Abstract
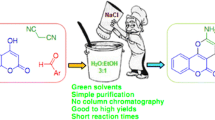
An efficient and practical protocol for the synthesis of chromenes derivatives catalyzed by a low-loading sodium ethylene diamine tetraacetate (2 mol%) as a catalyst via multicomponent reaction is reported. A wide range of aromatic aldehydes easily undergo condensations with malononitrile and 4-hydroxycoumarin (4-hydroxy-6-methyl-2-pyrone or dimedone) under mild conditions to afford the desired products of good purity in excellent yields. This protocol has several advantages, such as mild conditions, high yields, and an inexpensive catalyzed system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromenes are the specific selection owing to their important pharmaceutical and biological properties, whose derivatives exist widely in natural products and exhibit many features such as anticancer, anti-anaphylactin, anticoagulant, diuretic, and spasmolytic activitites [1,2,3,4]. Especially, 2-amino-4H-chromenes have been applied to cure cancer, psoriasis and rheumatoids, and are also widely used in laser dyes, optical brighteners, pigments, cosmetics and agrochemicals (Fig. 1) [1, 4,5,6,7]. Hence, the preparation of 4H-pyrans has been receiving renewed interest of researchers because of their wide range of biological, industrial and synthesis applications. Furthermore, the development of a practical methodology is available to synthesize biologically active heterocyclic compounds and often plays an important role in pharmaceutical discovery. The multicomponent reactions (MCRs) strategy, which has considerable merits over conventional two-component reactions in some aspects, such as variable and high bond forming efficiency and atom economy, have been broadly applied in combinatorial and medicinal chemistry [8].
Many protocols for synthesis have been reported, including the use of microwaves, [9] ultrasonic irradiation [10] and a variety of reagents such as cerium(III) chloride [11], acidic ionic liquids [12, 13], deep eutectic solvents [14, 15], Sc(OTf)3 [16], NbCl5 [17], l-proline [18], ZnO zeolite [19], meglumine [20], silica-bonded S-sulfonic acid [21], glycerol [22], Cu(II) oxymetasilicate [23], potassium phosphate [24], sodium selenate [25], S-proline [26], MgO [27], tetrabutylammonium bromide [28], CuO nanoparticles [29], Fe2O3 nanoparticles [30], starch solution [31], Fe3O4@SiO2 magnetic nanocatalyst [32], and RuBr2(PPh3)4 [33]. Although these methods show many advantages, some of them meet limitations in special aspects such as sekeletoncomplicated preparation of catalyst, long reaction times, relatively expensive catalyzed systems, harsh reaction conditions, and even tedious work-ups. As our goal is to design an inexpensive catalyzed system to promote the synthesis of various biologically active chromenes, we have to note that the price of EDTA-4Na is US$33 per 100 g. Hence, we hope to report here sodium ethylene diamine tetraacetate (EDTA-4Na) as a cheap and efficient catalyst for the synthesis of chromenes under mild conditions via MCRs strategy.
Experimental
Apparatus and analysis
Unless otherwise noted, all commercial materials and solvents were used without further purification. Melting points were measured on an Electrothemal X6 microscopy digital melting point apparatus and are corrected. IR spectra were obtained as potassium bromide pellets or as liquid films between two potassium bromide pellets with a Brucker Vector 22 spectrometer. 1H NMR spectra were recorded in DMSO at 300 or 400 MHz, and 13C NMR spectra were recorded in DMSO at 75 or 100 MHz, respectively. C, H and N analysis were performed by a Perkin-Elmer 2400 CHN elemental analyzer. HRMS was carried out on a MAT 95XP (Thermo).
