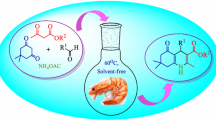
Abstract
New 3-(2-aryl-6-nitro-1H-indol-3-yl)quinoline-2,4(1H,3H)-diones were synthesized, and good-to-excellent yields were achieved through one-pot, three-component condensation of aryl glyoxal monohydrates, 4-hydroxyquinolin-2(1H)-one and 3-nitroaniline using sodium alginate and water/ethanol (1:1) as a green solvent at mild reaction conditions. The noticeable features of the present procedure are mild reaction conditions, economic procedure, availability of starting materials, very simple operation, easy isolation, no need for column chromatography separation and using sodium alginate as a natural polysaccharide that is a transition-metal-free, biodegradable, reusable and commercially affordable catalyst. In addition, sodium alginate was recycled up to five times with no remarkable loss of its catalytic properties.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Designing an efficient procedure for the synthesis of complex organic molecules with biological properties using available materials is an important region of research and development in pharmacological industries. One of the most favorable methods for synthesis of these compounds relies on the use of one-pot, multicomponent reactions. In this respect, a multicomponent reaction aims to reach the desired product through a single operation using three or more initiating compounds. This highly efficient approach has become very popular for synthesis of various materials such as heterocyclic and biological compounds used in different fields [1,2,3,4].
During recent years, some hazardous solvents have been replaced by water, known as a green solvent. Nontoxicity, nonflammability and economic issues are some of the reasons for using water as an alternative solvent in synthesis of heterocyclic compounds [5,6,7].
According to several reports [8,9,10,11], indole core unit is the abundant heterocyclic unit present in many natural products with varied medicinal and pharmaceutical properties [12,13,14]. Some drugs containing indole moieties such as serotonin, indomethacin, tryptophan, oxypertine, roxindole and arbidole [15] are shown in Fig. 1. Indole derivatives exhibit the broad range of biological properties such as antioxidant [16, 17], antimicrobial [18], antifungal [19], anticancer [20], antidiabetic [21], antiparkinsonian [22], anti-inflammatory [23], antiviral [24], COX-2 inhibitors [25] and cytotoxic agent [26].
There are several methods reported for the synthesis of indole derivatives in the literature, and the majority of these methods are based on condensation and cyclization technique by using diverse starting materials [27,28,29,30,31,32]. Many of the reported methods are restricted due to using costly reagents, multistep procedures, harmful solvents or catalysts and being time-consuming.
Alginates, as natural polysaccharides, are widely distributed in the wall of the cell or matrix of some types of algae such as brown algae. The alginate presented in these cells is in the form of sodium alginic acid salt, which is known as a valuable and safe component with great applications in the food industry as an emulsifier, thickener and gel-forming agent [33,34,35]. Synthesis of pyrano[3,2-c]chromenes has been reported recently using sodium alginate as catalyst [36]. Polymeric structure of sodium alginate catalyst is shown in Fig. 2.
Aryl glyoxal monohydrates are important precursors in synthesis of heterocyclic compound with biological and pharmaceutical activities [37].
In continuation of our previous studies on synthesis of novel heterocyclic compounds [38,39,40,41,42,43,44,45,46], herein, we report the reaction of aryl glyoxal monohydrates, 4-hydroxyquinolin-2(1H)-one and 3-nitroaniline to form a new series of 3-(2-aryl-6-nitro-1H-indol-3-yl)quinoline-2,4(1H,3H)-diones by one-pot, three-component reactions using sodium alginate as biopolymeric catalysts in H2O/EtOH (1:1) at room temperature. The structure of synthesized compounds was characterized by their spectral data, microanalysis and HRMS. These compounds may have potential biological and pharmacological properties.
Results and discussion
A new series of 3-(2-aryl-6-nitro-1H-indol-3-yl)quinoline-2,4(1H,3H)-diones 5a–i was synthesized by a one-pot, three-component reaction of aryl glyoxal monohydrates 1a–i, 4-hydroxyquinolin-2(1H)-one, (2) and 3-nitroaniline (3) in the presence of sodium alginate (4) as a catalyst in H2O/EtOH (1:1) at room temperature.
