Abstract
The effects of Zn/B nanofertilizer on the biophysical characteristics and growth of coffee seedlings in a greenhouse were investigated. Zn/B nanofertilizer was prepared by loading ZnSO4 and H3BO3 on a chitosan nanoparticles emulsion that was prepared by ionic gelation with tripolyphosphate. The nanofertilizer was characterized by TEM, SEM, zeta potential value and size distribution. The nanofertilizer was sprayed on the leaves of coffee seedlings in five different doses of 0, 10, 20, 30 and 40 ppm. Application of the nanofertilizer enhanced the uptake of zinc, nitrogen and phosphorus. The results were found to increase the chlorophyll content and photosynthesis of the coffee. Finally, the nanofertilizer promoted growth of the coffee plants in the leaf area, height of plant and stem diameter. The nanofertilizer seems to be a great potential foliar feed for the growth of coffee and other plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Micronutrients, such as Zn, Mn, B, Fe, etc., play very important roles in the metabolism and biophysical characteristics of plants because they act as cofactors to enhance the activity of enzymes. These roles significantly impact the growth of plants and crop yields [1,2,3].
Recently, nanotechnology has been widely used to produce nanofertilizers and for applications in agriculture. Nanofertilizers have a nanosize and can penetrate easily to the roots, cuticles and stomata of leaves, as well as being transported quickly in the xylem and phloem of plants [4,5,6,7,8]. Studies and applications of nanofertilizer to improve crops have been reported. Zinc oxide and titan oxide nanofertilizers increase the uptake of nutrition and chlorophyll content, and enhance the enzymatic activity that leads to improved growth, yields and quality of crops [9,10,11,12,13,14]. In addition, zinc oxide, titan oxide, manganese oxide and iron oxide nanoparticles are not only micronutrients for crops but also inhibit the growth of plant pathogen fungi [15,16,17].
Micronutrient nanofertilizers have been applied to some crops such as barley, spinach (Spinacia oleracea), soy beans (Glycin max L.), peanuts (Arachis hypogae), cluster beans (Cyamopsis tetragonoloba L.), chick peas (Cicer arietinum L.), pearl millet (Pennisetum americanum), etc. [10,11,12,13, 18, 19].
Generally, plants take up micronutrients from the soil. However, plants such as coffee may lack some micronutrients in a monocropping farm after a long period of cultivation. In fact, coffee plants cultivated in Central Highland, Vietnam, appear to have symptoms of zinc and boron deficiency. There are two ways to avoid deficiency of zinc and boron. Fertilizer containing the two compounds can be applied to the soil or sprayed as foliar fertilizer. However, these applications have low efficiency and any excess of zinc and boron may poison plants and pollute the environment [1].
Normally, materials used for prepared nanofertilizers are metal oxides such as iron oxide, titan oxide, manganese oxide, carbon nanotube, synthetic polymers, such as polyacrylic and polyacrylamit, or natural polymers such as chitin, chitosan, cellulose, etc. [9, 20,21,22,23,24]. Disadvantages of metal oxides and synthetic polymers are that they are not biocompatible or biodegradable. In contrast with synthetic polymers, natural polymers such as chitin and chitosan are biocompatible, biodegradable and friendly to the environment. Chitin, chitosan and chitosan nanoparticles have many bioactivities such as antibacterial and antifungal, are plant growth promoters and have the ability to increase the yield and quality of crops [25,26,27,28,29,30].
In this study, a chitosan nanoparticle emulsion loaded with zinc and boron are used to prepare a zinc/boron nanofertilizer. The aims of the study are to prepare a zinc/boron nanofertilizer, to evaluate the effects of the nanofertilizer on the biophysical properties and growth of coffee seedlings in greenhouses, and to determine the optimal dose of the nanofertilizer to enhance the growth of coffee seedlings.