General procedures for synthesis of chromenes
An equimolar (2 mmol) mixture of an aromatic aldehyde (1), malononitrile (2), 4-hydroxycoumarin (3) or 4-hydroxy-6-methyl-2-pyrone (5) or dimedone (7) and 2 mol% sodium ethylene diamine tetraacetate was vigorously stirred at 50°C in 5 mL EtOH:H2O (40:60) for the specific times indicated in Tables 2 and 3. The end of the reaction was monitored by TLC. Then, the crude product obtained was poured into cold water. The resulting precipitated solid was purified by recrystallization from hot methanol to afford the pure products 4 or 6 or 8. The structures of all the products were identified by IR, 1H NMR, 13C NMR, elemental analysis and HRMS spectra. The spectral data of selected products are given below:
2-Amino-5-oxo-4-phenyl-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile (4a)
IR (KBr): 3379, 3288, 3181, 2199, 1710, 1675, 1607 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 7.90(d, J = 7.8 Hz, 1H, ArH), 7.65–7.68(m, 1H, ArH), 7.40–7.48(m, 2H, ArH), 7.20–7.32(m, 5H, ArH), 4.41(s, H, CH). 13C NMR: (75 MHz, DMSO-d 6) δ 160.6, 158.9, 154.5, 153.2, 144.3, 133.9, 129.7, 128.7, 128.2, 125.8, 123.6, 120.4, 117.6, 114.3, 105.4, 59.1. HRMS Calculated for C19H12N2O3: 316.0848, found: 316.0842.
2-Amino-4-(4-chlorophenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile (4c)
IR (KBr): 3386, 3313, 3191, 2194, 1714, 1677, 1608 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 7.88(d, J = 7.9 Hz, 1H, ArH), 7.65–7.68(t, H, ArH), 7.39–7.47(m, 2H, ArH), 7.34(d, J = 8.5 Hz, 2H, ArH), 7.26(d, J = 8.5 Hz, 2H, ArH), 4.44(s, H, CH). 13C NMR: (75 MHz, DMSO-d 6) δ 160.6, 159.2, 154.6, 153.3, 143.3, 133.9, 132.8, 130.6, 129.4, 125.7, 123.6, 120.1, 117.3, 113.9, 104.6, 58.8. HRMS Calculated for C19H11ClN2O3: 350.0458, found: 350.0455.
2-Amino-4-(4-nitrophenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile (4f)
IR (KBr): 3480, 3430, 3370, 3337, 2196, 1718, 1674, 1606 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 8.15(d, J = 8.6 Hz, 1H, ArH), 7.91(d, J = 7.7 Hz, 1H, ArH), 7.67–7.72(m, 1H, ArH), 7.55(d, J = 8.6 Hz, 2H, ArH), 7.44–7.49(m, 2H, ArH), 4.63(s, H, CH). 13C NMR: (75 MHz, DMSO-d 6) δ 160.7, 159.3, 154.9, 153.5, 151.8, 147.6, 134.2, 130.3, 125.8, 124.7, 123.5, 119.9, 117.6, 113.9, 103.8, 57.9. HRMS Calculated for C19H11N3O5: 361.0699, found: 361.0695.
2-Amino-4-(4-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile (4j)
IR (KBr): 3391, 3314, 3194, 2196, 1715, 1676, 1609 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 7.87(d, J = 7.6 Hz, 1H, ArH), 7.54–7.57(m, 1H, ArH), 7.39–7.43(m, 2H, ArH), 7.13(d, J = 8.5 Hz, 2H, ArH), 6.83(d, J = 7.7 Hz, 2H, ArH), 4.41(s, H, CH), 3.73(s, 3H, CH3). 13C NMR: (75 MHz, DMSO-d 6) δ 160.8, 159.6, 158.9, 154.2, 152.9, 136.5, 133.8, 129.6, 125.3, 123.5, 120.5, 117.6, 114.9, 113.9, 105.3, 59.3, 56.4. HRMS Calculated for C20H14N2O4: 346.0954, found: 346.0948.
2-Amino-7-methyl-5-oxo-4-phenyl-4H,5H-[4,3-b]pyran-3-carbonitrile (6a)
IR (KBr): 3397, 3316, 3201, 2927, 2198, 1712, 1677, 1634 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 7.19–7.37(m, 5H, ArH), 6.29(s, 1H, CH), 4.28(1H, CH), 2.22(3H, CH3). 13C NMR: (75 MHz, DMSO-d 6) δ 162.7, 161.0, 158.7, 157.8, 143.2, 133.5, 128.1, 127.2, 126.6, 119.0, 112.5, 100.4, 97.6, 57.5, 36.0, 19.1; HRMS Calculated for C16H12N2O3: 280.0848, found: 280.0842.
