Abstract
A new acidic mesoporous catalyst (FSM-16/AEPC-SO3H) was successfully synthesized and characterized by FT-IR, TGA, XRD, SEM, TEM, EDS and BET techniques. The FSM-16/AEPC-SO3H showed excellent catalytic activity for the synthesis of 1,8-dioxo-octahydroxanthene and tetrahydrobenzo[b]pyran derivatives. The synthesized catalyst can also be recycled.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
FSM-16 is one of the mesoporous materials whose pore size in the range 2–50 nm in structure [1] and has attracted attention in the field of adsorption, catalytic chemistry [2], separation membrane, fixing agent for biomolecule and semiconductor cluster, functional electronics and photonics materials [1]. FSM-16 is structurally similar to the MCM-41 solid; both of them have uniform mesoporous channel structure [3] with a hexagonal array [1]. Despite the mentioned similarities, FSM-16 and MCM-41, have the differences such that FSM-16 has higher structural stability than MCM-41 [4]. Therefore, development of synthetic procedures that produce new heterogeneous catalysts based on FSM-16 is an ever-challenging objective.
Itaconic acid was discovered by Baup in 1837 and is a five carbon chemical building block, fully sustainable, non-toxic and inexpensive [5]. Uses for this compound include plastic, rubber, detergents, textile industries, production of biodegradable green plastics and in the synthesis of different heterocycles [6].
1,8-Dioxo-octahydroxanthenes are an important family of heterocycle compound containing phenyl substituted pyran ring which is attached on either side with two cyclohexanone rings [7]. Due to their numerous applications in leuco dyes, in laser technology, as pH-sensitive fluorescent materials for the visualization of biomolecular assemblies [8], in photodynamic therapy [9], in chiroptical molecular switches [10], also their many pharmacological properties such as antinociceptive activities [11], antibacteria, antiviral, anti-inflammatory, anti-depressants and antimalarial agents [9] have attracted much attention on their synthesis with the use of various catalysts. Basically, in order to synthesis the xanthenedione derivatives [7, 12,13,14,15] and benzoxanthene derivatives [16,17,18,19] in the presence of various catalysts have been reported.
Another group of heterocycle compounds containing oxygen are tetrahydrobenzo[b]pyran derivatives. Tetrahydrobenzo[b]pyran has attracted considerable attention not only for the biological properties such as pasmolytic, diuretic, anticoagulant, anticancer, antianaphylactic, antioxidant, antileishmanial, antibacterial, antifungal, hypotensive, antiviral, antiallergenic, and antitumor activities [20] but also for the treatment of neurodegenerative disease, for example, AIDS associated dementia, Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, amyotrophic lateral sclerosis and Down syndrome. Additionally, these compounds widely used as potential biodegradable agrochemicals, cosmetics, photoactive materials and pigments [21].
Thus, in a view of their wide applicability the development highly efficient procedures for the synthesis of tetrahydrobenzo[b]pyran have attracted a great deal of attention [20]. The conventional synthesis involves a three component condensation of malononitrile, an aldehyde and dimedone in different conditions [21,22,23]. Also synthesis of benzopyran derivatives by condensation reaction of substituted salicyaldehydes and substituted 1,3-hexanediones in the presence of catalyst have been reported [24].
2 Experimental
2.1 Materials and Physical Measurements
The chemicals used for this work, were purchased from Merck and Aldrich chemical companies. The FT-IR spectra were measured by FT-IR, Bruker, Germany spectroscopy. The thermogravimetric analysis of catalyst was determined by TGA, PerkinElmer Pyris Diamond, U.K. The analysis of XRD pattern used for the synthesized catalyst was recognized by XRD, X’Pert PRO MPD, PANalytical, Netherland. The energy dispersive X-ray was measured with instruments EDX, TESCAN MIRA, Czech. Also the instruments of Brunauer–Emmett–Teller and scanning electron microscopy have this character respectively: BET, Micromeritics, Asap2020, USA and SEM, FESEM-TESCAN MIRA3. The analysis of transmission electron microscopy (TEM), was done using a Philips 420 transmission microscopy, with an accelerating voltage of 60.0 kV.
2.2 Synthesis of FSM-16/AEPC-SO3H
The FSM-16 was prepared by the method that was presented in literature [25]. The itaconic acid (1 mmol) with ethylenediamine (1 mmol) was reacted in H2O under reflux condition for 24 h [26]. Then the solvent was evaporated to afford N-(2-aminoethyl)-2-pyrrolidone-4-carboxylic acid (AEPC) and recrystallized in EtOH. FSM-16 (1 g) was sonicated in deionized water for 20 min. Then AEPC (2 g) was added to reaction mixture under N2 atmosphere at 80 °C for 21 h. The reaction mixture was filtered, washed with H2O and dried in room temperature for overnight. Chlorosulfuric acid was applied for acidification of catalyst [27]. The 0.5 g of synthesized solid (FSM-16/AEPC) was sonicated in n-hexane for 20 min and then, chlorosulfuric acid (1.5 ml) was added drop of drop and stirred for 3 h in ice bath. Finally, the synthesized catalyst was washed with n-hexane, EtOH then CH2Cl2 and dried in room temperature.
2.3 General Procedure for the Synthesis of 1,8-Dioxo-octahydroxanthene Derivatives
A test tube including aldehyde (1 mol) and dimedone (2 mol) in the presence of FSM-16/AEPC-SO3H (0.45 mol%) as catalyst was heated at 120 °C. After the completion of reaction (monitored by TLC), the hot EtOH was added to reaction mixture and the catalyst was separated by filtration. The products were recrystallization with EtOH.
