Abstract
NPK nanofertilizer was prepared by loading nitrogen (N), phosphorous (P) and potassium (K) into chitosan nanoparticles. The chitosan nanoparticles were prepared via ionic gelation of tripolyphosphate and chitosan solution. The chitosan nanoparticles were characterized by SEM, TEM, zeta potential and size distribution. The results showed that size distribution was from 300 to 750 nm and zeta potential of around 50 mV. The released kinetics of nitrogen, phosphorous and potassium in nanofertilizer were also investigated for 240 h. The nanofertilizer was applied to coffee seedlings in a greenhouse condition. The results showed that the nanofertilizer enhanced uptake of nutrients, photosynthesis and growth of coffee plants. Application of the nanofertilizer improved 17.04% nitrogen, 16.31% phosphorous and 67.50% potassium content in the leaves of treated plots compared to the control; total chlorophyll content increased up to 30.68% and 71.7% of photosynthesis net rate. Application of nanofertilizer also enhanced leaf number, plant height and leaf area of the coffee seedlings. Using the nanofertilizer may be a potential way to enhance use efficiency of fertilizers for coffee.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fertilizers are indispensible to crops, provide necessary nutrients for crop growth, and increase crop yield and crop quality. The most important nutrients for almost all crops are macronutrients such as nitrogen, phosphorous and potassium. Nowadays, farmers have used much more chemical fertilizers to harvest higher yield and enhance economical effeciency. However, macronutrients use efficiency for crops is very low, such as use efficiency of nitrogen, potassium and phosphorous is about 30–60%; 30–50% and 10–20%, respectively. It means that there are about 50–70% nitrogen and potassium, and 80–90% phosphorous fertilizer that could not be taken up by crops and released to the environment. The result in this case is that it does not only increase input of production, but also reduces economical efficiency and enhances environmental pollution [1, 2].
Recently, there has been a new orientation to prepare and apply the nanofertilizers to enhance use efficiency of fertilizer, increase yield and quality of crops, reduce harmful effects of chemical fertilizers on environment and develop a green and sustainable agriculture [3,4,5,6,7,8,9,10,11,12]. Urea nanofertilizer prepared by coating urea into nanofilm has been successful using it for Canola [13]. Nano nitrogen fertilizer carried out by coating urea with sulfur and nano nitrogen chelate were effectively applied for potato to enhance yield and reduce nitrate leaching [12]. In recent years, Complex NPK nanofertilizer has been studied by many researchers. Wu and Liu [11] investigated loading NPK fertilizer into chitosan and coating the outer by poly (acrylic acid-co-acrylamide). Complex NPK nanofertilizer was prepared by trapping the fertilzer in polyacrylic hydrogel and invertigating the NPK slow release control of the nanofertilizer [14]. Chitosan and chitosan nanoparticles were applied as a useful matrix for loading nutrients for crops. Complex NPK fertilizer was loaded into chitosan nanoparticles, and chitosan nanoparticles with polymethacrylic acid (MMA) [15, 16].
The materials being used for preparation of nanofertilizer could be from inorganic, organic, synthesis polymer, natural polymer, and grafted copolymers between carbohydrate and synthetic polymer materials [4, 5, 9]. However, using synthetic polymer materials to prepare nanofertilizers seems to have some disadvantages such as their nonbiodegradable property that can be harmful to the environment and public health. In addition, synthetic polymers are much more expensive in producing nanofertilizer on an industrial scale.
Chitosan is a biopolymer of glucosamine and N-acetyl glucosamine residues, processed from seafood wastes. Chitosans are non-toxic, biocompatible, biodegradable, and friendly to the environment and have a great potential for agricultural application. Chitosan enhances growth and crop yield due to their bioactivities to plants such as stimulating growth of plants, seed germination, enhancing nutrient uptake, and antibacterial and antifungal activity [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. Moreover, chitosan nanoparticles have also a positive effect on biophysical aspects and growth of crops [34]. In our previous investigation, chitosan nanoparticles have effects on photosynthesis, nutrient uptake and growth of coffee seedlings [34]. Therefore, in this work chitosan nanoparticles were selected to use as nanomaterial for loading NPK with the idea of applying both bioactive effects of chitosan nanoparticles and NPK macronutrients to crops.