2-Amino-4-(4-methoxyphenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile (6d)
IR (KBr): 3398, 3321, 3202, 2925, 2197, 1713, 1675, 1642 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 7.13–6.83(m, 4H, ArH), 6.31(s, 1H, CH), 4.56(s, 1H, CH), 3.63(s, 3H, CH3), 2.20(s, 3H, CH3); 13C NMR (75 MHz, DMSO-d6): δ 163.8, 161.5, 159.9, 156.6, 132.7, 130.5, 129.4, 126.6, 122.3, 120.5, 113.8, 101.5, 99.6, 58.3, 55.6, 38.3, 20.8; HRMS Calculated for C17H14N2O4: 310.0954, found: 310.0947.
2-Amino-3-cyano-4-(4-benzyl)-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran (8a)
IR (KBr): 3396, 3325, 3252, 3212, 2964, 2200, 1681, 1605, 1215, 1038 cm−1; 1H NMR (400 M, DMSO-d6): δ (J, Hz): 0.90 (s, 3H, CH3), 0.99 (s, 3H, CH3), 2.05 (d, J = 16.2 Hz, 1H), 2.21 (d, J = 16.2 Hz, 1H), 3.57 (s, 2H, CH2), 4.12 (s, 1H, CH), 7.11–7.20 (m, 3H, ArH), 7.25–7.29 (m, 2H, ArH). 13C NMR (100 M, DMSO-d6): 196.7, 163.6, 159.6, 145.8, 129.4, 128.2, 127.7, 120.8, 113.8, 59.4, 51.1, 40.3, 36.7, 32.9, 29.5, 27.9. Found, %: C 73.39; H 6.33; N 9.59. C18H18N2O2. Calculated, %: C 73.45; H 6.16; N 9.52.
2-Amino-3-cyano-4-(4′-chlorobenzyl)-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran (8b)
IR (KBr):: 3382, 3184, 2189, 1675, 1605, 1217, 1034 cm−1; 1H NMR (400 M, DMSO-d6): (J, Hz): 0.91 (s, 3H, CH3), 0.99 (s, 3H, CH3), 2.09–2.24 (m, 2H, CH2), 2.48 (s, 2H, CH2), 4.16 (s, H, CH), 7.13 (d, J = 8.5 Hz, 2H, ArH), 7.31 (d, J = 8.6 Hz, 2H, ArH). 13C NMR (100 M, DMSO-d6): 196.8, 163.9, 159.7, 144.7, 132.2, 129.8, 129.1, 120.2, 113.8, 58.9, 51.2, 40.6, 36.8, 33.4, 29.7, 28.2. Found, %: C 65.70; H 5.31; N 8.58. C18H17ClN2O2. Calculated, %: C 65.75; H 5.21; N 8.52.
2-Amino-3-cyano-4-(4′-nitrobenzyl)-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran (8d)
IR (KBr): 3383, 3330, 2191, 1677, 1606, 1216, 1039, 1210 cm−1; 1H NMR (400 M, DMSO-d6): 0.91 (s, 3H, CH3), 1.00 (s, 3H, CH3), 2.10–2.25 (s, 3H, CH3), 2.38–2.36 (m, 2H, CH2), 2.44 (s, 2H, CH2), 4.32 (s, 1H, CH), 7.41 (d, J = 8.7 Hz, 2H, ArH), 8.14 (d, J = 8.6 Hz, 2H, ArH). 13C NMR (100 M, DMSO-d6): 197.3, 165.5, 159.7, 153.4, 147.5, 129.6, 124.8, 120.5, 112.7, 57.9, 50.6, 40.6, 36.8, 32.9, 29.5, 27.9. Found, %: C 63.68; H 4.98; N 12.45. C18H17N3O4. Calculated, %: C 63.71; H 5.05; N 12.38.
