Zr (Fig. 5).
To assess physicochemical and structural parameters of HMS/Pr-Rh-Zr, N2 adsorption–desorption technique was applied (Fig. 6). According to the IUPAC classification, N2 adsorption/desorption isotherm of HMS/Pr-Rh-Zr revealed typically reversible type IV isotherms [4]. BET surface area (SBET), total pore volumes (Vtotal) and pore diameters (DBJH) were evaluated using the adsorption–desorption of nitrogen at 77 K (Table 1). This analysis revealed a mesoporous synthesized catalyst has high surface area.
Investigation of the catalytic activity of HMS/Pr-Rh-Zr for synthesis of tetrahydrobenzo[b]pyran and 1,4-dihydropyrano[2,3-c]pyrazole
After the synthesis, evaluation and affirmation of catalyst, the catalytic efficacy of HMS/Pr-Rh-Zr was investigated for synthesis of tetrahydrobenzo[b]pyran and 1,4-dihydropyrano[2,3-c]pyrazole.
For optimization of the reaction conditions, initially, the catalytic amount was studied, and then, various solvents and different temperatures were optimized (Table 2). At the outset, we checked a different amount of catalyst such as 0, 0.03 g, 0.04 g, 0.05 g and 0.06 g for the reaction of 4-chlorobenzaldehyde (1 mmol), malononitrile (1 mmol), dimedone (1 mmol) and HMS/Pr-Rh-Zr as catalyst, in which 0.05 g of catalyst was found to be the best amount catalyst (90%, Table 2, entry 4). Significant increase in the yield of product was not observed when the amount of catalyst was increased from 0.05 g to 0.06 g. Also, to investigate the effect of metal in synthesized catalyst in promote of the reaction, the reaction was investigated in the presence of HMS/Pr-Rh. That result indicated that the effect of metal in catalytic activity is very important (Table 2, entry 12). Next, various solvents including PEG, H2O, EtOH, solvent-free and mixture of H2O:EtOH (3:1 mL) were checked. We also evaluated different temperatures for the model reaction; the results suggest that the 80 °C is a better temperature for the reaction.
The result clearly reveals that 0.05 g of HMS/Pr-Rh-Zr in PEG at 80 °C was found to be ideal reaction condition for producing an excellent yield of the desired product (Table 2, entry 4).
With the optimized reaction conditions, we next assessed the reaction of various aldehydes including electron-withdrawing and electron-donating substituted aldehyde. Twelve derivatives of tetrahydrobenzo[b]pyran were synthesized in optimal conditions, as reported in Table 3.
In this part a plausible reaction mechanism has been described for the formation of tetrahydrobenzo[b]pyran in the presence of HMS/Pr-Rh-Zr (Scheme 3).
The reaction begins with the generation of arylidenemalononitrile intermediate (I) from the reaction of malononitrile with activated aldehyde. In the next step enolized dimedone (II) reacts with the arylidenemalononitrile intermediate (I) generated from the previous step. Ultimately, intramolecular cyclization and rearrangement occurs leading to the formation of tetrahydrobenzo[b]pyran.
In this part we optimized the reaction by screening various effective factors including: catalyst dosing, type of solvent and temperature for synthesis of 1,4-dihydropyrano[2,3-c]pyrazole. There for, the reaction of 4-chlorobenzaldehyde (1 mmol), malononitrile (1 mmol), ethyl acetoacetate (1 mmol) and hydrazine hydrate (1 mmol) in the presence of HMS/Pr-Rh-Zr as catalyst was chosen as the model reaction. With the evaluation of amount of catalyst (catalyst free, 0.008 g, 0.01 g, 0.015 g and 0.02 g), solvents (H2O, EtOH, PEG, H2O:EtOH (3:1 mL) and solvent-free) and the reaction temperature (25, 35 and 60 °C), we found that 0.01 g of catalyst, mixture of H2O:EtOH (3:1 mL) at 35 °C were the most effective condition for synthesis of 1,4-dihydropyrano[2,3-c]pyrazole (Table 4, entry 3). For indicating necessity of the presence of zirconium in the catalyst, the model reaction undertaken in the presence of HMS/Pr-Rh instead of HMS/Pr-Rh-Zr (Table 4, entry12). The yield of this reaction was obtained 42%.