Initially, a reaction of phenyl glyoxal monohydrate (1a), 4-hydroxyquinolin-2(1H)-one (2) and 3-nitroaniline (3) was chosen as a trial reaction (Table 1). The model reaction was carried out in the absence of any catalysts using different solvents, and no product was achieved after 24 h under reflux conditions (Table 1, entries 1–7). By using K2CO3, NaOH, KOH as basic catalysts, no product was formed (Table 1, entries 8–10). The product was obtained (23–38% yield) using H2O or EtOH in the presence of DABCO or Et3N as organocatalysts under reflux condition (Table 1, entries 11–14). In addition, using DBU, p-TSA, l-proline and sulfamic acid as catalysts in H2O, EtOH, and MeOH under reflux conditions afforded the desired product in 28–59% yield, respectively (Table 1, entries 15–22). The favorable result (81% yield) was attained using sodium alginate in H2O/EtOH (1:1) at room temperature after 2 h reaction time (Table 1, entry 24).

Furthermore, using different molar ratios of catalyst and room temperature to 50 °C within 1–8 h reaction times afforded product in 63–82% yield (Table 2, entries 1–8). The optimum result (82% yields and 1 h reaction time) was observed using sodium alginate (20 mol%) in H2O/EtOH (1:1) at room temperature (Table 2, entry 6). Decreasing the molar ratio of catalysts to 15 mol% and stirring at 50 °C for 4 h reduce the yield of product to 78% yield (Table 2, entry 5).
After optimization of reaction conditions, the generality and the scope of this reaction were examined to a range of substituted aryl glyoxal monohydrates 1a–i to produce 1H-indole quinolinediones 5a–i in 78–84% yields. The reaction times, yields and melting points of all final products 5a–i are listed in Table 3.

Furthermore, the reusability as one of the most important factors of a catalyst was examined in the synthesis of 5a. For this purpose, the recovered catalyst was used at least up to five times and the catalytic performance was measured (Fig. 3). The results show that the sodium alginate (4) can be used several times with no remarkable loss of its activity.
A proposed mechanism for the synthesis of 1H-indole quinolinediones 5a–i is shown in Scheme 1. The reaction involved the Knoevenagel condensation of aryl glyoxals 1a–i with 4-hydroxyquinolin-2(1H)-one (2) using sodium alginate (4) to form the corresponding intermediate A. The Michael addition of 3-nitroaniline to aforementioned intermediate A afforded the intermediate B, which formed the desired product 5a–i by intramolecular condensation followed by dehydration and aromatization in the presence of sodium alginate catalyst.
The structure of 3-(2-aryl-6-nitro-1H-indol-3-yl)quinoline-2,4(1H,3H)-diones 5a–i was confirmed by their FTIR, 1H-NMR, 13C-NMR spectral data, microanalysis and HRMS. The FTIR spectra showed the absorptions at ῡ = 1600–1697 cm−1 due to carbonyl groups and absorptions at ῡ = 1341–1550 cm−1 attributed to nitro group. The 1H-NMR spectra showed singlets at δ = 6.35–6.91 ppm due to CH and broad singlets at δ = 10.88–11.66 ppm as NH peaks. The 13C-NMR spectra showed the carbonyl peaks at δ = 162.8–201.0 ppm.
Conclusions
In this article, a new and highly effective procedure was reported for the synthesis of a new series of 3-(2-aryl-6-nitro-1H-indol-3-yl)quinoline-2,4(1H,3H)-diones in the presence of sodium alginate using H2O/EtOH (1:1) as solvent at room temperature by one-pot, three-component reaction. The simple workup, green solvent, simple operational conditions and high output are some advantages of the reported procedure. The obtained compounds may have pharmaceutical and biological activities.