2.4 General Procedure for the Synthesis of Tetrahydrobenzo[b]pyran Derivatives
Aldehyde (1 mol), dimedone (1 mol), malononitrile (1 mol) and FSM-16/AEPC-SO3H (0.6 mol%) as catalyst were mixed in H2O: EtOH (3:1 ml) at 80 °C. Upon completion of the reaction checked by TLC, the reaction mixture was cooled at room temperature, filtered and washed by H2O. Then hot EtOH was poured on precipitate until the product is solved. Finally, products were recrystallized for more purification.
2.5 Characterization Data of All Compounds
2.5.1 3,3,6,6-Tetramethyl-9-(4-chloro-phenyl)-1,8-dioxo octahydroxanthene
1H NMR (300 MHz, CDCl3): δ = 0.99 (s, 6H), 1.1 (s, 6H), 2.13–2.27 (q, J = 16.2 Hz, 4H), 2.46 (s, 4H), 4.71 (s, 1H), 7.17–7.26 (m, 4H) ppm. 13C NMR (CDCl3, 75 MHz): δ = 27.3, 29.3, 31.4, 32.2, 40.8, 50.7, 115.3, 128.2, 129.8, 132, 142.7, 162.4, 196.4 ppm. IR (KBr): 3026, 2927, 2876, 1662, 1470, 1363, 1198, 1151, 1007, 846 cm−1.
2.5.2 3,3,6,6-Tetramethyl-9-(4-bromo-phenyl)-1,8-dioxo octahydroxanthene
1H NMR (400 MHz, CDCl3): δ = 1.00 (s, 6H), 1.1 (s, 6H), 2.16–2.28 (q, J = 16 Hz, 4H), 2.48 (s, 4H), 4.72 (s, 1H), 7.18–7.20 (d, J = 8 Hz, 2H), 7.35–7.37 (d, J = 8 Hz, 2H) ppm. 13C NMR (CDCl3, 100 MHz): δ = 27.3, 29.3, 31.5, 32.2, 40.8, 50.7, 115.2, 120.2, 130.2, 131.1, 143.2, 162.4, 196.3 ppm. IR (KBr): 3026, 2955, 2873, 1666, 1628, 1487, 1467, 1362, 1198, 1165, 1005, 846 cm−1.
2.5.3 3,3,6,6-Tetramethyl-9-(4-fluoro-phenyl)-1,8-dioxo octahydroxanthene
1H NMR (400 MHz, CDCl3): δ = 1.02 (s, 6H), 1.1 (s, 6H), 2.17–2.26 (q, J = 16 Hz, 4H), 2.48 (s, 4H), 4.75 (s, 1H), 6.89–7.29 (m, 4H) ppm.13C NMR (CDCl3, 100 MHz): δ = 27.3, 29.3, 31.2, 32.2, 40.8, 50.7, 114.7, 115.5, 129.8, 160, 162.3, 162.6, 196.4 ppm. IR (KBr): 2958, 2927, 2873, 2819, 1661, 1629, 1508, 1398, 1364, 1223, 1198, 1141, 1002, 851 cm−1.
2.5.4 3,3,6,6-Tetramethyl-9-(4-methoxy-phenyl)-1,8-dioxo octahydroxanthene
1H NMR (400 MHz, CDCl3): δ = 1.01 (s, 6H), 1.1 (s, 6H), 2.16–2.27 (q, J = 16 Hz, 4H), 2.47 (s, 4H), 3.75 (s, 3H), 4.71 (s, 1H), 6.76–6.78 (d, J = 8 Hz, 2H), 7.21–7.23 (d, J = 8 Hz, 2H) ppm.13C NMR (CDCl3, 100 MHz): δ = 27.3, 29.3, 30, 32, 40.9, 50.8, 55.1, 113.4, 115.8, 129.3, 136.5, 157.9, 162.1, 196.5 ppm. IR (KBr): 3059, 2961, 2882, 2875, 1664, 1628, 1511, 1462, 1357, 1260, 1194, 1137, 1032, 931 cm−1.
2.5.5 3,3,6,6-Tetramethyl-9-(3,4-dimethoxy-phenyl)-1,8-dioxo octahydroxanthene
1H NMR (300 MHz, CDCl3): δ = 0.99 (s, 6H), 1.09 (s, 6H), 2.14–2.26 (q, J = 16.5 Hz, 4H), 2.45 (s, 4H), 3.79 (s, 3H), 3.85(s,3H) 4.69 (s, 1H), 6.69–6.77 (m, 2H), 6.89 (s, 1H) ppm. 13C NMR (CDCl3, 75 MHz): δ = 27.3, 29.3, 31.2, 32.2, 40.9, 50.7, 55.7, 110.8, 112.2, 115.7, 120.1, 137, 147.4, 148.4, 162.1, 196.5 ppm. IR (KBr): 3072, 3025, 2932, 2877, 1669, 1622, 1513, 1459, 1360, 1262, 1196, 1140, 1024, 933 cm−1.
2.5.6 3,3,6,6-Tetramethyl-9-(3-nitro-phenyl)-1,8-dioxo octahydroxanthene
1H NMR (400 MHz, CDCl3): δ = 1.02 (s, 6H), 1.1 (s, 6H), 2.15–2.28 (q, J = 16 Hz, 4H), 2.5 (s, 4H), 4.85 (s, 1H), 7.28–7.43 (m, 1H), 7.80–7.82 (m, 1H), 7.98–8.04 (m, 2H) ppm. 13C NMR (CDCl3, 100 MHz): δ = 27.3, 29.3, 32.1, 32.2, 40.8, 50.6, 114.5, 121.6, 128.8, 135.6, 146.3, 148.3, 163, 196.4 ppm. IR (KBr): 2958, 2934, 1657, 1624, 1524, 1472, 1396, 1357, 1201, 1166, 816 cm−1.