After optimization of the reaction conditions, a range of functionalized aldehydes (substitute of electron-withdrawing and electron-donating) are used and 1,4-dihydropyrano[2,3-c]pyrazole derivatives were obtained in good yields as summarized in Table 5 (Scheme 4).
A plausible reaction mechanism for the synthesis of 1,4-dihydropyrano[2,3-c]pyrazole using HMS/Pr-Rh-Zr as catalyst is demonstrated in Scheme 5.
The Knoevenagel condensation occurs between malononitrile and activated aldehyde with catalyst, whereupon the intermediate of arylidenemalononitrile (intermediate I) was generated. The condensation reaction between hydrazine and activated ethyl acetoacetate carried out that consequently pyrazolone (intermediate II) was obtained. Finally, the enolized pyrazolone reacted to the arylidenmalononitrile by Michael addition reaction. Then, tautomerization of the intermediate leads to produce 1,4-dihydropyrano[2,3-c]pyrazole [38].
Reusability of the HMS/Pr-Rh-Zr
In the synthesis of catalyst, its recyclability is the most crucial aspects of organic synthesis. In this light, recyclability of HMS/Pr-Rh-Zr toward the synthesis of 1,4-dihydropyrano[2,3-c]pyrazole was studied to establish the reusability of the synthesized catalyst.
After completion of the reaction, hot ethanol was added and the catalyst was separated from the reaction mixture by centrifuge instrument followed drying for overnight. Then, the dried separated catalyst was used for next catalytic run. By this experimental, the results were defined that HMS/Pr-Rh-Zr can be reused for five consecutive runs with minimal decrease in the yield of product (Fig. 7).
Characterization of recycled catalyst
Characterization of the catalyst after the reuse process was done by XRD technique in order to study its stability. The XRD pattern of reused and fresh catalyst is indicated in Fig. 8. As shown in this figure, there is not any change in XRD pattern of the recovered catalyst with fresh catalyst. In addition, the XRD pattern of recovered catalyst showed a good agreement with standard XRD pattern of HMS. This analysis confirmed that the crystalline structure of recovered catalyst has not changed and was strong evidence for the stability of recovered HMS/Pr-Rh-Zr.
Hot filtration test
The hot filtration test was applied to investigate leaching of zirconium in the reaction mixture and to show the nature heterogeneous of catalyst. In this light, the reaction between 4-chlorobenzaldehyde, malononitrile, ethyl acetoacetate, hydrazine hydrate and HMS/Pr-Rh-Zr in H2O: EtOH (3:1 mL) at 35 °C was investigated. In this experiment we found the yield of correspond product in the half time of the reaction (10 min) was 58%. Then, the same reaction was repeated meanwhile after half‐time of reaction (after 10 min), the catalyst was separated and the reaction mixture was permitted to react for another 10 min. In this stage the product was obtained in 63% yield. These results confirmed that leaching of zirconium during the reaction did not occur.
Comparison results of HMS/Pr-Rh-Zr
On the basis of earlier reports, the comparison results of HMS/Pr-Rh-Zr with other catalysts have been collected for synthesis of 2-amino–4-(4-chlorophenyl)-3–cyano-7,7–dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran (Table 6, entry 1–9) and 6-amino-4-(4-chlorophenyl)-3-methyl-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (Table 6, entry 10–15). As shown in Table 6, we find that in the present work, our catalytic system has benefit such as: mild reaction conditions, good to high yields and low reaction times.