Experimental
Materials and plants
Chitosan was purchased from Biosics, Canada. The degree of chitosan deacetylation was approximately 80–90%, as determined by IR spectroscopy. Sodium tripolyphosphate (TPP), ZnSO4, H3BO3 and acetic acid were the analytical chemicals (Merck, Germany) used. R4 hybrid coffee seedlings were purchased from the Western Highland Institute of Agricultural Science and Technology, Buon Ma Thuot City, Dak Lak Province, Vietnam. All seedlings used for our experiment had two pairs of leaves.
Preparation of Zn/B nanofertilizer
Chitosan nanoparticle emulsion was prepared by TPP ionic gelation [30]. In brief, 0.1% chitosan solution was added to acetic acid (adjusted by NaOH 1 M to pH 5.5). Then, 0.25% sodium tripolyphosphate (TPP) was dissolved in deionic water, and this solution was added to the chitosan solution and stirred by a magnetic stirrer at 900 rpm for 60 min at room temperature. The ratio of chitosan to TPP was 6:1 (w/w). The Zn/B nanofertilizer was prepared by dissolving 0.25% ZnSO4 and 0.25% H3BO3 in the chitosan nanoparticles suspension.
The chitosan nanoparticle suspension and Zn/B nanofertilizer were characterized by TEM (JEM-1400, Japan) and FE-SEM (JSM 7401F, Japan), and the size distribution and zeta potential were characterized by a Zetasizer Nano ZS ( Malvern, UK).
Slow release kinetic of Zn and B from nanofertilizer
A chitosan nanoparticle emulsion loaded with 0.25% ZnSO4 and 0.25% H3BO3 were investigated for the slow release kinetic of Zn and B every 24 h during a 240-h period at room temperature and pH 5.5. Every 24 h, 1 mL of the nanofertilizer was collected and then centrifuged at 15,000 rpm. The Zn and B content in the supernatant were determined by AAS (A 700; Shimadzu, Japan).
Experimental application of the nanofertilizer on the coffee seedlings in the greenhouse
To find the suitable dose of the nanofertilizer for the coffee seedlings, five different doses of the nanofertilizer were applied. The five doses were made up of 75 mL of 0 ppm (water), plus 10, 20, 30 and 40 ppm of the nanofertilizer based on the chitosan nanoparticle concentration applied for each plot. They were sprayed on both sides of the two leaves. The experiment was conducted in triplicate and contained 15 plots, each containing 15 plants. All the coffee seedlings were planted in black PE bags, consisting of 95% ferrasols and 5% degraded manure. During the experiment, the nanofertilizer was used three times within a period of 20 days. Irrigation and culture technique control assured that all the conditions for the coffee seedlings in all the plots were similar. In the greenhouse, air temperature was around 25 °C, humidity was 85% and light intensity was approximately 15,000 lx.
The growth of the coffee seedlings was determined by the number of leaves, the height of each plant, the area of the leaves and the diameter of the stems. The height of the coffee seedlings was measured with a ruler from the ground to the tip of the plants. The diameter of the stem was measured using a caliper. The area of the leaf was determined by measuring the length and diameter of the leaf using the following equation:
where S is the area of the leaf, a and b are the length and diameter of the leaf, and K is 0.66 (specific index for Robusta coffee leaf). All the parameters were measured from five plants for each plot, and all the data were the mean of each group of five plants. All the parameters of photosynthesis, such as CO2 concentration in integrated cells, stomatal conductance, transpiration rate and photosynthesis net rate, were measured by the PPsystem, PTS 2 (USA) at 0900–1000 hours each day.
Plant analysis
After 60 and 100 days of spraying with the nanofertilizer, 15 matured leaves from each plot were collected to analyze the nutritional content. The content of chlorophyll and carotenoid was determined following the spectrophotometry method of Yoshida and Forno (UV-Vis; Jasco V 630; Japan) [31]. After collecting the leaves, all the samples were dried at 105 °C until their weight was unchanged, and were then ground up for analysis. The content of P, K, Ca, Mg and Zn were analyzed by AAS (A 700; Shimadzu). The total amount of nitrogen was determined by the Kjeldahl method (Velp; UDK 139, Italy).