This work is to evaluate loading efficiency, slow release control of NPK from the nanoparticles and their effects on biophysical characteristics and the growth of coffee seedlings in greenhouse conditions.
Experimental
Materials and Plants
Chitosan was purchased from Biosics, Canada. The degree of chitosan deacetylation was approximately 80–90%, as determined by IR spectroscopy. The molecular weight of chitosan was 130 cPs, determined via its viscosity (Brookfield viscometer).
TPP (tripolyphosphate), acetic acid and KNO3 were high grade chemicals and purchased from Merck (Germany).
In this work, the TR4 clones of coffee seedlings were purchased from Western Highland Agricultural Scientific Technological Institute (WASI), Buon Ma Thuot, Vietnam to make sure that all the plants are not different genetically.
Preparation of nanofertilizer
Chitosan nanoparticles were prepared according to a modified ionic gelation method described by Gan et al. [35]. In brief, 0.1% chitosan solution was prepared by dissolving 0.1 g chitosan powder in 100 mL of 0.35% (w/v) acetic acid solution and kept overnight at room temperature. The chitosan solution was adjusted to pH 5.5 by 0.5 M NaOH solutions. 0.25% (w/v) TPP solution was dissolved in deionized water. And then, chitosan nanoparticles were prepared by dropping 0.25% TPP solution into the chitosan solution at a chitosan: TPP ratio of 6: 1 (w/w) while stirring at 900 rpm with a magnetic stirrer for 60 min at room temperature.
The nanofertilizer was prepared by adding 0.3% KNO3 into chitosan nanoparticles emulsion.
Particles size, zeta potential value and morphology of the nanoparticles
The morphology of the nanoparticles was investigated by field emission scanning electron microscopy (FE-SEM; model JSM 7401F, JEOL, Ltd., Japan) at an acceleration voltage of 15 kV. Zeta potential value and size distribution were determined by a Zetasizer Nano ZS (Malvern, UK).
Slow released control of NPK from nanofertilizer
Chitosan nanoparticles were loaded with 0.3% KNO3 (w/v) and studied for slow release for 240 h. After every 24 h, 1 mL of the nanofertilizer emulsion was collected and centrifuged at 15,000 rpm. The supernatant was measured for content of N, P and K to determine kinetics of slow release of N, P and K elements. Total phosphorous was measured by the colorimetric molybdate blue method by Spectrophotometer, Jasco, V630, Japan. Total potassium was determined by AAS (A7000, Shimazu, Japan). Total nitrogen was determined by the micro Kjeldahl method (Velp, Italia).
Experiment for coffee seedlings in greenhouse
The experiment was conducted in the greenhouse of the Faculty of Agriculture, Tay Nguyen University. The experiment was performed with six plots sprayed with water for control, 10, 20, 30, 40 and 50 ppm of NPK nanofertilizer emulsion on two sides of the leaves in triplicate and volume of 50 mL for 20 coffee seedlings of each plot. Coffee seedlings were planted in black polyethylene bags (size 15 × 25 cm), consisting of Ferrasols mixed with 5% degraded manure. During the experiment, application of NPK nanofertilizer was used three times with period of 15 days. The irrigation control assured that water content in the soil of all plots were similar. Fertilizing regime and the microclimate conditions were identical (average air temperature was 25 °C, humidity was 85% and light intensity was around 5000 lx). The growth of the coffee seedlings was determined by height of the plants, and diameter of the stem. The height of the coffee seedlings was measured by a ruler from ground to pinnacle of the plants. Diameter of the stem of coffee seedlings was measured by Palmer. Area of the leaf was determined by measuring length and diameter of the leaf by the following equation:
where S is leaf area; a and b are length and diameter of the leaf. K is 0.66 (specific index of Robusta coffee leaf). All parameters of the growth of coffee seedlings were measured for ten plants for each plot.
Parameters of photosynthesis such as CO2 concentration in cells, stomatal conductance, transpiration and photosynthesis intensity were determined by PPsystem, PTS 2 (USA).