Experimental: materials and methods
A Philip Harris C4954718 apparatus was used to measure the melting points, and no correction was imposed. Thermo-Nicolet Nexus 670 instrument was used to obtain FTIR spectra using KBr disks. Both 1H-NMR at 300 MHz and 13C-NMR at 75.5 MHz spectra were attained through NMR spectrometer (Bruker Avance AQS 300 MHz). Chemical shifts were detected in DMSO-d6 using Si(CH3)4 as the standard. The reaction progress was investigated and tracked by TLC on silica gel plates (Polygram SILG/UV254). Elemental analyses were performed using a Leco Analyzer 932. High-resolution mass was recorded on a Kratos MS 25RF spectrometer.
General synthesis procedure of the new series of 3-(2-aryl-6-nitro-1H-indol-3-yl)quinoline-2,4(1H,3H)-dione derivatives 5a–i
Suspensions of aryl glyoxal monohydrates 1a–i (1 mmol), 4-hydroxyquinolin-2(1H)-one (2, 1 mmol) and 3-nitroaniline (3, 1 mmol) in water/ethanol (1:1, 10 mL) and sodium alginate (4, 20 mol%) all were kept under stirring at room temperature for 23–62 min. Finally, the completed reactions were affirmed by TLC using CHCl3:MeOH (10:1) as eluent and the obtained precipitate was separated using filtration following with rinsing using H2O and cold EtOH to obtain the final product in 78–84% yields.
Separation of catalyst The solvent of filtrate was evaporated, and the residue was washed with cold ethanol (2 × 2 mL) and dried to give the catalyst as white solid, which was used for checking its reusability (Fig. 3).
Spectral data of compounds 5a–i
3-(6-Nitro-2-phenyl-1H-indol-3-yl)quinoline-2,4(1H,3H)-dione (5a)
Yellow powder; yield 82% (326 mg); m.p. 194–195 °C; FTIR (KBr) ῡ (cm−1): 3418, 3369, 3067, 2968, 2856, 1697, 1643, 1612, 1526, 1404, 1343, 1218, 871, 737; 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 11.57 (s, 2H, exchanged by D2O, 2 × NH), 8.01–7.85 (m, 2H, ArH), 7.74 (s, 1H, Ar), 7.60–6.93 (m, 9H, ArH), 6.42 (s, 1H, CH); 13C-NMR (75.5 MHz, DMSO-d6) δ (ppm): 196.5 (C=O), 162.9 (C=O), 160.3 (C, Ar), 149.0 (C, Ar), 138.6 (C, Ar), 136.1 (C, Ar), 134.2 (C, Ar), 132.3 (C, Ar), 131.2 (C, Ar), 129.2 (C, Ar), 127.8 (C, Ar), 126.9 (C, Ar), 122.7 (C, Ar), 121.0 (C, Ar), 116.9 (C, Ar), 115.0 (C, Ar), 111.9 (C, Ar), 110.0 (C, Ar), 108.0 (N–C=C), 105.8 (C=C), 54.1 (CH). Anal. calcd for C23H15N3O4: C, 69.52; H, 3.80; N, 10.57; found: C, 69.41; H, 3.89; N, 10.45%. HRMS (ESI): m/z (M)+ calcd. for C23H15N3O4+: 397.1063; found: 397.1055.