2.5.7 3,3,6,6-Tetramethyl-9-(3-hydroxy-phenyl)-1,8-dioxo octahydroxanthene
1H NMR (300 MHz, CDCl3): δ = 0.99 (s, 6H), 1.09 (s, 6H), 2.16–2.28 (q, J = 16.5 Hz, 4H), 2.45 (s, 4H), 4.76 (s, 1H), 6.54–6.58 (m, 1H), 6.68–6.70 (d, J = 9 Hz, 1H), 7.0-7.03 (m, 2H), 7.06–7.08 (m, 1H) ppm. 13C NMR (CDCl3, 75 MHz): δ = 27.4, 29.2, 31.7, 32.2, 40.8, 50.6, 113.6, 115.6, 116.4, 119.5, 129.12, 145.5, 156, 162.6, 197 ppm. IR (KBr): 3382, 3035, 2959, 2927, 1661, 1605, 1458, 1363, 1255, 1199, 977 cm−1.
2.5.8 3,3,6,6-Tetramethyl-9-(4-hydroxy-phenyl)-1,8-dioxo octahydroxanthene
1H NMR (400 MHz, CDCl3): δ = 1.02 (s, 6H), 1.11 (s, 6H), 2.18–2.28 (q, J = 16 Hz, 4H), 2.48 (s, 4H), 4.69 (s, 1H), 6.56–6.58 (d, J = 8 Hz, 2H), 7.08–7.10 (d, J = 8 Hz, 2H), 7.28 (s,1H) ppm. 13C NMR (CDCl3, 100 MHz): δ = 27.4, 29.2, 30, 32.3, 40.9, 50.8, 115.2, 115.8, 129.3, 135.7, 154.6, 162, 197 ppm. IR (KBr): 3405, 2962, 2930, 1662, 1616, 1513, 1361, 1247, 1165, 1003, 838 cm−1.
2.5.9 3,3,6,6-Tetramethyl-9-(2,4-dichloro-phenyl)-1,8-dioxo octahydroxanthene
1H NMR (400 MHz, CDCl3): δ = 1.04 (s, 6H), 1.1 (s, 6H), 2.16–2.27 (q, J = 16 Hz, 4H), 2.47 (s, 4H), 4.97 (s, 1H), 7.15–7.45 (m, 3H) ppm. 13C NMR (CDCl3, 100 MHz): δ = 27.4, 29.3, 32, 40.8, 50.7, 113.3, 126.7, 129.8, 132.8, 134, 138.6, 163.2, 196.5 ppm. IR (KBr): 3072, 3026, 2981, 2876, 1661, 1624, 1586, 1471, 1389, 1360, 1199, 1102, 860 cm−1.
2.5.10 3,3,6,6-Tetramethyl-9-(2-nitro-phenyl)-1,8-dioxo octahydroxanthene
1H NMR (400 MHz, CDCl3): δ = 1.03 (s, 6H), 1.12 (s, 6H), 2.14–2.27 (q, J = 16 Hz, 4H), 2.49 (s, 4H), 5.53 (s, 1H), 7.24–7.47 (m, 3H), 7.76–7.78(d, J = 8 Hz, 1H) ppm. 13C NMR (CDCl3, 100 MHz): δ = 27.6, 28.9, 29.4, 29.6, 29.7, 32, 40.9, 50.6, 114, 124.6, 127.2, 131.9, 137.8, 149.9, 162, 196 ppm. IR (KBr): 2959, 2844, 1665, 1626, 1528, 1384, 1354, 1205, 1150, 797 cm−1.
2.5.11 3,3,6,6-Tetramethyl-9-(4-nitro-phenyl)-1,8-dioxo octahydroxanthene
1H NMR (400 MHz, CDCl3): δ = 1.01 (s, 6H), 1.14 (s, 6H), 2.17–2.30 (q, J = 16 Hz, 4H), 2.52 (s, 4H), 4.85 (s, 1H), 7.48–7.50 (d, J = 8 Hz, 2H), 8.10–8.12 (d, J = 8 Hz, 2H) ppm. 13C NMR (CDCl3, 100 MHz): δ = 27.3, 29.3, 32.2, 32.3, 40.8, 50.6, 114.5, 123.4, 129.3, 146.5, 151.5, 162.1, 196.2 ppm. IR (KBr): 2924, 2854, 1659, 1515, 1467, 1398, 1201, 1165, 1001, 865 cm−1.
2.5.12 2-Amino-3-cyano-7,7-dimethyl-4-(4-chlorophenyl)-5-oxo-4H-5,6,7,8-tetrahydro benzopyran
1H NMR (400 MHz, DMSO-d6): δ = 0.95 (s, 3H), 1.04 (s, 3H), 2.09–2.13 (d, J = 16 Hz, 1H), 2.24–2.28 (d, J = 16 Hz, 1H), 2.47–2.56 (m, 2H), 4.21 (s, 1H), 7.07 (s, 2H), 7.17–7.19 (d, J = 8 Hz, 2H), 7.34–7.36 (d, J = 8 Hz, 2H) ppm. 13C NMR (DMSO, 100 MHz): δ = 27.3, 28.8, 31.1, 32.3, 35.6, 50.4, 58.2, 112.8, 120, 128.7, 129.6, 131.6, 144.2, 158.7, 163, 196.1 ppm. IR (KBr): 3382, 3323, 3264, 2189, 1677, 1638, 1491, 1407, 1217, 1162, 884 cm−1.
2.5.13 2-Amino-3-cyano-7,7-dimethyl-4-(4-bromophenyl)-5-oxo-4H-5,6,7,8-tetrahydro benzopyran
1H NMR (400 MHz, DMSO-d6): δ = 0.95 (s, 3H), 1.06 (s, 3H), 2.09–2.13 (d, J = 16 Hz, 1H), 2.23–2.27 (d, J = 16 Hz, 1H), 2.74–2.56 (m, 2H), 4.19 (s, 1H), 7.07 (s, 2H), 7.11–7.13 (d, J = 8 Hz, 2H), 7.48–7.50 (d, J = 8 Hz, 2H) ppm. 13C NMR (DMSO, 100 MHz): δ = 19, 27.3, 28.8, 32.3, 35.6, 50.4, 56.5, 58.1, 112.7, 120, 121, 129.9, 131.7, 144.6, 158.7, 160, 196.1 ppm. IR (KBr): 3361, 3317, 3255, 3193, 2960, 2852, 2189, 1685, 1650, 1478, 1404, 1379, 1252, 1142, 1083, 840, 564 cm−1.