Plant analysis
After 8 weeks spraying the NPK nanofertilizer with three applications, the matured leaves of the coffee seedlings were collected to analyze the nutrient content. The leaves were dried at 105 °C in the convention oven until their weight was unchanged and ground up for analysis. Total phosphorous was measured by the colorimetric molybdate blue method following Kjeldahl digestion (UV Vis spectrophotometer, Jasco V630, Japan). Total potassium, calcium and magnesium were measured by AAS (A7000, Shimazu, Japan).
Total nitrogen of the leaves was determined by the micro Kjeldahl method (Velp, Italia). Content of chlorophyll and carotenoid was determined from Yoshida and Forno [36] as follows: The leaves were cut in small pieces and immersed in 80% acetone solvent to extract pigments. The extraction solvent was diluted and measured with a spectrophotometer (UV Vis, Jasco V 630, Japan) at 663, 645 and 440.5 nm. The content of pigments was calculated by the equation:
where Ca, Cb (mg g−1 fresh leaf) are content of chlorophyll a and b; and Ccar (mg g−1 fresh leaf) is content of carotenoid. D663, D645 and D440.5 are optical density of the extract solution at 663, 645 and 440.5 nm.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) and followed by Duncan’s multiple range tests with triplicate by MSTATC software. α ≤ 0.05 considered as significant.
Results and discussion
Characteristics of nanofertilizer prepared by loading NPK on chitosan nanoparticles
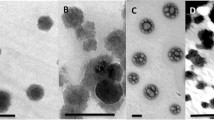
Preparation of Chitosan nanoparticles by ionic gelation with TPP is a relatively simple method, and cheap in expense. Therefore, it is a suitable method for agriculture application. The morphology of chitosan nanoparticles, characterized by SEM, TEM, size distribution and zeta potential value, affected the loading capacity of NPK nutrients. The results shown in Fig. 1 indicated that chitosan nanoparticles were a spherical surface with homogenous size distribution, stable and of fine shape. The spherical shape of chitosan nanoparticles possesses the highest surface area that is suitable for loading NPK nutrients on the surface of the nanoparticles. Sizes of the chitosan nanoparticles were distributed from 300 nm to 750 nm and mean size was around 500 nm (Fig. 2) and the zeta potential value of 50 mV. The higher zeta potential value nanoparticles have more adsorption capacity of negative charge ions such as NO3− and PO43− on their surface.
SEM of chitosan nanoparticles prepared by ionic gelation with TPP under condition: 0.1% chitosan solution, 0.25% TPP; Chitosan: TPP ratio 6:1 (w/w). The chitosan nanoparticle images were taken by field emission scanning electron microscopy FE-SEM (left) model JSM 7401F, JEOL, Japan) at an acceleration voltage of 15 kV. TEM (right) was taken by JEM-1400, JEOL, Japan
Chitosan nanoparticles, prepared by different methods such as ionic gelation with TPP, spray drying, nanocomposite of chitosan with polyacrylic acid, polymethacrylic acid…have different characteristics [4, 5, 7]. Moreover, the size distribution of the nanoparticles also depended on molecular weight, concentration and ratio of chitosan with other polymer and cross-linking agents. La et al. [25] that reported that the size distribution of chitosan nanoparticles prepared by spray drying is in a range of 95.5–395 nm and by spray drying combined with ionic gelation was in the size distribution of 166–1230 nm with the zeta potential value ranging from 34.9 to 59 mV [29]. In our previous work it was indicated that the the medium size distribution of chitosan nanoparticles (average size of 750 nm) had better effect on nutrient uptake, and growth of the coffee than bigger and smaller sizes [34]. Recent works that reported that a smaller size of nanofertilzer prepared by chitosan and polymethacrylic acid was distributed from 80 to 100 nm [15, 37]. According to the review of Ghormade et al. [4], the size of nanoparticles applied for nanofertilizers, nanopesticides and nanoherbicides in agriculture fields was normally from 100 to 500 nm. Therefore, the mean size of nanoparticles in this work was around 500 nm to be suitable for preparation of NPK nanofertilizer.