Conclusion
In conclusion, in this work we synthesized new complex of zirconium supported in the pores of functionalized HMS which showed that HMS/Pr-Rh-Zr catalyst significantly improved activity. In the next step for confirmation and characterization of synthesized catalyst, several techniques were applied. Finally, the catalytic activity of prepared catalyst assessed for the synthesis of tetrahydrobenzo[b]pyran and 1,4-dihydropyrano[2,3-c]pyrazole derivatives. Additionally, the influences of metal of Zr were investigated on the catalytic activity of HMS/Pr-Rh-Zr that result indicated that in the absence of metal, the reaction underdeveloped. Ultimately, the result of recovery test of synthesized catalyst defined that catalyst could be recovered after the reaction and reused without any noticeable loss in activity.
References
Q. Zhang, Y.H. Gao, S.L. Qin, H.X. Wei, Catalysts 7, 351 (2017)
S. Jafari, H. Derakhshankhah, L. Alaei, A. Fattahi, B.S. Varnamkhasti, A.A. Saboury, Biomed. Pharma. 109, 1100 (2019)
L. Hu, B. Yue, X. Chen, H. He, Catal. Commun. 43, 179 (2014)
F. Gholamian, M. Hajjami, Polyhedron 170, 649 (2019)
B. Moongraksathum, Y.W. Chen, J. Porous. Mat. 26, 51 (2019)
F. Farjadian, S. Azadi, S. Mohammadi-Samani, H. Ashrafi, A. Azadi, Heliyon 4, 1e00930 (2018)
D.F. Enache, E. Vasile, C.M. Simonescu, D. Culita, E. Vasile, O. Oprea, A.M. Pandele, A. Razvan, F. Dumitru, G. Nechifor, RSC Adv. 8, 176 (2018)
T.L. Lambat, S.H. Mahmood, P.V. Ledade, S. Banerjee, Chem. Sel. 5, 8864 (2020)
P.T. Mistry, N.R. Kamdar, D.D. Haveliwala, S.K. Patel, J. Heterocycl. Chem. 49, 349 (2012)
P.M. Ronad, M.N. Noolvi, S. Sapkal, S. Dharbhamulla, V.S. Maddi, Eur. J. Med. Chem. 45, 85 (2010)
J.L. Wang, D. Liu, Z.J. Zhang, S. Shan, X. Han, S.M. Srinivasula, C.M. Croce, E.S. Alnemri, Z. Huang, Proc. Natl. Acad. Sci. 97, 7124 (2000)
S. Singh, A. Ahmad, D.S. Raghuvanshi, M. Hasanain, K. Agarwal, V. Dubey, K. Fatima, S. Alam, J. Sarkar, S. Luqman, F. Khan, S. Tandon, A. Gupta, Bioorg. Med. Chem. Lett. 26, 5322 (2016)
M.E. Zaki, H.A. Soliman, O.A. Hiekal, A.E. Rashad, Z. Naturforsch C 61, 1 (2006)
S.M. Hasan, M.M. Alam, A. Husain, S. Khanna, M. Akhtar, M.S. Zaman, Eur. J. Med. Chem. 44, 4896 (2009)
R.S. Aliabadi, N.O. Mahmoodi, RSC Adv. 6, 85877 (2016)
M. Grazul, A. Kufelnicki, M. Wozniczka, I. Lorenz, P. Mayer, A. Jozwiak, M. Czyz, E. Budzisz, Polyhedron 31, 150 (2012)
F. Gholamian, M. Hajjami, A.M. Sanati, Silicon 18, 1 (2019)
M. Hajjami, F. Gholamian, R.H. Hudson, A.M. Sanati, Catal. Lett. 149, 228 (2018)
F. Gholamian, M. Hajjami, React. Kinet. Mech. Catal. 128, 867 (2019)
M. Hajjami, F. Gholamian, RSC Adv. 6, 87950 (2016)