2.5.14 2-Amino-3-cyano-7,7-dimethyl-4-(4-fluorophenyll)-5-oxo-4H-5,6,7,8-tetrahydro benzopyran
1H NMR (400 MHz, DMSO-d6): δ = 0.95 (s, 3H), 1.04 (s, 3H), 2.09–2.13 (d, J = 16 Hz, 1H), 2.23–2.27 (d, J = 16 Hz, 1H), 2.50–2.52 (m, 2H), 4.21 (s, 1H), 7.03 (s,2H), 7.09–7.19 (m, 4H) ppm. 13C NMR (DMSO, 100 MHz): δ = 27.3, 28.8, 32.3, 35.4, 50.4, 58.5, 113, 115.4, 115.6, 120, 129.5, 141, 158, 160, 162, 196.1 ppm. IR (KBr): 3358, 3180, 2959, 2894, 2190, 1676, 1637, 1604, 1506, 1466, 1368, 1216, 1161, 1033, 858, 776 cm−1.
2.5.15 2-Amino-3-cyano-7,7-dimethyl-4-(4-nitrophenyl)-5-oxo-4H-5,6,7,8-tetrahydro benzopyran
1H NMR (400 MHz, DMSO-d6): δ = 0.97 (s, 3H), 1.06 (s, 3H), 2.10–2.14 (d, J = 16 Hz, 1H), 2.25–2.29 (d, J = 16 Hz, 1H), 2.50–2.59 (m, 2H), 4.35 (s, 1H), 7.18 (s, 2H), 7.44–7.46 (d, J = 8 Hz, 2H), 8.17–8.19 (d, J = 8 Hz, 2H) ppm. 13C NMR (DMSO, 100 MHz): δ = 27.4, 28.7, 32.3, 36.1, 50.3, 56.5, 57.4, 112.2, 119.8, 124.1, 129.1, 146.7, 152.7, 159, 163.5, 196 ppm. IR (KBr): 3506, 3385, 3135, 2966, 2934, 2189, 1683, 1657, 1663, 1518, 1369, 1344, 1254, 1216, 1143, 1041, 828, 564 cm−1.
2.5.16 2-Amino-3-cyano-7,7-dimethyl-4-(3-nitrophenyl)-5-oxo-4H-5,6,7,8-tetrahydro benzopyran
1H NMR (400 MHz, DMSO-d6): δ = 0.96 (s, 3H), 1.05 (s, 3H), 2.10–2.14 (d, J = 16 Hz, 1H), 2.26–2.30(d, J = 16 Hz, 1H), 2.51–2.60 (m, 2H), 4.43 (s, 1H), 7.19 (s, 2H), 7.61–7.69 (m, 2H), 7.99–8.1 (m, 2H) ppm. 13C NMR (DMSO, 100 MHz): δ = 27.2, 28.8, 32.3, 35.8, 50.3, 57.6, 112.2, 119.8, 122.1, 122.2, 130.5, 134.6, 147.5, 148.2, 159.1, 163.6, 196.2 ppm. IR (KBr): 3432, 3327, 2958, 2875, 2204, 1681, 1662, 1600, 1531, 1372, 1349, 1210, 1093, 1038, 902, 822 cm−1.
2.5.17 2-Amino-3-cyano-7,7-dimethyl-4-(2-nitrophenyl)-5-oxo-4H-5,6,7,8-tetrahydro benzopyran
1H NMR (400 MHz, DMSO-d6): δ = 0.9 (s, 3H), 1.02 (s, 3H), 2.00-2.04 (d, J = 16 Hz, 1H), 2.19–2.23 (d, J = 16 Hz, 1H), 2.48–2.57 (m, 2H), 4.94 (s, 1H), 7.20 (s, 2H), 7.35–7.37 (d, J = 8 Hz, 1H), 7.41–7.45 (t, J = 8 Hz, 1H), 7.67–7.69 (m, 1H), 7.81–7.83(d, J = 8 Hz, 1H) ppm. 13C NMR (DMSO, 100 MHz): δ = 27.2, 28.8, 30.4, 32.3, 50, 56.8, 112.8, 119.5, 124.2, 128.3, 130.7, 139.4, 149.4, 159, 163, 196 ppm. IR (KBr): 3471, 3335, 2961, 2895, 2194, 1689, 1663, 1598, 1525, 1409, 1360, 1254, 1215, 1043, 862 cm−1.
2.5.18 2-Amino-3-cyano-7,7-dimethyl-4-(4-methoxyphenyl)-5-oxo-4H-5,6,7,8-tetrahydro benzopyran
1H NMR (300 MHz, DMSO-d6): δ = 0.93 (s, 3H), 1.02 (s, 3H), 2.04–2.10 (d, J = 18 Hz, 1H), 2.20–2.26(d, J = 18 Hz, 1H), 2.47–2.49 (m, 2H), 3.69 (s, 3H), 4.01 (s, 1H), 6.81–6.84 (d, J = 9 Hz, 2H), 6.95 (s, 2H), 7.02–7.05 (d, J = 9 Hz, 2H) ppm. 13C NMR (DMSO, 100 MHz): δ = 26.8, 28.5, 31.8, 34.8, 50, 55, 58.6, 113, 113.7, 119.9, 128.3, 136.9, 157.9, 158.4, 162.2, 195.7 ppm. IR (KBr): 3361, 3186, 2960, 2192, 1652, 1606, 1507, 1463, 1252, 1030 cm−1.