Statistical analysis
Statistical analysis was performed using the one-way analysis of variance followed by Duncan’s multiple range tests with triplicate using SAS 9.1 software. P value ≤ 0.05 were considered significant.
Results and Discussion
Characteristics of Zn/B nanofertilizer prepared by chitosan nanoparticle-loaded zinc and boron
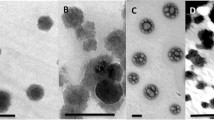
Chitosan nanoparticles were prepared by ionic gelation with TPP. The chitosan nanoparticles formed a suspension with slight turbidity. The morphology of chitosan nanoparticles was characterized by TEM and FE SEM images, as shown in Fig. 1, which, before loading zinc and boron, showed that they were nanospheres with a smooth and uniform surface. Size distribution of the chitosan nanoparticles had three ranges from 200 to 2000 nm and a mean size of 700 nm (Fig. 1a, b). The obtained results are the same as those in previously published works [24, 27, 30, 32].
After loading 0.25% ZnSO4 and 0.25% H3BO3 to make the nanofertilizer, the morphology changed, as shown in Fig. 1c, d. FE-SEM images indicate that the surface of the nanofertilizer was slightly swollen. The nanoparticles seemed not to be smooth, but had changed to rough after adsorption of the zinc and boron. The zeta potential of the nanofertilizer, shown in Fig. 2, indicates that the value was around 35 mV, which is as low as the chitosan nanoparticles after the same method and conditions used in our previous work. Nguyen et al. [30] indicated that the zeta potential values were around 47.7–54.5 mV because the chitosan nanoparticles have easily created a chelate form with the zinc and boron [25].
Size distribution of the nanofertilizer. Chitosan nanoparticles prepared under the following conditions: 0.1% chitosan solution; 0.25% TPP; chitosan: TPP ratio 6:1 (w/w). The Zn/B nanofertilizer was prepared by loading 0.25% ZnSO4 and 0.25% H3BO3 on chitosan nanoparticles. Size distribution of the nanofertilizer was determined by a Zetasizer Nano ZS (Malvern)
Size distribution of the nanofertilizer showed three sharp peaks in which almost all the particles of the nanofertilizer were focused on a peak at 1000 nm and a peak at 250 nm. There was a peak with a very small size of about 1–2 nm (Fig. 3). The smaller the size of the nanofertilizer, the more easily it penetrated into the stomata and cuticle of the plant cells to enhance the effectiveness of the nanofertilizer. Nair et al. [33] reported that particles may enter plant cells directly if the particle sizes are smaller than the size of the cell wall’s pores (5–20 nm). Recently, the size of micronutrient nanofertilizer prepared with metal oxides such as FeO, ZnO, MnO and Mo was around 20–250 nm [34].
Zeta potential of the nanofertilizer. Chitosan nanoparticles prepared under the following conditions: 0.1% chitosan solution; 0.25% TPP; chitosan: TPP ratio 6:1 (w/w). The Zn/B nanofertilizer was prepared by loading 0.25% ZnSO4 and 0.25% H3BO3 on chitosan nanoparticles. The zeta potential value was determined by a Zetasizer Nano ZS (Malvern)
Released kinetic of Zn and B from the nanofertilizer
After the chitosan nanoparticle emulsion was prepared by ionic gelation with 0.25% TPP, 0.25% ZnSO4 and 0.25% H3BO3 were completely dissolved in the emulsion, equivalent to 1000 mg/L zinc and 450 mg/L boron. The release kinetic of zinc and boron from the nanofertilizer (Fig. 4) showed that the loading efficiency of the zinc was around 65%, and the loading efficiency of the boron was 30% lower than that of zinc. Next, 650 mg/L zinc and 135 mg/L boron was loaded on the nanofertilizer. The results shown in Fig. 4 also indicate that the zinc and boron were released slowly from the nanofertilizer for 240 h. There was around 5% zinc and boron released from the nanofertilizer during 240 h. The release of micronutrients, such as zinc and boron, is very important for plants because they require very small amounts of micronutrients, and providing overdoses may be toxic to them [14].