NPK nanoparticles were prepared by loading a suitable amount of KNO3 solution at pH 5.5 into chitosan nanoparticles. After loading, the total contents of N, P and K nutrients in nanofertilzer were analyzed for the loading capacity. The results indicated that loading capacity of NPK in nanofertilizer was in the amount of 0.21% N, 0.0004% P2O5 and 0.116% K2O which was higher than that of other NPK nanofertilizers. Hasaneen [38] and Corradini [15] prepared NPK nanofertilizer by a combination of chitosan nanoparticles with polymethacrylic acid and then loading urea, calcium phosphate and potassium chloride into the chitosan nanoparticles. The results indicated that the maximum N, P, K loaded into chitosan nanoparticles was 500 ppm N (0.05%), 400 ppm K (0.04%) and 60 ppm P (0.006%) which was lower than current study works. It could be because the chitosan nanoparticles will precipate at higher concentration [15, 16]. Wu and Liu [11] reported that a new NPK fertilizer granular prepared by coating three layers of chitosan with poly (acrylic -co- polyacrylmide) contained 7.98% K2O, 8.14% P2O5 and 8.06% N which was relatively high compared to current work and Hassaneen and Corradini’s works. However, this NPK fertilizer in a granular shape was of a bigger size than nanoparticles. Moreover, NPK fertilizers prepared by Wu & Liu and Hassaneen were synthesized in nonbiodegradable polymers as polymethacrylic acid, polyacrylic acid and polyacrylamide. Therefore, these NPK fertilizers can be harmful to the environment and public health [11, 16].
Slow release kinetics of the NPK nanofertilizer
The slow release kinetics of NPK nanofertilizer were analyzed by immersing it in pH 5.5 solution. The result of N, P and K slow release from the nanofertilizer is shown in Table 1. It indicated that nitrogen nutrient (NO3−) was slowly released from nanoparticles for the first 48 h with the relative release of 13.3%, and then quickly increased up to 60.0% from 48 h to 72 h later. Afterward, the relative release of nitrogen seemed to be unchanged with the release percentage from 60.0 to 63.2% from 72 to 192 h and got the release of about 66.7% after 240 h. There was, according to the results of Chao et al., [21] indication that the release of nitrogen of NPK fertilizer prepared by loading on the membrane of chitosan cross linked with suberoyl chloride was 60% nitrogen after the third h. The results in this work were accordance with Wu and Liu’s work (2008) that indicated that NPK released from chitosan nanoparticles was 15% by the third day and 75% by the 30th day, respectively [11]. The slow release of nitrogen in NPK fertilizer could be explained due to the ionic bond force between chitosan nanoparticles in positive charge (around 50 mV as shown in Fig. 2) and nitrogen in negative charge (NO3−). The slow release kinetics of potassium ions (Table 1) indicated that the fast release of K from chitosan nanoparticles was shown in the first 72 h, with the percentage of about 55.4% and stable from 55 to 58% for 240 h later. These results were in accordance with Santos [39]. In this work, chitosan nanospheres were prepared with montmorillonite and their mean size was of 200 nm and then, loading with KNO3. The result showed that release of K was also fast for first 3 days.
The slowest release kinetic amongst the NPK in naofertilizer was found in phosphorous nutrient with the relative release of about 3% and unchanged for the first 240 h. This result could be explained by content of phosphorous in the NPK nanofertilizer coming from TPP which was cross-linked with NH3+ residues in the chitosan chain.
Effect of NPK nanofertilizer on biophysical characteristics and growth of coffee seedlings in a greenhouse
NPK nanofertilizer was sprayed with different doses on the leaves of the coffee seedlings in a greenhouse to evaluate its effect on nutrient uptake, photosynthesis and growth of the coffee which are shown in Table 2. Results represented that NPK nanofertilizer significantly enhanced nitrogen and potassium content in the leaves of investigated coffee plants compared to that of control. Nitrogen content in the leaves increased from 3.15 to 3.57% compared to 3.05% in the control and increase in the doses trended to improving the uptake of nitrogen in the coffee leaves. Potassium content in the leaves increased from 1.169% in the control (NF0) to 1.959% in the NF30 experiment. In contrast, uptake of phosphorous, calcium and magnesium nutrients was not affected by spraying NPK nanofertilizer. The results shown in Table 2 also indicated that suitable doses of NPK nanofertilizer for improvement of nutrients uptake of coffee plant seedings in a green house was of 30 to 40 ppm.