2.5.19 2-Amino-3-cyano-7,7-dimethyl-4-(3,4-dimethoxyphenyl)-5-oxo-4H-5,6,7,8-tetrahydro benzopyran
1H NMR (400 MHz, DMSO-d6): δ = 0.98 (s, 3H), 1.05 (s, 3H), 2.10–2.14 (d, J = 16 Hz, 1H), 2.25–2.29 (d, J = 16 Hz, 1H), 2.50–2.53 (m, 2H), 3.71 (s, 3H), 3.72 (s, 3H), 4.14 (s, 1H), 6.65–6.70 (m, 2H), 6.86–6.88 (d, J = 8 Hz, 1H), 6.95 (s, 2H) ppm. 13C NMR (DMSO, 100 MHz): δ = 19, 27.1, 28.9, 32.2, 35.5, 50.5, 55.9, 56, 59, 111.5, 112.3, 113.3, 119.6, 120.3, 137.8, 148, 148.9, 158.8, 162.7, 196.1 ppm. IR (KBr): 3395, 3330, 3258, 3215, 2934, 2194, 1680, 1658, 1512, 1465, 1367, 1214, 1141, 1033, 749 cm−1.
2.5.20 2-Amino-3-cyano-7,7-dimethyl-4-(4-methylphenyl)-5-oxo-4H-5,6,7,8-tetrahydro benzopyran
1H NMR (400 MHz, DMSO-d6): δ = 0.95 (s, 3H), 1.04 (s, 3H), 2.08–2.12 (d, J = 16 Hz, 1H), 2.23–2.27 (d, J = 16 Hz, 1H), 2.45–2.56 (m, 2H), 3.72 (s, 3H), 4.13 (s, 1H), 6.84–6.86 (d, J = 8 Hz, 2H), 6.96 (s, 2H), 7.05–7.07 (d, J = 8 Hz, 2H) ppm. 13C NMR (DMSO, 100 MHz): δ = 27.2, 28.9, 32.3, 35.2, 50.5, 55.5, 59, 113, 114.1, 120.3, 128.7, 137.3, 158.4, 158.9, 162.6, 196.1 ppm. IR (KBr): 3434, 3382, 3188, 2925, 2193, 1684, 1654, 1509, 1403, 1370, 1254, 1214, 1140, 1035, 569 cm−1.
2.5.21 2-Amino-3-cyano-7,7-dimethyl-4-(2,4-dichlorophenyl)-5-oxo-4H-5,6,7,8-tetrahydro benzopyran
1H NMR (300 MHz, DMSO-d6): δ = 0.94 (s, 3H), 1.00 (s, 3H), 2.01–2.07 (d, J = 18 Hz, 1H), 2.18–2.24 (d, J = 18 Hz, 1H), 2.47–2.49 (m, 2H), 4.64 (s, 1H), 7.09 (s, 2H), 7.16–7.19 (d, J = 9 Hz, 1H), 7.31–7.34 (dd, J = 9 Hz, 1H), 7.49–7.50 (d, J = 3 Hz, 1H) ppm. 13C NMR (DMSO, 75 MHz): δ = 26.9, 28.3, 31.8, 32.6, 49.9, 56.2, 111.3, 119.1, 127.7, 128.7, 131.4, 131.8, 133, 140.7, 158.7, 163.3, 195.6. IR (KBr): 3405, 3331, 3258, 3214, 2931, 2868, 2193, 1685, 1652, 1469, 1419, 1366, 1252, 1213, 1039, 856, 795 cm−1.
2.5.22 2-Amino-3-cyano-7,7-dimethyl-4(phenyl)-5-oxo-4H-5,6,7,8-tetrahydro benzopyran
1H NMR (400 MHz, DMSO-d6): δ = 0.96 (s, 3H), 1.05(s, 3H), 2.09–2.13 (d, J = 16 Hz, 1H), 2.24–2.28 (d, J = 16 Hz, 1H), 2.50–2.57 (m, 2H), 4.18 (s, 1H), 7.00 (s, 2H), 7.14–7.17 (m, 2H), 7.19–7.21 (d, J = 8 Hz, 1H), 7.27–7.31 (t,, J = 8 Hz, 2H) ppm. 13C NMR (DMSO, 100 MHz): δ = 27.3, 28.9, 32.3, 36, 50.4, 58.8, 113.2, 120.2, 127, 127.6, 128.8, 146.2, 158.9, 162.9, 196 ppm. IR (KBr): 3397, 3326, 3252, 3212, 2924, 2884, 2199, 1681, 1660, 1604, 1410, 1370, 1249, 1138, 1035, 697, 531 cm−1.
2.5.23 2-Amino-3-cyano-7,7-dimethyl-4-(4-hydroxyphenyl)-5-oxo-4H-5,6,7,8-tetrahydro benzopyran
1H NMR (400 MHz, DMSO-d6): δ = 0.95 (s, 3H), 1.04 (s, 3H), 2.07–2.11 (d, J = 16 Hz, 1H), 2.22–2.26 (d, J = 16 Hz, 1H), 2.48–2.51 (m, 2H), 4.07 (s, 1H), 6.65–6.94 (m, 6H), 9.25 (s,1H) ppm. 13C NMR (DMSO, 100 MHz): δ = 27.2, 28.9, 32.3, 35.2, 50.5, 56.5, 59.2, 113.6, 115.4, 120.3, 128.6, 135.6, 156.4, 158.8, 162.4, 196.1 ppm. IR (KBr): 3438, 3375, 3195, 2963, 2195, 1684, 1649, 1512, 1454, 1371, 1253, 1215, 1144, 1120, 1040, 880 cm−1.