J. Lee, S. Yoon, S.M. Oh, C.H. Shin, T. Hyeon, Adv. Mater. 12, 359 (2000)
E. Sheikhhosseini, D. Ghazanfari, V. Nezamabadi, Iran. J. Catal. 3, 197 (2013)
J. Lu, X.W. Fu, G. Zhang, C. Wang, Res. Chem. Intermed. 42, 417 (2016)
G.M. Ziarani, A. Abbasi, A. Badiei, Z. Aslani, J. Chem. 8, 293 (2011)
M.A. Bodaghifard, M. Solimannejad, S. Asadbegi, S. Dolatabadifarahani, Res. Chem. Intermed. 42, 1165 (2016)
H. Hu, F. Qiu, A. Ying, J. Yang, H. Meng, Int. J. Molecul. Sci. 15, 6897 (2014)
A.K. Kshirsagar, S.S. Bankar, A.U. Khandebharad, S.R. Sarda, M.G. Soni, B.R. Agrawal, J. Med. Chem. Drug Discov. 1, 720 (2016)
N. Hazeri, M. Lashkari, H. Faroughi Niya, H. Pourbalouch, J. Appl. Chem. Res. 14, 51 (2020)
M. Haghighat, F. Shirini, M. Golshekan, J. Nanosci. Nanotech. 19, 3447 (2019)
A. Saha, S. Payra, S. Banerjee, Green Chem. 17, 2859 (2015)
M. Fatahpour, F.N. Sadeh, N. Hazeri, M.T. Aghsoodlou, M.S. Hadavi, S. Mahnae, J. Saudi Chem. Soc. 21, 998 (2017)
C.S. Maheswari, V. Tamilselvi, R. Ramesh, A. Lalitha, Org. Prep. Proced. Int. 52, 22 (2020)
Y.A. Tayade, S.A. Padvi, Y.B. Wagh, D.S. Dalal, Tetrahedron. Lett. 56, 2441 (2015)
M. Kangani, N. Hazeri, M.T. Mghsoodlou, S.M. Habibi-khorasani, S. Salahi, Res. Chem. Intermed. 41, 2513 (2015)
K.S. Dalal, Y.A. Tayade, Y.B. Wagh, D.R. Trivedi, D.S. Dalal, B.L. Chaudhari, RSC Adv. 6, 14868 (2016)
F. Tamaddon, M.A. Alizadeh, Tetrahedron. Lett. 55, 3588 (2014)
S.S. Ghodk, S.U. Tekale, R.D. Pathrikar, P.M. Khandare, L. Kotai, R.P. Pawar, Eur. Chem. Bull. 9, 38 (2020)
M. Dadaei, H. Naeimi, Polycycl. Aromat. Comp. 1, 14 (2020)
D. Azarifar, Y. Abbasi, Synth. Commun. 46, 745 (2016)
J. Davarpanah, A.R. Kiasat, S. Noorizadeh, M. Ghahremani, J. Mol. Catal. A Chem. 376, 78 (2013)
A.A. Mohammadi, M.R. Asghariganjeh, A. Hadadzahmatkesh, Arab. J. Chem. 10, S2213 (2017)
N. Montazeri, T. Noghani, M. Ghorchibeigy, R. Zoghi, J. Chem. 1, 2014 (2014)
D. Azarifar, M. Khaleghi-Abbasabadi, Res. Chem. Intermed. 45, 199 (2019)
T.L. Lambat, J. Chin. Adv. Mater. Soc. 6, 134 (2018)
G. Brahmachari, B. Banerjee, ACS. Sustain. Chem. Eng. 2, 411 (2014)
Acknowledgements
Authors thank Ilam University and Bu-Ali Sina University for financial support of this research project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Abdolahi, S., Hajjami, M. & Gholamian, F. An approach to the synthesis and characterization of HMS/Pr-Rh-Zr as efficient catalyst for synthesis of tetrahydrobenzo[b]pyran and 1,4-dihydropyrano[2,3-c]pyrazole derivatives. Res Chem Intermed 47, 1883–1904 (2021). https://doi.org/10.1007/s11164-021-04411-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-021-04411-z