Effect of the Zn/B nanofertilizer on biophysical characteristics of coffee seedlings in a greenhouse
Zinc and boron were found to be activators of enzymes that have a strong effect on the metabolism and biophysical characteristics of plants. Therefore, zinc and boron play very important roles in the growth and development of plants, such as the flowering and pollination of coffee. Zinc, boron and other micronutrients impact the metabolism of plants and affect the nutritional uptake. After application of the nanofertilizer, the coffee leaves were collected and their nutritional content was analyzed to evaluate the effect of the nanofertilizer on nutrient uptake. The results shown in Table 1 indicate that the application of a nanofertilizer enhanced the zinc content from 28 ppm (control) to 93 ppm (VL 30), and the zinc content increased when increasing the nanofertilizer concentration from 0, 10, 20 and 30 ppm. Wintgens [1] proposed these observations for four levels of zinc for Robusta coffee (Coffea canephora) as follows: < 20 ppm show a lack; 20–35 ppm is low; 35–60 ppm is optimal and > 65 ppm is excessive [1]. In this experiment, the zinc content was 28 ppm in the control which was found to be lower than the optimal level. Therefore, applying nanofertilizer containing zinc is necessary for the coffee. After comparison with the results shown in Table 1, it was found that application of the nanofertilizer at concentrations of 10–20 ppm supplied enough zinc for the coffee. Increasing the concentration to 30–40 ppm of the nanofertilizer was redundant for the requirements of the plants. The boron content was not determined because of an under-limit analysis. Recently, Wang et al. [35] sprayed nanofertilizer on watermelon plants and found that the Zn content in the leaves and roots increased by up to 13.9% compared by using ZnSO4.
The results (Table 1) show that there was an increase in N and P content of the leaves with application of the nanofertilizer. They increased when the nanofertilizer concentration was increased from 0 to 40 ppm. N content was 2.87% in VL0 whereas with VL10, N was 3.01% and increased up to 3.29% in VL20 and VL30 and up to 3.42% in VL40 (40 ppm) after the 60-day application. Nitrogen content at 3.2–3.3% is considered to be optimal for the growth and development of the coffee.
The results (Table 1) also show that P and Mg content increased with the increase in concentration of nanofertilizer, and that VL20 was the optimal concentration for P and Mg uptake.
Nguyen et al. [28] applied chitosan oligomer to coffee seedlings and found that it enhanced N, P and Mg content in the coffee leaves [24]. Nguyen et al. [24] reported that chitosan nanoparticles strongly affected the uptake of N, P and Mg in the coffee leaves [28]. This means that both chitosan in the nanofertilizer and the zinc and boron were reasons for the increase in nutritional uptake. Zinc nanofertilizer may stimulate activity of some enzymes with results such as 72.7% phytase, 22.58% alkaline phosphatase, 14.18% acid phosphatase and 9.22% dehydrogenase [19]. An increase in the enzymatic activity contributes to the enhancement of the metabolism and nutritional uptake of plants.
Chlorophyll is a very important component in the photosynthesis process in plants and it contributes significantly to the growth and yield of crops. The results shown in Table 2 demonstrate that the nanofertilizer enhanced the chlorophyll content in the leaves, increasing the chlorophyll when the concentration of the nanofertilizer was increased from 0 (1.07 mg g−1 fresh leaf) to 30 ppm (1.37 mg g−1 fresh leaf). With an increase to 40 ppm at VL40, the chlorophyll content did not increase but actually decreased to 1.33 mg g−1 in a fresh leaf. These results were the same as those for carotenoids. Compared to the results shown in Table 1, it was found that there was a relationship between N and Mg content and chlorophyll content. N and Mg are two extremely important elements in the chemical structure of chlorophyll: increasing N and Mg content in the leaves leads to an increase in chlorophyll content. These results are the same as those in our previous works and inother referenced works [24, 28].