3-(2-(4-Bromophenyl)-6-nitro-1H-indol-3-yl)quinoline-2,4(1H,3H)-dione (5b)
Yellow powder; yield 78% (371 mg); m.p. 202–203 °C; FTIR (KBr) ῡ (cm−1): 3424, 3107, 2969, 2854, 1693, 1648, 1615, 1582, 1524, 1402, 1341, 1261, 1181, 998, 748; 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 11.58 (s, 2H, exchanged by D2O, 2 × NH), 7.95 (d, J = 7.8 Hz, 1H, ArH), 7.86 (d, J = 7.5 Hz, 2H, ArH), 7.74 (s, 1H, ArH), 7.61 (d, J = 7.8 Hz, 2H, ArH), 7.39–7.08 (m, 5H, ArH), 6.40 (s, 1H, CH); 13C-NMR (75.5 MHz, DMSO-d6) δ (ppm): 195.8 (C=O), 172.5 (C=O), 162.8 (C, Ar), 160.4 (C, Ar), 149.1 (C, Ar), 149.0 (C, Ar), 138.6 (C, Ar), 135.3 (C, Ar), 131.1 (C, Ar), 130.7 (C, Ar), 128.8 (C, Ar), 127.2 (C, Ar), 124.5 (C, Ar), 120.9 (C, Ar), 118.9 (C, Ar), 117.0 (C, Ar), 115.0 (C, Ar), 111.8 (C, Ar), 109.8 (N–C=C), 105.8 (C=C), 54.1 (CH). Anal. calcd for C23H14BrN3O4: C, 58.00; H, 2.96; N, 8.82; found: C, 58.19; H, 2.88; N, 8.71%. HRMS (ESI): m/z (M)+ calcd. for C23H14BrN3O4+: 475.0168; found: 475.0140.
3-(2-(4-Chlorophenyl)-6-nitro-1H-indol-3-yl)quinoline-2,4(1H,3H)-dione (5c)
Yellow powder; yield 79% (341 mg); m.p. 249–250 °C; FTIR (KBr) ῡ (cm−1): 3396, 3097, 2962, 2869, 1653, 1604, 1550, 1535, 1496, 1350, 1318, 1233, 1089, 801, 766; 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 10.88 (s, 2H, exchanged by D2O, 2 × NH), 8.15–7.80 (m, 6H, ArH), 7.70–7.10 (m, 5H, ArH), 6.91 (s, 1H, CH); 13C-NMR (75.5 MHz, DMSO-d6) δ (ppm): 201.0 (C=O), 178.9 (C=O), 177.8 (C, Ar), 167.1 (C, Ar), 148.1 (C, Ar), 141.4 (C, Ar), 141.0 (C, Ar), 139.2 (C, Ar), 137.4 (C, Ar), 134.4 (C, Ar), 134.0 (C, Ar), 133.4 (C, Ar), 132.2 (C, Ar), 131.3 (C, Ar), 130.0 (C, Ar), 128.8 (C, Ar), 125.1 (C, Ar), 121.6 (C, Ar), 119.7 (N–C=C), 114.6 (C=C), 67.8 (CH). Anal. calcd for C23H14ClN3O4: C, 63.97; H, 3.27; N, 9.73; found: C, 64.07; H, 3.11; N, 9.80%. HRMS (ESI): m/z (M)+ calcd. for C23H14ClN3O4+: 431.0673; found: 431.0668.
3-(2-(4-Fluorophenyl)-6-nitro-1H-indol-3-yl)quinoline-2,4(1H,3H)-dione (5d)
Yellow powder; yield 79% (328 mg); m.p. 193–194 °C; FTIR (KBr) ῡ (cm−1): 3396, 3334, 3109, 2873, 1672, 1600, 1530, 1343, 1233, 1161, 1104, 996, 760; 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 11.56 (s, 2H, exchanged by D2O, 2 × NH), 8.11–7.90 (m, 2H, ArH), 7.85–7.64 (m, 2H, ArH), 7.50–6.86 (m, 7H, ArH), 6.39 (s, 1H, CH); 13C-NMR (75.5 MHz, DMSO-d6) δ (ppm): 195.0 (C=O), 162.8 (C=O), 160.5 (C, Ar), 149.0 (C, Ar), 142.9 (C, Ar), 138.7 (C, Ar), 132.7 (C, Ar), 132.0 (C, Ar), 131.1 (C, Ar), 130.6 (C, Ar), 129.7 (C, Ar), 124.4 (C, Ar), 122.7 (C, Ar), 120.9 (C, Ar), 119.1 (C, Ar), 116.8 (C, Ar), 115.0 (C, Ar), 111.8 (C, Ar), 109.8 (N–C=C), 105.7 (C=C), 54.1 (CH). Anal. calcd for C23H14FN3O4: C, 66.51; H, 3.40; N, 10.12; found: C, 66.42; H, 3.49; N, 10.02%. HRMS (ESI): m/z (M)+ calcd. for C23H14FN3O4+: 415.0968; found: 415.0981.