2-Amino-3-cyano-4-(4′-methylbenzyl)-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran (8l)
IR (KBr): 3427, 3331, 3222, 2958, 2192, 1677, 1603, 1207, 1034 cm−1; 1H NMR (400 M, DMSO-d6): 0.90 (s, 3H, CH3), 0.99 (s, 3H, CH3), 2.20(s, 3H, CH3), 2.04–2.21 (m, 2H, CH2), 2.48 (s, 2H, CH2), 4.08 (s, 1H, CH), 6.98(d, J = 8.1 Hz, 2H, ArH), 7.05 (d, J = 8.1 Hz, 2H, ArH). 13C NMR(100 M, DMSO-d6): 196.7, 163.4, 159.5, 142.9, 136.6, 129.9, 128.2, 120.8, 113.9, 59.4, 50.8, 40.3, 36.3, 32.8, 29.5, 27.8, 21.6. Found, %: C 73.96; H 6.52; N 9.16.C19H20N2O2. Calculated, %: C 74.00; H 6.54; N 9.08.
2-Amino-3-cyano-4-(4′-methoxybenzyl)-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran (8m)
IR (KBr): 3374, 3323, 3183, 2194, 1684, 1607, 1214, 1035 cm−1; 1H NMR (400 M, DMSO-d6): 0.90 (s, 3H, CH3), 0.99 (s, 3H, CH3), 2.04–2.21 (m, 2H, CH2), 2.26 (s, 2H, CH2), 2.47 (s, 2H, CH2), 3.67 (s, 3H, OCH3), 4.08 (s, 1H, CH), 6.80 (d, J = 8.7 Hz, 2H, ArH), 7.01 (d, J = 8.7 Hz, 2H, ArH). 13C NMR(100 M, DMSO-d6): 196.7, 163.5, 128.3, 127.3, 120.8, 113.8, 59.4, 51.3, 40.3, 36.6, 32.6, 29.2, 27.6. Found, %: C 70.38; H 6.28; N 8.59.C19H20N2O3. Calculated, %: C 70.35; H 6.21; N 8.64.
Results and discussion
We initially began our investigation with the reaction of an equimolar (2 mmol) mixture of 4-bromo-benzaldehyde 1, malononitrile 2, and 4-hydroxycoumarin 3 and 10 mol% EDTA-4Na at 80 °C in a Schlenk tube with 5 mL 95% ethanol. The target product 4a was obtained with 75% yield smoothly. Under the inspiration of this good result, we optimized this model reaction with the different conditions such as ratio (EtOH:H2O), reaction temperature and catalyst loading. After screening of this reaction, the yield of the desired compounds 4a was improved to 91% (Table 1, Entry 14).
When the mixture of aldehyde 1, malononitrile 2, and 4-hydroxycoumarin 3 were employed in the solution of 5 ml EtOH:H2O (40:60) in the presence of 2 mol% sodium ethylene diamine tetraacetate at 50 °C, high yields of 2-amino-4-aryl-3-cyano-5-oxo-4H,5H-pyrano-[3,2-c]chromenes 4 were obtained within 40 min, and representative compounds are shown in Table 2, respectively (Scheme 1).
The effects of electrons and the nature of substituents on the aromatic ring indicated obvious influences in terms of yields and reaction times. From Table 2, we found that the aromatic aldehydes with electron-withdrawing functional groups are employed rapidly to obtain the desired target compounds (4b, 4c), and the aromatic aldehyde with electron-donating groups (such as 4j, 4k) was employed for formation of desired product with low yields. On the other hand, the yields of 4l, 4m and 4n are relatively lower mostly because of their stronger steric hindrance.
Then, we employed 4-hydroxy-6-methyl-2-pyrones as the starting material in order to broaden the scope of synthesis of 3,4-dihydropyrano[c]chromene, and high yields of 2-amino-4-aryl-7-methyl-5-oxo-4H,5H- pyrano[4,3-b]pyran-3-carbonitrile 6 were obtained within 45 min, respectively (see Table 3) (Scheme 2).