2.5.24 2-Amino-3-cyano-7,7-dimethyl-4-(thiophen-2-yl)-5-oxo-4H-5,6,7,8-tetrahydro benzopyran
1H NMR (400 MHz, DMSO-d6): δ = 1.00 (s, 3H), 1.1 (s, 3H), 2.15–2.19 (d, J = 16 Hz, 1H), 2.3–2.34 (d, J = 16 Hz, 1H), 2.42–2.46 (d, J = 16 Hz, 1H), 2.52–2.59 (m, 1H), 4.56 (s, 1H), 6.88–6.89 (d, J = 4 Hz, 1H), 6.92–6.94 (m, 1H), 7.13 (s, 2H), 7.33–7.34 (dd, J = 4 Hz, 1H) ppm. 13C NMR (100 MHz, DMSO-d6): δ = 26.9, 29.1, 30.9, 32.2, 50.3, 58.5, 113.4, 120.1, 124.5, 124.9, 127.3, 149.8, 159.4, 163, 196 ppm. IR (KBr): 3383, 3322, 3208, 2962, 2928, 2883, 2196, 1679, 1661, 1602, 1417, 1375, 1215, 1171, 1036, 699 cm−1.
3 Result and discussion
3.1 Preparation of FSM-16/AEPC-SO3H
The procedure for the synthesis of FSM-16 was presented in literature [25]. For the production of N-(2-aminoethyl)-2-pyrrolidone-4-carboxylic acid (AEPC), the reaction of itaconic acid with ethylenediamine was carried out in water at the reflux condition [26]. Then treatment of the FSM-16 with AEPC led to production of FSM-16/AEPC. Finally, to prepare of catalyst, chlorosulfuric acid was added to FSM-16/AEPC (Scheme 1). Eventually, FSM-16/AEPC-SO3H was obtained and is fully characterized with FT-IR, TGA, XRD, SEM, TEM, BET and EDX spectrum.
3.2 Characterization of FSM-16/AEPC-SO3H
Figure 1 represents the FT-IR spectrum of FSM-16 (a), FSM-16/AEPC (b) and FSM-16/AEPC-SO3H (c). The FT-IR spectra of FSM-16 (a) indicate the band in the 802 and 1085 cm−1 region assigned to the symmetric and asymmetric stretching modes of Si–O–Si. The another bond in the wavenumber of 3457 cm−1 assigned to OH stretching vibrations on the surface of FSM-16. In the spectra of FSM-16/AEPC (b), the peak at 3449 cm−1 can be contributed to the NH2 stretching vibrations. The vibration of stretching modes of CH2 and carbonyl of amid group demonstrate at 2926–2979 cm−1 and 1634 cm−1 respectively. Also, the bands that appear in the 801 and 1094 cm−1 assigned to the symmetric and asymmetric stretching modes of Si–O–Si. As shown in the FT-IR spectrum of FSM-16/AEPC-SO3H (c), the peaks that appear at 3450, 2931–2892 and 1636 cm−1 can be related to NH2 stretching vibrations, CH2 stretching and carbonyl of amid group respectively. Additionally, the vibration signals in region of 961–1164 cm−1 are typical SO3–H groups [28].
Figure 2 shows the thermogravimetric analysis (TGA) of FSM-16 and FSM-16/AEPC-SO3H. The TGA was used to determinate the amount of organic groups on the surface of FSM-16. The weight loss occurs at temperature below 100 °C that is related to the loss of solvent, adsorbed surface hydroxyl group and water in synthesized catalyst. As shown in Fig. 2, the total amount of organic content of synthesized catalyst was obtained to be 10 wt% at 200–600 °C.
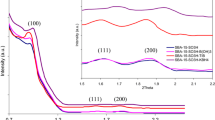
The XRD pattern of FSM-16 and FSM-16/AEPC-SO3H have been recorded in Fig. 3. Diffraction peaks are observed in the lower angle region 2θ < 10 reflections of the two dimensional hexagonal structure of the FSM-16 material. Due to partial loss synthesized catalyst’s ordered structure and the presence of organic moieties onto the mesoporous of FSM-16, the intensity of peaks for the catalyst is lower than FSM-16 [2].
The synthesized catalyst was analyzed using EDS to obtain the existence elements in the catalyst. As shown in Fig. 4, the Si, O, N, C and S showed in the EDX spectrum of FSM-16/AEPC-SO3H. Also the SEM images of FSM-16/AEPC-SO3H and recovered catalyst indicated in Figs. 5 and 6. As shown in this Figure, nanometer-sized particles were confirmed also the morphology of synthesized catalyst is clear.
The transmission electron microscopy (TEM) images of catalyst and recovered catalyst are shown in Fig. 7. The image of FSM-16/AEPC-SO3H showed the hexagonal channels with uniform pore size. The structure of the recovered catalyst did not change the general shape of catalyst and no considerable shape change was observed in recovered catalyst.
Due to obtained physicochemical and structural parameters of FSM-16/AEPC-SO3H and FSM-16, the nitrogen adsorption–desorption isotherms was applied (Fig. 8). The type of nitrogen adsorption–desorption isotherm of synthesized catalyst and FSM-16, based on the IUPAC classification, are a typical type IV isotherm, which is characteristic of mesoporous materials. Brunauer–Emmett–Teller (BET), surface area for the synthesized catalyst and FSM-16 are 603 m2 g−1 and 1001 m2 g−1 respectively. Also total volume for synthesized catalyst and FSM-16 obtain 0.71 cm3 g−1 and 1.21 cm3 g−1.
The back titration of the catalyst was applied for determination of the acid amount on the surface of synthesised catalyst. In this light, 0.1 g of the synthesized catalyst was added to aqueous NaCl solution (1 mol/l, 10 ml) with an initial pH 7.4. The mixture was stirred for 30 min, until the pH of the solution had decreased to 2.81. This result indicates ion exchange between sulfamic acid protons and sodium ions that is equal to a loading of 0.15 mmol/g of sulfuric acid group.