Nguyen and Nguyen [36] reported that spraying 30 ppm chitosan oligomer on leaves increased the chlorophyll content 17.9% in soy beans and 23.0% in peanuts [36]. Limpanavech et al. [37] found that chitosan oligomer had an influence on the size of the stomata and chlorophyll of Dendrobium orchids at a concentration of 10–30 ppm.
The effect of zinc on chlorophyll has been reported by Tarafda et al. [19] with zinc nanofertilizer enhancing the chlorophyll of Pennisetum americanum by 18.4%. Raliya and Tarafdar [38] applied ZnO nanofertilizer to Cyamopsis tetragonoloba, increasing the chlorophyll by 54.5% and the protein ny 17.2%. Prasad et al. [39] sprayed nano-ZnO at 400–1000 mg/L concentration on peanut plants (Arachis hypogaea) and found an increase of up to 41% in leaf chlorophyll content compared with the control.
The effects of Zn/B nanofertilizer on the photosynthesis of coffee plants under greenhouse conditions are shown in Table 3. It has been found that zinc, boron and chitosan nanoparticles in the nanofertilizer increased the transpiration from 0.15 up to 0.35 mmol m−1 s−1, stomatal conductance increased from 16 to 32 mmol m−2 s−1 and CO2 concentration in integrated cells increased from 234 to 335 µmol mol−1. Our study found a relationship between the increase in stomatal conductance with CO2 concentration in integrated cells and the photosynthesis net rate, which increased from 2.4 (control) to 4.1 (VL30) and 7.2 µmol m−2 s−1 (VL30). When applying higher concentrations (VL40), the value of the photosynthesis net rate was found to decrease to 6.8 µmol m−2 s−1. Prasad et al. [39] also found that using an overdose of nano-ZnO for peanut plants could be toxic and decrease their growth. Burman et al. [18] applied nanoscale ZnO to cluster beans, Cicer arietinum L., and found that a concentration of 20 mg/L inhibited their growth.
These results are the same as those from our previous works [24, 28]. When Zn/B nanofertilizer promotes photosynthesis in coffee seedlings, it is possible that zinc and boron activates and enhances the enzymatic activity, as discussed in Tarafda’s work [19]. Tarafda et al. [19] researched nanoscale ZnO’s effects on peanut plants and found that zinc activated some enzymes and also increased the activity of phosphatase and dehydrogenase [19].
Effectiveness of Zn/B nanofertilizer on the growth of coffee seedlings in a greenhouse
The growth data for coffee plants treated with Zn/B nanofertilizer, such as the number of leaves, leaf area, the height of plants and diameter of stems, were observed after 100 days of spraying, as shown in Table 4. The results show that the data for growth before the fertilizer application were not significant between all of the plots (Table 4). After 100 days of application, all data for growth in the treated plots were found to improve in comparison with the control group. The average number of leaves increased from 5.60 to 6.73, compared with the control group (5.37). Leaf area of 5 plants in the treated plots was found to be 751.46–846.84 cm2/5 plants; this increased by 22.1–38.0% compared with the control group. These differences were significant between all the plots at P < 0.05.
The height of plants and the diameter of their stems are very important growth data used to evaluate the quality of coffee seedlings. The heights of the plants and diameters of the stems of the coffee seedlings undergoing application of the nanofertilizer were higher than those of the control group. The data for coffee in VL30 and VL40 were significantly different from the control group (Table 4).
Nguyen et al. [24] applied chitosan nanoparticles to coffee seedlings in a greenhouse and found that the growth of the coffee plants was strongly affected. After evaluation of data in this work, the results indicate that the effects of Zn/B nanofertilizer on the growth of coffee were stronger than the effects of chitosan nanoparticles. The nanofertilizer not only contains chitosan nanoparticles but also a supply of zinc and boron micronutrients.