From Table 3, the results indicated that the reaction speed of the aromatic aldehyde with electron-withdrawing functional groups is faster than with electron-donating functional groups (6b, 6c vs. 6d, 6f). On the other hand, the ortho-substituted aldehydes were employed to obtain relatively low yields compare with para-substituted aldehydes.
To further explore the potential of EDTA-4Na as a catalyst for heterocyclic synthesis, we investigated the MCRs reactions involving aromatic aldehyde, malononitriles and dimedone and acquired the high yields of 2-amino-4-aryl-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile 8 in about 40 min, respectively (Table 4). Obviously, the catalyst (EDTA-4Na) plays a crucial role in the success of the reaction in terms of the rate and the yields, and catalyst loading (2 mol%) was sufficient to push the reaction forward to complete and to examine activity of the different aromatic aldehydes, and the results are summarized in Table 4 (Scheme 3).
The results clearly demonstrated that electronic effects and the nature of substituents on the aromatic ring show strongly obvious effects in terms of reaction time under the reaction conditions mentioned above. When aromatic aldehydes containing electron-donating groups (such as the dimethylamino, methoxy and methyl groups) were employed (Table 4, Entries 11–17), a longer reaction time was required in most cases than those of electron-withdrawing groups (such as the nitro group, and halide) on aromatic rings (Table 4, Entries 2–10).
Conclusions
In conclusion, we have employed the inexpensive EDTA-4Na as a catalyst and developed an efficient and practical strategy for the synthesis of chromenes via a MCRs strategy in good to excellent yields. This procedure offers several advantages including mild reaction conditions, cleaner reaction, and higher yields of products, and will be used as a useful and attractive method for the synthesis of these compounds.
References
M. Curini, G. Cravotto, F. Epofano, G. Giannone, Curr. Med. Chem. 13, 199 (2006)
Y.M. Litvinov, A.M. Shestopalov, Adv. Hetero Chem. 103, 289 (2011)
A.A. Patchet, R.P. Nargund, Ann. Rep. Med. Chem. 35, 289 (2000)
L. Bonsignore, G. Loy, D. Secci, A. Calignano, Eur. J. Med. Chem. 28, 517 (1993)
Y. Gao, W. Yang, D.M. Du, Tetrahedron Asymmetry 23, 339 (2012)
G.A. Reynolds, K.H. Drexhage, Opt. Commun. 13, 222 (1975)
E.R. Bissell, A.R. Mitchell, R.E. Smith, J. Org. Chem. 45, 2283 (1980)
A. Domling, I. Ugi, Angew. Chem. Int. Ed. 39, 3168 (2000)
I. Devi, P.J. Bhuyan, Tetrahedron Lett. 45, 8625 (2004)
S.J. Tu, H. Jiang, Q.Y. Zhung, C.B. Miu, D.Q. Shi, X.S. Wang, Y. Gao, Chin. J. Org. Chem. 23, 488 (2003)
G. Shabitha, K. Harundhathi, K. Sudhakar, B.S. Sartry, J.S. Yadav, Synth. Commun. 39, 433 (2009)
D. Fang, H.B. Zhang, Z.L. Liu, J. Heterocycl. Chem. 47, 63 (2010)
N. Azizi, F. Shirdel, J. Mol. Liq. 222, 783 (2016)