3.3 Investigation of the Catalytic Activity of FSM-16/AEPC-SO3H for Multi Component Reactions
After the characterization of catalyst and confirmation of structure, we were investigated the catalyst activity in synthesis of 1,8-dioxo-octahydroxanthene and tetrahydrobenzo[b]pyran derivatives.
In our first experiments, condensation reaction between 4-chlorobenzaldehyde (1 mol) and dimedone (2 mol) in the presence of FSM-16/AEPC-SO3H as catalyst was chosen as a model reaction.
During the optimization of the reaction conditions for the synthesis of 1,8-dioxo-octahydroxanthene, we have studied the amount of the catalyst. for this reason, the amounts of 0.75 mol%, 0.6 mol%, 0.45 mol%, 0.3 mol% and catalyst free condition was investigated. The 0.45 mol% of FSM-16/AEPC-SO3H was the best result. Also we were tested the solvents of EtOH, H2O, PEG, CH3CN and solvent free condition. As is evident from Table 1 entry 3, the best results were achieved in the solvent-free conditions. Finally, the temperature effect on the model reaction checked. The results show that temperature of 120 °C is the best (Table 1, entry 3).
In the obtained optimum conditions, a wide range of aldehydes were used for synthesis of 1,8-dioxo-octahydroxanthene (Scheme 2). Among the various aldehydes compounds, electron-withdrawing groups reacted faster than electron-donating groups. The results summarized in Table 2.
The suggested mechanism for the synthesis of 1,8-dioxo-octahydroxanthene in the presence of FSM-16/AEPC-SO3H, on the basis of the literature [32], is proposed in Scheme 3. The condensation of the one molecule of dimedone with activated aldehyde give intermediate (I) that with dehydration, give intermediate (II). Then another molecule of dimedone reacts with (II) by Michael addition to afford the intermediate (III). Finally, cyclodehydration of (IV) produc 1,8-dioxo-octahydroxanthenes. As shown in Fig. 9, justification mechanism of catalyst was investigated by FT-IR spectrum of product and intermediate.
Then the catalytic ability of FSM-16/AEPC-SO3H was investigated for the synthesis of tetrahydrobenzo[b]pyran derivatives. In this light, the one pot synthesis of 4-chlorobenzaldehyde (1 mol), malononitrile (1 mol) and dimedone (1 mol) in the presence of FSM-16/AEPC-SO3H was choosing as model reaction.
Initially, we assessed the amount of catalyst. Hence the amounts of 0.45 mol%, 0.6 mol%, 0.75 mol%, and catalyst free conditions were applied. According to the obtained results (Table 3, entry 3), the amount of 0.6 mol% of synthesized catalyst, produced the high yield.
Then, we screened different solvents for the synthesis of 2-amino-4-(4-chlorophenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8 tetrahydro-benzo[b]pyran. As shown in Table 3, the mixture of H2O:EtOH with ratio of 3:1 ml resulted in the highest yield. In order to demonstrate the effect of temperature on the synthesis of tetrahydrobenzo[b]pyran, we tested temperatures of 60, 80 and 100 °C. The temperature of 80 °C was choosen.
Having established the reaction conditions, treatment of many aldehydes (1 mol) with malononitrile (1 mol) and dimedone (1 mol) in the presence of FSM-16/AEPC-SO3H (0.6 mol%) in H2O: EtOH (ratio of 3:1 ml) at 80 °C to generate tetrahydrobenzo[b]pyran derivatives (Scheme 4). The activity of aldehydes compounds in synthesis of tetrahydrobenzo[b]pyran is very important. As shown in Table 4 among the various aldehydes compounds, electron-withdrawing groups reacted faster than electron-donating groups and also the yields of product were better. When aldehydes containing an electron-donating group were used (Table 4, entries 7–9 and 12), the corresponding tetrahydrobenzo[b]pyran was produced in moderate to good yields, but the reaction time was longer compared to aldehydes with electron withdrawing groups. The results are in Table 4.
A possible mechanism for the synthesis of tetrahydrobenzo[b]pyran catalyzed by FSM-16/AEPC-SO3H is indicated in Scheme 5 [38]. As shown in this Scheme 5, malononitrile first condenses with activated aldehyde followed by dehydration to afford arylidenemalononitrile intermediate (I). Subsequently, the nucleophilic addition of the enolizable dimedone to the arylidenemalononitrile intermediate (I) followed by consecutive intramolecular cyclization occur to afford the intermediate (IV) which rearranges to afford the expected tetrahydrobenzo[b]pyran.
Also in Fig. 10, FT-IR spectrum of intermediates and product of tetrahydrobenzo[b]pyran indicated.
3.4 Reusability of the FSM-16/AEPC-SO3H
For practical purposes, the reusability of the catalyst is highly favorable. In this light, the reusability of FSM-16/AEPC-SO3H was tested for synthesis of 2-amino-4-(4-chlorophenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydro-benzo[b]pyran. After the completion of the reaction, the reaction mixture was cooled to room temperature and washed by H2O. Then hot EtOH was added and centrifuged. The remaining catalyst was washed, dried and applied for the next run. The results were indicated that the heterogeneous catalysis of FSM-16/AEPC-SO3H has reusability for at least five times (Fig. 11).
To extend the scope of merit of synthesised catalyst, the FSM-16/AEPC-SO3H was compared with other catalysts for synthesis of 2-amino-4-(4-chlorophenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydro-benzo[b]pyran (Table 5, entry 1–7) and 3,3,6,6-tetramethyl-9-(4-chloro-phenyl)-1,8-dioxo octahydroxanthene (Table 5, entry 8–11).
4 Conclusion
In summary, we have developed a new, efficient and heterogonous catalyst of FSM-16/AEPC-SO3H for the one pot synthesis of 1,8-dioxo-octahydroxanthene and tetrahydrobenzo[b]pyran derivatives. The prepared catalyst was confirmed by several analyses including: FT-IR, TGA, XRD, SEM, TEM, EDS and BET techniques. The advantages of this method are: utilization of green solvent and solvent free condition, short time reaction, high yield of products, simple work-up and purification, simple separation of catalyst and reusability of catalyst for five time.