3-(6-Nitro-2-(p-tolyl)-1H-indol-3-yl)quinoline-2,4(1H,3H)-dione (5e)
Yellow powder; yield 84% (345 mg); m.p. 197–198 °C; FTIR (KBr) ῡ (cm−1): 3328, 3154, 3064, 2972, 2871, 1643, 1606, 1531, 1490, 1407, 1342, 1265, 1183, 1001, 871, 827, 759; 1H- NMR (300 MHz, DMSO-d6) δ (ppm): 11.55 (s, 2H, exchanged by D2O, 2 × NH), 7.94–7.53 (m, 4H, ArH), 7.50–6.87 (m, 7H, ArH), 6.36 (s, 1H, CH), 2.49 (s, 3H, Me); 13C-NMR (75.5 MHz, DMSO-d6) δ (ppm): 195.9 (C=O), 174.2 (C=O), 162.9 (C, Ar), 160.4 (C, Ar), 151.7 (C, Ar), 149.4 (C, Ar), 149.0 (C, Ar), 143.8 (C, Ar), 138.6 (C, Ar), 133.4 (C, Ar), 130.4 (C, Ar), 129.3 (C, Ar), 128.4 (C, Ar), 127.1 (C, Ar), 124.4 (C, Ar), 122.7 (C, Ar), 121.0 (C, Ar), 116.9 (C, Ar), 115.0 (N–C=C), 110.0 (C=C), 55.4 (CH), 33.3 (Me). Anal. calcd for C24H17N3O4: C, 70.07; H, 4.17; N, 10.21; found: C, 70.25; H, 4.03; N, 10.16%. HRMS (ESI): m/z (M)+ calcd. for C24H17N3O4+: 411.1219; found: 411.1200.
3-(2-(3-Methoxyphenyl)-6-nitro-1H-indol-3-yl)quinoline-2,4(1H,3H)-dione (5f)
Yellow powder; yield 82% (350 mg); m.p. 208–209 °C; FTIR (KBr) ῡ (cm−1): 3379, 3143, 3070, 2931, 2853, 1692, 1646, 1612, 1526, 1404, 1344, 1266, 1230, 1177, 873, 792, 741, 673; 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 11.60 (s, 2H, exchanged by D2O, 2 × NH), 8.05–7.90 (m, 1H, ArH), 7.74 (s, 1H, ArH), 7.54–6.88 (m, 9H, ArH), 6.39 (s, 1H, CH), 3.74 (s, 3H, OMe); 13C-NMR (75.5 MHz, DMSO-d6) δ (ppm): 196.0 (C=O), 163.0 (C=O), 160.5 (C, Ar), 159.4 (C, Ar), 149.3 (C, Ar), 149.0 (C, Ar), 138.7 (C, Ar), 137.3 (C, Ar), 132.6 (C, Ar), 131.2 (C, Ar), 129.2 (C, Ar), 124.4 (C, Ar), 121.0 (C, Ar), 120.3 (C, Ar), 119.0 (C, Ar), 116.9 (C, Ar), 115.0 (C, Ar), 111.9 (C, Ar), 109.9 (C, Ar), 108.0 (N–C=C), 105.7 (C=C), 56.6 (CH), 54.3 (OMe). Anal. calcd for C24H17N3O5: C, 67.44; H, 4.01; N, 9.83; found: C, 67.31; H, 4.12; 9.78%. HRMS (ESI): m/z (M)+ calcd. for C24H17N3O5+: 427.1168; found: 427.1186.