The results in the current study are in accordance with some other works on using chitosan nanoparticles and nanofertilizer [34, 37]. Using only chitosan nanoparticles increased nutrient uptake as 9.8–27.4% N and 30–45% K [34]. Some works have reported that chitosan may directly affect gene expression, metabolism and induce many biological responses in the plants [3, 4]. These affects may directly or indirectly make an increase in uptake of nutrients in the leaves of plants. Ledezma-Delgadillo [37] reported that using N nanofertilizer based on chitosan nanoparticles and methacrylic acid possessed a higher N use efficiency compared to using free urea in the control. Nitrogen content in the leaves of lettuce in the use of urea nanofertilzer was the same as free urea in the control, but the amount of urea used in nanofertilizer accounted for only 16% of the control. It indicated that increasing uptake of nutrients may be effected by both chitosan nanoparticles and N, P and K nutrients loaded into chitosan nanoparticles.
Effects of the NPK nanofertilizer on photosynthesis of the coffee, the content of pigments and data of photosynthesis process were investigated and shown in Tables 3 and 4.
The results shown in Table 3 indicated that the nanofertilizer enhanced contents of chlorophyll and carotenoids in the coffee leaves with an incease in chlorophyll a, chlorophyll b and carotenoid of the percentage of 14.8–27.7% of, 14.7–35.2% of and 10.7–25.0%, respectively, compared to the control. These results may be due to the improvement of the nutrients uptake of the nanofertilizer as shown in Table 2, and in accordance with some previous works [28, 34]. Limpanavech [40] proposed that chitosan can induce expression of chloroplast gene in plants that lead to the increase in chlorophyll content of leaves [26].
Photosynthesis is the most important physiological process of plants; it affects directly the growth, biomass, yield and quality of crops. Effect of NPK nanofertilizer on data of photosynthesis is shown in Table 4 which indicates that the nanofertilizer had a remarkable effect on photosynthesis of coffee as photosynthesis net rate, CO2 concentration integrated cells, stomatal conductance and transpiration rate. All photosynthesis data in Table 4 were increased when treating with NPK nanofertilizer. Stomatal conductance improved from 12.5 to 54.1% of treated plots compared to the control. Increase in stomatal conductance lead to the improvement of CO2 concentration in integrated cells up to 31.3–47.8% of NF30, NF40 and NF50 plots. These results can be explained that positive charge of chitosan nanoparticles and potassium may increase osmosis pressure of stomatal cells that lead to improving the opening of stomatal cells and stomatal conductance. As CO2 gas is a very important component for photosynthesis reaction of plants, these results contributed to the enhancement of the photosynthesis net rate of the coffee. Photosynthesis net rate was of 4.6 µmol m−2 s−1 in the control and increased from 6.2 to 7.9 µmol m−2 s−1 (34.7–71.7%) in the NF20 to NF50 plots. In comparison to our previous work, the results also showed that NPK nanofertilizer had a greater effect on the photosynthesis process of the coffee than only chitosan nanoparticles [34].
The affects of NPK nanofertilizer on the enhancement of nutrient uptake, chlorophyll content and photosynthesis of the coffee contributed to the improvement of growth data as shown in Table 5.
The results shown in Table 5 indicate that before experiments, all growth data such as leaf number, leaf area, and plant height and stem diameter were not significantly different between all plots but after 60 days of the application of the nanofertilizer, all growth data of the coffee were higher than the control. Leaf number was of 3.07 in the control compared to 3.77 in the NF30 plot and leaf area dramatically increased from 540.13 cm2 in the control to 793.36 cm2 in the NF30 plot. The difference of leaf area and leaf number between plots was of significant difference (α < 0.05) (see Table 5). Moreover, the plant height in the treated plots was from 36.13 to 40.27 cm which was higher than the 35.72 cm of the control. The application of nanofertilizer also increased stem diameter of the coffee up to 0.59 cm of NF30 compared to 0.46 cm of the not treated coffee seedlings. The results shown in Table 5 also indicate that the suitable dose of the nanofertilizer for the growth of the coffee seedlings was of 30 ppm and with each two weeks for application.