The results obtained in this work were the same as for previously published works. Tarafdar et al. [19] applied Zn nanofertilizer at a concentration of 10 mg/L for Pennisetum americanum, and it was found to increase bud length by 10.8%, dry biomass by 10.2%, stem diameter by 18.4%, yield by 29.5% and leaf protein by 19.9% [19]. Raliya and Tarafdar [38] applied ZnO nanofertilizer to Cyamopsis tetragonoloba with a concentration of 10 mg/L which enhanced the phosphorous uptake, the biomass by 27.1%, the shoot length by 31.5%, the root length by 66.3% and the chlorophyll content by 276.2% [39].
Conclusions
It is concluded that chitosan nanoparticles prepared by ionic gelation are suitable for the synthesis of Zn/B nanofertilizer. The Zn/B nanofertilizer was adsorbed easily by the plant anbd has positive effects on the growth of coffee seedlings in a greenhouse by enhancing the nutritional uptake, cholorophyll content and photosynthesis. The suitable concentration of Zn/B nanofertilizer is 20–30 mg/L. Zn/B nanofertilizer is a high-tech fertilizer that can be studied on other crops.
References
J.N. Wintgens, Coffee: Growing, Processing Sustainable Production (Wiley VCH, Weinheim, 2004), pp. 246–269. ISBN 3-527-30731-1
Y. Poltronieri, H.E.P. Martines, P.R. Cecon, J. Sci. Food Agric. 91, 2431 (2011)
S. Pradhan, P. Patra, S. Das, S. Chandra, S. Mitra, K.K. Dey, S. Akbar, P. Palit, A. Goswami, Environ. Sci. Technol. 47, 13122 (2013)
T. Eichert, A. Kurtz, U. Steiner, H.E. Goldbach, Physiol. Plant. 134, 151 (2008)
E. Corredor, P.S. Testillano, M. José Coronado, P. González-Melendi, R. Fernández-Pacheco, C. Marquina, M. Ricardo Ibarra, J.M. de la Fuente, D. Rubiales, A.P. de Luque, M. Carmen Risueño, BMC Plant Biol. 9, 1 (2009)
M.C. De Rosa, C. Monreal, M. Schitzer, R. Walsh, Y. Sultan, Nat. Nanotechnol. 5, 91 (2010)
Z. Wang, X. Xie, J. Zhao, X. Liu, W. Feng, J.C. White, B. Xing, Environ. Sci. Technol. 46, 4434 (2012)
S. Huang, L. Wang, Y. Hou, L. Li, Agron. Sustain. Dev. 35, 369 (2015)
V. Ghormade, M.V. Deshpande, K.M. Paknikar, Biotechnol. Adv. 29, 792 (2011)
R. Liu, R. Lal, Sci. Rep. 4(5686), 1 (2014)
L. Zheng, F.S. Hong, S.P. Lu, C. Liu, Biol. Trace Elem. Res. 104, 83 (2005)
J.H. Priester, Y. Ge, R.E. Mielke, S.C. Moritz, K. Espinosa, Proc. Natl. Acad. Sci. 109, 2451 (2012)
P. Pradhan, P. Patra, S. Mitra, K.K. Dey, S. Jain, S. Sarkar, S. Roy, P. Palit, A. Goswami, J. Agric. Food Chem. 62, 8777 (2014)
R. Mohamadipoor, S. Sedaghathoor, A.M. Khomami, Eur. J. Exp. Biol. 3, 232 (2013)
C. Chen, Z. Gao, X. Qiu, S. Hu, Molecules 18, 7239 (2013)
K. Lamsal, S.W. Kim, J.H. Jung, Y.S. Kim, K.S. Kim, Y.S. Lee, Mycobiology 29, 26 (2011)
K. Giannousi, I. Avramidis, C. Dendrinou-Samara, RSC Adv. 3, 21743 (2013)