3-(2-(4-Methoxyphenyl)-6-nitro-1H-indol-3-yl)quinoline-2,4(1H,3H)-dione (5g)
Yellow powder; yield 81% (346 mg); m.p. 198–199 °C; FTIR (KBr) ῡ (cm−1): 3348, 3153, 3067, 2937, 2842, 1645, 1600, 1529, 1490, 1410, 1339, 1261, 1172, 1125, 1030, 995, 875, 841, 761; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 11.59 (s, 2H, exchanged by D2O, 2 × NH), 7.98 (d, J = 7.2 Hz, 2H, Ar), 7.18 (s, 1H, ArH), 7.46–7.11 (m, 5H, ArH), 6.94 (d, J = 6.9 Hz, 2H, ArH), 6.72–6.58 (m, 1H, ArH), 6.35 (s, 1H, CH), 3.75 (s, 3H, OMe); 13C NMR (75.5 MHz, DMSO-d6) δ (ppm): 194.6 (C=O), 163.5 (C=O), 162.9 (C, Ar), 160.4 (C, Ar), 149.4 (C, Ar), 148.9 (C, Ar), 138.6 (C, Ar), 132.6 (C, Ar), 131.6 (C, Ar), 130.5 (C, Ar), 129.3 (C, Ar), 129.2 (C, Ar), 128.4 (C, Ar), 124.4 (C, Ar), 122.1 (C, Ar), 121.0 (C, Ar), 119.0 (C, Ar), 116.9 (C, Ar), 115.1 (N–C=C), 110.0 (C=C), 56.5 (CH), 55.2 (OMe). Anal. calcd for C24H17N3O5: C, 67.44; H, 4.01; N, 9.83; found: C, 67.29; H, 3.93; N, 10.19%. HRMS (ESI): m/z (M)+ calcd. for C24H17N3O5+: 427.1168; found: 427.1189.
3-(2-(3,4-Dimethoxyphenyl)-6-nitro-1H-indol-3-yl)quinoline-2,4(1H,3H)-dione (5h)
Yellow powder; yield 80% (366 mg); m.p. 192–193 °C; FTIR (KBr) ῡ (cm−1): 3359, 2972, 2942, 2866, 1641, 1605, 1527, 1460, 1347, 1272, 1213, 1173, 1132, 1018, 887, 765, 668; 1H- NMR (300 MHz, DMSO-d6) δ (ppm): 11.66 (s, 2H, exchanged by D2O, 2 × NH), 7.95–7.55 (m, 4H, ArH), 7.52–6.91 (m, 6H, ArH), 6.38 (s, 1H, CH), 3.76 (s, 3H, OMe), 3.74 (s, 3H, OMe); 13C-NMR (75.5 MHz, DMSO-d6) δ (ppm): 194.5 (C=O), 163.0 (C=O), 160.4 (C, Ar), 153.4 (C, Ar), 149.3 (C, Ar), 148.5 (C, Ar), 138.6 (C, Ar), 131.2 (C, Ar), 130.5 (C, Ar), 129.2 (C, Ar), 128.1 (C, Ar), 127.8 (C, Ar), 124.2 (C, Ar), 122.1 (C, Ar), 120.9 (C, Ar), 119.0 (C, Ar), 116.9 (C, Ar), 115.0 (C, Ar), 111.9 (C, Ar), 110.1 (C, Ar), 108.0 (N–C=C), 105.7 (C=C), 56.9 (CH), 55.3 (OMe), 53.7 (OMe). Anal. calcd for C25H19N3O6: C, 69.64; H, 4.19; N, 9.19; found: C, 69.87; H, 4.00; N, 9.13%. HRMS (ESI): m/z (M)+ calcd. for C25H19N3O6+: 457.1274; found: 457.1265.