References
Nagata H, Takimura M, Yamasaki Y, Nakahira A (2006) Mater Trans 47:2103
Jakdetchai O, Takayama N, Nakajima T (2005) Kinet Catal 46:56
Das P, Silva AR, Carvalho AP, Pires J, Freire C (2009) Catal Lett 129:367
Das P, Silva AR, Carvalho AP, Pires J, Freire C (2008) Colloid Surf A 329:190
Medway AM, Sperry J (2014) Green Chem 16:2084
Quadri SA, Das TC, Malik MS, Seddigi ZS, Farooqui M (2016) Chem Select 1:4602
Makone S, Mahurkar S (2013) Green Sustain Chem 3:27
Arbosara FS, Shirini F, Abedini M, Moafi HF (2015) J Nanostruct Chem 5:55
Javid A, Heravi MM, Bamoharram FF (2011) J Chem 8:910
Kahandal SS, Burange AS, Kale SR, Prinsen P, Luque R, Jayaram RV (2017) Catal Commun 97:138
Sadat SN, Hatamjafari F (2015) Orient J Chem 31:1191
Zare A, Moosavi-Zare AR, Merajoddin M, Zolfigol MA, Hekmat-Zadeh T, Hasaninejad A, Khazaei A, Mokhlesi M, Khakyzadeh V, Derakhshan-Panah F, Beyzavi MH, Rostami E, Arghoon A, Roohandeh R (2012) J Mol Liq 167:69
Kumbhar A, Kamble S, Rashinkar G, Mote K, Salunkhe R (2010) Arch Appl Sci Res 2:235
Li JJ, Tao XY, Zhang ZH (2008) Phosphorus Sulfur Silicon 183:1672
dos Santos WH, Da Silva-Filho LC (2016) Chem Pap 70:1658
Li GC (2008) J Chem Res 1:484
Zhang ZH, Zhang P, Yang SH, Wang HJ, Deng J (2010) J Chem Sci 122:427
Davoodnia A, Yadegarian S, Nakhaei A, Tavakoli-Hoseini N (2016) Russ J Gen Chem 86:2849
Maleki A, Aghaei M, Ghamari N (2016) Appl Organomet Chem 30:939
Kshirsagar AK, Bankar SS, Khandebharad AU, Sarda SR, Soni MG, Agrawal BR (2016) J Med Chem Drug Discov 1:720
Hu H, Qiu F, Ying A, Yang J, Meng H (2014) Int J Mol Sci 15:6897
Azarifar D, Khatami SM, Zolfigol MA, NejatYami R (2014) J Iran Chem Soc 11:1223
Bodaghifard MA, Solimannejad M, Asadbegi S, Dolatabadifarahani S (2016) Res Chem Intermed 42:1165
Zhang P, Yu YD, Zhang ZH (2008) Synth Commun 38:4474
Matsumoto A, Sasaki T, Nishimiya N, Tsutsumi K (2002) Colloids Surf A 203:185
Qi P, Chen HL, Nguyen HTH, Lin CC, Miller SA (2016) Green Chem 18:4170
Zolfigol MA, Madrakian E, Ghaemi E (2002) Molecules 7:734
Hajjami M, Tahmasbi B (2015) RSC Adv 5:59194
Lu HY, Li JJ, Zhang ZH (2009) Appl Organomet Chem 23:165
Bayat M, Imanieh H, Hossieni SH (2009) Chin J Chem 27:2203
Nisar M, Ali I, Shah MR, Badshah A, Qayum M, Khan H, Khan I, Ali S (2013) RSC Adv 3:21753
Hazeri N, Masoumnia A, Mghsoodlou MT, Salahi S, Kangani M, Kianpour S, Kiaee S, Abonajmi J (2015) Res Chem Intermed 41:4123
Sheikhhosseini E, Ghazanfari D, Nezamabadi V (2013) Iran J Catal 3:197
Beheshtiha S, Oskoole H, Pourebrahimi F, Zadsirjan V (2015) Chem Sci Trans 4:689
Montazeri N, Noghani T, Ghorchibeigy M, Zoghi R (2014) J Chem 2014:1
Lu J, Fu XW, Zhang G, Wang C (2016) Res Chem Intermed 42:417
Ziarani GM, Abbasi A, Badiei A, Aslani Z (2011) J Chem 8:293
Azarifar D, Abbasi Y (2016) Synth Commun 46:745
Davarpanah J, Kiasat AR, Noorizadeh S, Ghahremani M (2013) J Mol Catal A 376:78
Esmaeilpour M, Javidi J, Dehghani F, Nowroozi Dodeji F (2015) RSC Adv 5:34:26625
Esmaeilpour M, Javidi J, Dehghani F, Nowroozi Dodeji F (2014) New J Chem 38:5453
Karami B, Hoseini SJ, Eskandari K, Ghasemib A, Nasrabadi H (2012) Catal Sci Technol 2:331
Mobinikhaledi A, Khajeh-Amiri A (2014) React Kinet Mech Catal 112:131
Acknowledgements
Financial support for this work by the Ilam University, Ilam, Iran is gratefully acknowledged. The Natural Sciences and Engineering Research Council of Canada is gratefully acknowledged for support (RHEH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there is no conflict of interest involved.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hajjami, M., Gholamian, F., Hudson, R.H.E. et al. FSM-16/AEPC-SO3H: Synthesis, Characterization and Its Application for the Catalytic Preparation of 1,8-Dioxo-octahydroxanthene and Tetrahydrobenzo[b]pyran Derivatives. Catal Lett 149, 228–247 (2019). https://doi.org/10.1007/s10562-018-2623-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-018-2623-x