U. Burman, M. Saini, P. Kumar, Toxicol. Environ. Chem. 95, 605 (2013)
J.C. Tarafdar, R. Raliya, H. Mahawar, I. Rathore, Agric. Res. 3, 257 (2014)
L. Delagadino, C. Gonzalez, Rev. Mex. Ing. Quin. 15, 423 (2016)
E. Corradini, M.R. de Moura, L.H.C. Mattoso, Express Polym. Lett. 4, 509 (2010)
A. Servin, W. Elmer, A. Mukherjee, R. De la Torre-Roche, H. Hamdi, J.C. White, P. Bindranban, C. Dimkpa, J. Nanopart. Res. 17, 92 (2015)
M. Teodorescu, A. Lungu, P.O. Stanescu, C. Neamtu, Ind. Eng. Chem. Res. 48, 6527 (2009)
V.S. Nguyen, M.H. Dinh, A.D. Nguyen, Biocatal. Agric. Biotechnol. 2, 289 (2013)
M. Rinaudo, Prog. Polym. Sci. 31, 603 (2006)
L. Wu, M. Liu, Carbohydr. Polym. 72, 240 (2008)
T.K.N. La, S.L. Wang, M.H. Dinh, M.L. Phung, T.V. Nguyen, M.D. Tran, A.D. Nguyen, Res. Chem. Intermed. 40, 2165 (2014)
A.D. Nguyen, T.P.K. Vo, T.P. Khanh, T.D. Tran, Carbohydr. Polym. 84, 751 (2011)
A.D. Nguyen, Enhancing crop production with chitosan and its derivatives, in Chitin, Chitosan, Oligosaccharides and Their Derivatives: Biological Activity and Application, ed. by S.K. Kim (CRC Press, Taylor & Francis, Boca Raton, 2010), pp. 619–632
T.V. Nguyen, T.T.H. Nguyen, S.L. Wang, T.P.K. Vo, A.D. Nguyen, Res. Chem. Intermed. 43, 3527 (2017)
S. Yoshida, D. Forno, Laboratory Manual for Physiological Studies of Rice (IRRI, Los Baños, 1976), pp. 43–45
Q. Gan, T. Wang, C. Cochrane, P. McCarron, Colloids Surf. B Biointerface 44, 65 (2005)
R. Nair, S.H. Varghese, B.G. Nair, T. Maekawa, Y. Yoshida, D.S. Kuma. Plant Sci. 179, 154 (2010)
R. Liu, R. Lan, Sci. Tot. Env. 514, 131 (2015)
W.N. Wang, J.C. Tarafdar, P. Biswas, J. Nanopart. Res. 15, 1 (2013)
A.D. Nguyen, T.T. Nguyen, Adv. Chitin Sci. V, 463 (2002)
P. Limpanavech, S. Chaiyasuta, R. Vongpromek, R. Pichyangkura, C. Khunwasi, S. Chadchawan, P. Lotrakul, R. Bunjongrat, A. Chaidee, T. Bangyeekhun, Sci. Hortic. 116, 65 (2008)
R. Raliya, J.C. Tarafdar, Agric. Res. 2, 48 (2013)
T.N.V.K.V. Prasad, P. Sudahka, Y. Sreenivasulu, P. Latha, V. Munaswamy, K. Raja Reddy, T.S. Sreeprasat, S.R. Panikkanvalappil, P. Thalappil, J. Plant Nutr. 35, 905 (2012)
Acknowledgements
The authors are grateful to the Ministry of Education and Training, Vietnam, which supported a grant for this work (B2014-15-71) and the Ministry of Science and Technology, Taiwan (NSC 105-2313-B-032-001) which supported part of the grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, SL., Nguyen, A.D. Effects of Zn/B nanofertilizer on biophysical characteristics and growth of coffee seedlings in a greenhouse. Res Chem Intermed 44, 4889–4901 (2018). https://doi.org/10.1007/s11164-018-3342-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3342-z