3-(6-Nitro-2-(4-nitrophenyl)-1H-indol-3-yl)quinoline-2,4(1H,3H)-dione (5i)
Orange powder; yield 78% (345 mg); m.p. 165–166 °C; FTIR (KBr) ῡ (cm−1): 3310, 3107, 3084, 2855, 1648, 1614, 1527, 1431, 1348, 1233, 1107, 852, 756; 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 11.54 (s, 2H, exchanged by D2O, 2 × NH), 8.89–8.23 (m, 5H, ArH), 8.80–6.70 (m, 6H, ArH), 6.46 (s, 1H, CH); 13C-NMR (75.5 MHz, DMSO-d6) δ (ppm): 196.3 (C=O), 160.5 (C=O), 149.8 (C, Ar), 149.1 (C, Ar), 146.8 (C, Ar), 143.2 (C, Ar), 141.9 (C, Ar), 141.8 (C, Ar), 138.8 (C, Ar), 131.2 (C, Ar), 130.6 (C, Ar), 130.3 (C, Ar), 128.1 (C, Ar), 127.8 (C, Ar), 125.0 (C, Ar), 122.7 (C, Ar), 119.4 (C, Ar), 117.0 (C, Ar), 115.0 (N–C=C) 109.6 (C=C), 66.2 (CH). Anal. calcd for C23H14N4O6: C, 62.45; H, 3.19; N, 12.66; found: C, 62.36; H, 3.37; N, 12.42%. HRMS (ESI): m/z (M)+ calcd. for C23H14N4O6+: 442.0913; found: 442.0924.
References
B.H. Rotstein, V. Zaretsky, R. Yudia, A.K. Yudia, Chem. Rev. 114, 8323 (2014)
S.B. Azimi, J. Azizian, Tetrahedon Lett. 57, 181 (2016)
Z. Fu, K. Qian, S. Li, T. Shen, Q. Song, Tetrahedron Lett. 57, 1104 (2016)
S.R. Vidadala, H. Waldmann, Tetrahedron Lett. 56, 3358 (2015)
P.G. Jessop, Green Chem. 13, 1391 (2011)
R.A. Sheldon, Green Chem. 7, 267 (2005)
K. Shanab, C. Neudorfer, E. Schirmer, H. Spreitzer, Curr. Org. Chem. 17, 1179 (2013)
J. Su, M. Zhuang, F. Ma, W.Q. Zhang, H. Sun, G. Zhang, Y. Jian, Z. Gao, Asian J. Org. Chem. 8, 482 (2019)
J. Mao, Z. Wang, X. Xu, G. Liu, R. Jiang, H. Guan, Z. Zheng, P.J. Walsh, Angew. Chem. 131, 11149 (2019)
R. Lai, X. Wu, S. Lv, C. Zhang, M. He, Y. Chen, Q. Wang, Y. Wu, Chem. Commun. 55, 4039 (2019)
J. Pan, R. Zhao, J. Guo, D. Ma, Y. Xia, Y. Gao, P. Xu, Y. Zhao, Green Chem. 21, 792 (2019)
H.R. Hudson, N.J. Wardle, S.W. Bligh, I. Greiner, A. Grun, G. Keglevich, Mini-Rev. Med. Chem. 12, 313 (2012)
C. Queffélec, M. Petit, P. Janvier, D.A. Knight, B. Bujoli, Chem. Rev. 112, 3777 (2012)
C.S. Demmer, N. Krogsgaard-Larsen, L. Bunch, Chem. Rev. 111, 7981 (2011)
T.V. Sravanthi, S.L. Manju, Eur. J. Pharm. Sci. 91, 1 (2016)
I. Andreadou, A. Tasouli, E. Bofilis, M. Chrysselis, E. Rekka, A. Tsantili-Kakoulidou, E. Iliodromitis, T. Siatra, D.T. Kremastinos, Chem. Pharm. Bull. 50, 165 (2002)
C. Karaaslan, H. Kadri, T. Coban, S. Suzen, A.D. Westwell, Bioorg. Med. Chem. Lett. 23, 2671 (2013)
M.M. Sayed, Y.M. Moustafa, A.N. Mohamed, Med. Chem. Res. 23, 3377 (2014)
T.C. Leboho, J.P. Michael, W.A.L. van Otterlo, S.F. van Vuuren, C.B. de Koning, Bioorg. Med. Chem. Lett. 19, 4948 (2009)
N.D. Kumar, M. Kumar, S. Sundaree, O.E. Johnson, K. Shah, Eur. J. Med. Chem. 45, 1244 (2010)