In recent years, the effects of chitosan, chitosan nanoparticles and nanofertilizer based on chitosan nanoparticles on growth and yield crops have been reported [4, 5, 12, 34, 37, 41, 42]. To enhance N use efficiency of nano nitrogen chelate, sulfur coated nano nitrogen chelate and sulfur coated urea were applied for potatoes in the field. The results indicated that potato yield increased by 56.10, 59.61 and 49.76%, respectively. Application of nano nitrogen fertilizer also reduced nitrate leaching in potato to improve the quality [12]. Ledezma-Delgadillo [37] applied nitrogen nanofertilizer based on chitosan nanoparticles and polymethacrylic acid. Amount of nitrogen (urea) use was only 16% compared to free urea for lettuce. It means that nanofertilizer can be slow release and this property leads to using fertilizer with higher efficiency [37]. Foliar application of chitosan oligomer with molecular weight of 2 kDa and concentration of 40 ppm increased the growth and yield of coffee in the field [28]. Application of chitosan oligomer also improved the growth and yield of peanut, soybean and other crops [7, 17].
Conclusions
Chitosan nanoparticles prepared by ionic gelation with TPP can be a potential controlled slow release carrier to produce NPK nanofertilizer. Moreover, the NPK nanofertlizer had a strong effect on biophysical characteristics such as nutrient uptake, chlorophyll content, photosynthesis process and growth of the coffee seedlings in a greenhouse. These results concluded that NPK nanofertilizer can be a novel fertilizer for developing a green and sustainable agriculture in the near future.
References
B. Sun, L. Zhang, L. Yang, F. Zhang, D. Norse, Z. Zhu, Ambio 41, 370 (2012)
M.M. Trenkel, Controlled release and stabilized fertilizer in agriculture (International Fertilizer Industry Association, Paris, 1997)
E. Corredor, P.S. Testillano, M. José Coronado, P. González-Melendi, R. Fernández-Pacheco, C. Marquina, M. Ricardo Ibarra, J.M. de la Fuente, D. Rubiales, A.P. de Luque, M. Carmen Risueño, BMC Plant Biol. 9, 1 (2009)
V. Ghormade, M.V. Deshpande, K.M. Paknikar, Biotechnol. Adv. 29, 792 (2011)
S. Huang, L. Wang, Y. Hou, L. Li, Agron. Sustain. Dev. 35, 369 (2015)
M. Khodakovskaya, E. Dervishi, M. Mahmood, Y. Xu, Z. Li, F. Watanabe, A.S. Bris, ACS Nano 3, 3221 (2009)
R. Liu, R. Lal, Sci. Rep. 4(5686), 1 (2014)
T.N.V.K.V. Prasad, P. Sudahka, Y. Sreenivasulu, P. Latha, V. Munaswamy, K. Raja Reddy, T.S. Sreeprasat, S.R. Panikkanvalappil, P. Thalappil, J. Plant Nutr. 35, 905 (2012)
A. Servin, W. Elmer, A. Mukherjee, R. De la Torre-Roche, H. Hamdi, J.C. White, P. Bindranban, C. Dimkpa, J. Nanopart. Res. 17, 92 (2015)
J.C. Tarafdar, R. Raliya, H. Mahawar, I. Rathore, Agric. Res. 3, 257 (2014)
L. Wu, M. Liu, Carbohydr. Polym. 72, 240 (2008)
H. Zareabyaneth, M. Bayatvarkeshi, Environ. Earth Sci. 74, 3385 (2015)
M.C. De Rosa, C. Monreal, M. Schitzer, R. Walsh, Y. Sultan, Nat. Nanotechnol. 5, 91 (2010)
M. Teodorescu, A. Lungu, P.O. Stanescu, C. Neamtu, Ind. Eng. Chem. Res. 48, 6527 (2009)