N. Azizi, S. Dezfooli, M. Khajeh, M.M. Hashemi, J. Mol. Liq. 186, 76 (2013)
N. Azizi, S. Dezfooli, M.M. Hashemi, J. Mol. Liq. 194, 62 (2014)
S. Dong, C. Fang, W. Tang, T. Lu, D. Du, Org. Lett. 2016(18), 3882 (2016)
M.S. Siqueira, L.C. Silva-Filho, Tetrahedron Lett. 57, 5050 (2016)
Y. Li, H. Chen, C. Shi, S. Ji, J. Comb. Chem. 12, 231 (2010)
S.S. Katkar, M.K. Lande, B.R. Arbad, S.T. Gaikwad, Chin. J. Chem. 29, 199 (2011)
R.Y. Guo, Z.M. An, L.P. Mo, R.Z. Wang, H.X. Liu, S.X. Wan, Z.H. Zhang, ACS. Comb. Sci. 15, 557 (2013)
K. Aswin, S.S. Mansoor, K. Logaiya, S.P.N. Sudhan, V.S. Malik, H. Ramadoss, Res. Chem. Intermed. 40, 2583 (2014)
H.R. Safaei, M. Shekouhy, S. Rahmanpur, S. Athar, Green Chem. 14, 1696 (2012)
M.M. Heravi, Y.S. Beheshtiha, Z. Pirnia, S. Sadjadi, M. Adibi, Synth. Commun. 39, 3663 (2009)
D.M. Pore, K.A. Undale, B.B. Dongare, U.V. Desai, Catal. Lett. 132, 104 (2009)
R. Hekmatshoar, S. Majedi, K. Bakhtiari, Catal. Commun. 9, 307 (2008)
S. Abdolmohammadi, S. Balalaie, Tetrahedron Lett. 48, 3299 (2007)
M. Seifi, H. Sheibani, Catal. Lett. 126, 275 (2008)
J.M. Khurana, S. Kumar, Tetrahedron Lett. 50, 4125 (2009)
H. Mehrabi, M. Kazemi-Mireki, Chin. Chem. Lett. 22, 1419 (2011)
H. Nagabhushana, S.S. Saundalkar, L. Muralidhar, B.M. Nagab-hushana, C.R. Girija, D. Nagaraja, M.A. Pasha, V.P. Jayashankara, Chin. Chem. Lett. 22, 143 (2011)
N. Hazeri, M.T. Maghsoodlou, F. Mir, M. Kangani, Chin. J. Catal. 35, 391 (2014)
M. Esmaeilpour, J. Javidi, F. Dehghani, F.N. Dodeji, RSC. Adv. 5, 26625 (2015)
K. Tabatabaeian, H. Heidari, M. Mamaghani, N.O. Mahmoodi, Appl. Organometal. Chem. 26, 56 (2012)
H. Jiang, S. Tu, F. Fang, Chin. J. Org. Chem. 24, 1468 (2004)
D. Shi, J. Wang, Q. Zhuang, Chin. J. Org. Chem. 26, 643 (2006)
A.M. El-Agrody, A.H. Fakery, A.H. Bedair, J. Chem. Res. 26 (2000)
M.G. Dekamin, M. Eslami, A. Maleki, Tetrahedron 69, 1074 (2013)
G. Zhang, Y. Zhang, J. Yan, R. Chen, S. Wang, Y. Ma, R. Wang, J. Org. Chem. 77, 878 (2012)
H. Wang, J. Lu, Z. Zhang, Monatsh. Chem. 141, 1107 (2010)
M. Abaszadeh, M. Seifi, Res. Chem. Intermed. 41, 7715 (2015)
A. Shaabani, S. Samadi, Z. Badri, A. Rahmati, Cata. Lett. 104, 39 (2005)
S. Balalaie, M. Bararjanian, A.M. Amani, B. Movassagh, Synlett 2, 263 (2006)
S. Banerjee, A. Horn, H. Khatri, G. Sereda, Tetrahedron Lett. 52, 1878 (2011)
S.J. Tu, Y. Gao, C. Guo, D. Shi, Z. Lu, Synth. Commun. 32, 2137 (2002)
Y. Gao, D.M. Du, Tetrahedron Asymmetry 23, 1343 (2012)
S.M. Baghbanian, N. Rezaeia, H. Tashakkorianb, Green Chem. 15, 3446 (2013)
Acknowledgement
We thank Science Foundation for Young Teachers of Wuyi University (2016zk03).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, L., Lin, J., Chen, B. et al. Sodium ethylene diamine tetraacetate catalyzed synthesis of chromene derivatives via multi-component reactions at low catalyst loading. Res Chem Intermed 43, 6691–6700 (2017). https://doi.org/10.1007/s11164-017-3015-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3015-3