Y.Y. Li, H.S. Wu, L. Tang, C.R. Feng, H.Y. Yu, Y.S. Yang, B.J. Yang, Pharmacol. Res. 56, 335 (2007)
S. Kumar, H. Kaur, K.K. Saxena, M. Sharma, P. Vishwakarma, A. Kumar, Indian J. Chem. 49, 1398 (2010)
P. Rani, V.K. Srivastava, A. Kumar, Eur. J. Med. Chem. 39, 449 (2004)
H. Abdel-Gawad, H.A. Mohamed, K.M. Dawood, F.A. Badria, Chem. Pharm. Bull. 58, 1529 (2010)
W. Hu, Z. Guo, X. Yi, C. Guo, F. Chu, G. Cheng, Bioorg. Med. Chem. 11, 5539 (2003)
N.D. Kumar, M. Kumar, B. Noel, K. Shah, Eur. J. Med. Chem. 55, 432 (2012)
D.B. England, G. Merey, A. Padwa, Org. Lett. 19, 3805 (2007)
X. Ban, Y. Pan, Y.F. Lin, S.Q. Wang, Y.F. Du, K. Zhao, Org. Biomol. Chem. 10, 3606 (2012)
J.T. Kuethe, A. Wong, I.W. Davies, Org. Lett. 5, 3975 (2003)
G.W. Gribble, J. Chem. Soc. Perkin Trans. 1, 1045 (2000)
Z. Liang, J. Zhao, Y.J. Zhang, Org. Chem. 75, 170 (2010)
J.E. DeLorbe, S.Y. Jabri, S.M. Mennen, L.E. Overman, F.-L. Zhang, J. Am. Chem. Soc. 133, 6549 (2011)
T.A. Fenoradosoa, G. Ali, C. Delattre, C. Laroche, E. Petit, A. Wadouachi, P. Michaud, J. Appl. Phtcol. 22, 131 (2010)
P. Vauchel, R. Kaas, A. Arhaliass, R. Baron, J. Legrand, Food Bioprocess Tech. 1, 297 (2008)
M. Glicksman, Adv. Food Res. 11, 109 (1963)
S. Ilkhanizadeh, J. Khalafy, M.G. Dekamin, Int. J. Biol. Macromol. 140, 605 (2019)
B. Eftekhari-Sis, M. Zirak, A. Akbari, Chem. Rev. 113, 2958 (2013)
J. Khalafy, N. Etivand, S. Dilmaghani, M. Ezzati, A. Poursattar Marjani, Tetrahedron Lett. 55, 3781 (2014)
M. Ezzati, J. Khalafy, A. Poursattar Marjani, R.H. Prager, Tetrahedron 73, 6587 (2017)
A. Poursattar Marjani, J. Khalafy, F. Majidi Arlan, E. Eyni, ARKIVOC v, 1 (2019)
N. Etivand, J. Khalafy, A. Poursattar Marjani, Res. Chem. Intermed. 45, 3379 (2019)
J. Khalafy, N. Etivand, A. Poursattar Marjani, N. Khalillou, J. Heterocycl. Chem. 56, 1857 (2019)
A. Poursattar Marjani, J. Khalafy, S. Akbarzadeh, Green Process Synth. 8, 533 (2019)
A. Nouri, A. Poursattar Marjani, J. Khalafy, J. Heterocycl. Chem. 56, 2912 (2019)
A. Poursattar Marjani, J. Khalafy, P. Eslamipour, M. Ahmadi Sabegh, Iran J. Chem. Chem. Eng. 38, 51 (2019)
M. Aslanpanjeh, A. Poursattar Marjani, J. Khalafy, N. Etivand, Res. Chem. Intermed. 46, 165 (2020)
Acknowledgements
The authors are appreciatively thankful for the support provided by the Urmia University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nouri, A., Marjani, A.P., Khalafy, J. et al. A new and facile synthesis of 3-(2-aryl-6-nitro-1H-indol-3-yl)quinoline-2,4(1H,3H)-diones by sodium alginate as biopolymeric catalyst. Res Chem Intermed 46, 3025–3036 (2020). https://doi.org/10.1007/s11164-020-04129-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04129-4