E. Corradini, M.R. de Moura, L.H.C. Mattoso, Express Polym. Lett. 4, 509 (2010)
M. Khodakovskaya, E. Dervishi, M. Mahmood, Y. Xu, Z. Li, F. Watanabe, A.S. Biris, ACS Nano 3, 3221 (2009)
A.M.R. Abdel-Mawgoud, A.S. Tantawy, M.A. El-Nemr, Y.N. Sassine, Eur. J. Sci. Res. 39, 161 (2010)
C. Akimoto Tomiyama, K. Sakata, J. Yasaki, J. Yazaki, K. Nakamura, F. Fujii, K. Shimbo, K. Yamamoto, T. Sasaki, S. Kikuchi, N. Shibuya, E. Minami, Plant Mol. Biol. 52, 537 (2003)
N.M. Alves, J.F. Mano, Int. J. Biol. Macromol. 43, 401 (2008)
M. Bitelli, M. Flury, G.S. Campbell, E.J. Nichols, Agric. For. Meteorol. 107, 167 (2001)
C. Chao, Z. Gao, X. Qui, S. Hu, Molecules 18, 7239 (2013)
A.G. Chmielewski, W. Migdal, J. Swietoslawski, U. Gryczka, T. Tarnowski, Radiat. Phys. Chem. 76, 1840 (2007)
Z. Gou, R. Xing, S. Liu, Carbohydr. Polym. 71, 694 (2008)
A. El Hadrami, L.R. Adam, I. El Hadrami, F. Daayf, Mar. Drugs. 8, 968 (2010)
T.K.N. La, S.L. Wang, M.H. Dinh, M.L. Phung, T.V. Nguyen, M.D. Tran, A.D. Nguyen, J. Res. Chem. Intermed. 40, 2165 (2014)
R. Liu, R. Lal, Sci. Rep. 4(5686), 1 (2014)
M. Rinaudo, Prog. Polym. Sci. 31, 603 (2006)
A.D. Nguyen, T.P.K. Vo, T.D. Tran, Carbohydr. Polym. 84, 751 (2011)
T.V. Nguyen, T.T.H. Nguyen, S.L. Wang, T.P.K. Vo, A.D. Nguyen, Res. Chem. Intermed. 43(6), 2537 (2017).
K.L. Nge, N. Nitar, S. Chandrkrachang, W.F. Steven, Plant Sci. 170, 1185 (2006)
R.G. Sarathchandra, S.N. Jaj, Crop. Prot. 23, 881 (2004)
A.F.M.J. Uddin, F. Hashimoto, K. Shimizu, Sci. Hortic. 100, 127 (2004)
H. Yin, X. Zhao, Y. Du, Carbohydr. Polym. 82, 1 (2010)
V.S. Nguyen, M.H. Dinh, A.D. Nguyen, Biocatal. Agric. Biotechnol. 2, 289 (2013)
Q. Gan, T. Wang, C. Cochrane, P. McCarron, Colloids Surf. B 44(2–3), 65 (2005)
S. Yoshida, S.D. Forno, Laboratory Manual for Physiological Studies of Rice (IRRI, Los Banos, 1976), p. 43
A. Ledezma-Delgadillo, R. Carrillo-Gonzalez, E. San Martin-Martinez, M.R. Jaime-Fonseca, M.A. Chacon-Lopez, Rev. Mex. Ing. Quim. 15, 423 (2016)
M.N.A. Hasaneen, H.M.M. Abdel-Aziz, D.M.A. El-Bialy, A.M. Omer, Afr. J. Biotechnol. 13(31), 3158 (2014)
B.R.D. Santos, F.B. Bacalhau, T.D.S. Pereira, C.F. Souza, R. Faez, Carbohydr. Polym. 127, 340 (2015)
P. Limpanavech, S. Chaiyasuta, R. Vongpromek, R. Pichyangkura, C. Khunwasi, S. Chadchanwan, Sci. Hort. 116, 65 (2008)
D. Liu, E. Xing, Envron. Sci. Technol. 42, 5580 (2008)
R. Liu, R. Lal, Sci. Rep. 4(5686), 1 (2014)
Acknowledgements
The authors express to thank Ministry of Education and Training, Vietnam supported a grant for this work (Code: B2014-15-71) and a part of the grant by the Ministry of Science and Technology, Taiwan (NSC 102-2313-B-032-001-MY3).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ha, N.M.C., Nguyen, T.H., Wang, SL. et al. Preparation of NPK nanofertilizer based on chitosan nanoparticles and its effect on biophysical characteristics and growth of coffee in green house. Res Chem Intermed 45, 51–63 (2019). https://doi.org/10.1007/s11164-018-3630-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3630-7