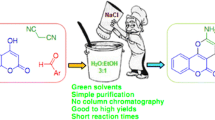
Abstract
Three-component processes for the synthesis of 2-amino-4H-chromenes catalyzed by potassium carbonate/cyanuric acid in water as a safe solvent were developed. These reactions were performed at room temperature through one-pot reactions. The benefits of this research are short reaction time, excellent yield, clean reaction media, simple work-up and easy purification.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The main challenges in recent decades in organic chemistry are the synthesis of new compounds with economical, high-efficiency and eco-friendly processes. According to the principles of “Green Chemistry”, energy requirements should be recognized for their environmental impacts and should be minimized. Synthetic methods should be conducted at ambient pressure and temperature, also using solvent-free or recyclable environmentally benign solvent systems. In this regard, water can be the best replacement for an rganic solvent, as it is nonvolatile, nonflammable, nontoxic, and inexpensive [1, 2].
Chromenes consist of bicyclic oxygen heterocycles resulting from the fusion of the benzene ring with 5, 6-positions of either a 2H- or a 4H-pyran ring system are designated as 2H-chromene and 4H-chromene (Fig. 1) [3]. The diverse pharmacological activities of this class of molecules, with antimicrobial [4] anti-antiviral [5], anti-inflammatory [6], anti-influenza [7] and anticancer activity [8] have been reported. Moreover, in recent decades, among various chromene derivatives, 2-amino-4H-chromene with cyano-functionality has a wide range of applications in the many fields of chemistry such as lasers, optical brighteners, fluorescence markers, pigments, and cosmetics [9].
Generally, the synthesis of 2-amino-4H-chromene performs by the cyclization of an aromatic or aliphatic aldehyde, malononitrile (or ethyl cyanoacetate), with enolizable C–H activated acidic compounds, such as phenol [10], naphthol [1], resorsinol [11], 4-hydroxycoumarin [12] or dimedone [13, 14]. Various catalysts have been reported for the synthesis of these compounds, such as DABCO [15], PEG-400 [16], thiourea dioxide [17], LiOH·H2O [18], poly (4-vinylpyridine) [19], sulfamic acid [20], Ca(OH)2 [21], Fe3O4 nanoparticles [22], bakers’ yeast [23], sodium acetate [24], ceric ammonium nitrate (CAN) [25], and nanocomposites such as Fe3O4@SiO2–NH2 [26], nano-powder ZnAl2O4–Bi2O3 [27] nano-cellulose/Ag [28], but most of them are associated with several drawbacks, such as long reaction time, high cost, harsh conditions, toxic organic solvents, difficult work-up procedures and multi-step reaction conditions with reduced yields.
In this paper, we studied the one-pot and multicomponent synthesis of 2-amino-4H-chromenes by the reaction of malononitrile and various aldehydes with phenols, resorsinol and dimedone in the presence of potassium carbonate/cyanuric acid as an available, economic and nontoxic catalyst in water
Experimental
Materials and solvents
The reagents and solvents were purchased from Merck and Aldrich and were used as received. The samples were analyzed by FT-IR spectroscopy (JASCO FT/IR-460 plus spectrometer) and melting point by Electrothermal 9100 apparatus. 1H NMR and 13C NMR spectra of compounds were recorded on a Bruker DRX-400 Avance instrument in DMSO-d6.
General procedure for the synthesis of 2-amino-4H-chromenes
A mixture of aldehyde (1 mmol), malononitrile (1 mmol) and 1 mmol of enolizable C–H activated acidic compound (resorcinol, phenol, or dimedone) was stirred in the presence of 10 mol% of potassium carbonate/cyanuric acid (3:1 mol%) in water at room temperature. The reaction process was monitored by thin layer chromatography. After completion of the reaction, the precipitate was filtered, washed with distilled water and crystallized from ethanol.
Selected spectral data
2 - amino - 4 - (2,4 - dichlorophenyl) - 7 - hydroxy - 4H - chromene - 3 - carbonitrile (Table 4 , Entry 3d)
m.p. = 252–254 °C, literature 256–258 °C [11], 1H NMR (400 MHz, DMSO-d6): 5.12 (s, 1H), 6.40 (d, 1H), 6.46 (d, 1H), 6.70 (d, 1H), 6.96 (s, 2H, NH2), 7.17 (d, 1H), 7.38 (d, 1H), 7.55 (d, 1H), 9.87 (s, 1H, OH) ppm, 13C NMR (100 MHz, CDCl3 DMSO-d6): δ (ppm) 56.63, 102.72, 112.71, 112.91, 113.83, 113.97, 121.13, 121.63, 129.75, 129.95, 130.37, 142.04, 149.35, 155.62, 159.94, 160.69.
2 - amino - 7,7 - dimethyl - 5 - oxo - 4 - phenyl - 5,6,7,8 - tetrahydro - 4H - chromene - 3 - carbonitrile (Table 6 , Entry 5a)
m.p. = 228–230 °C, literature 227–229 [33], 1H NMR (400 MHz, CDCl3): δ (ppm):1.07 (s, 3H), 1.13 (s, 3H), 2.21 (d, 1H, J = 16.4 Hz), 2.27 (d, 1H, J = 16.4 Hz), 2.45 (s, 2H), 4.41 (s, 1H), 4.56 (s, 2H, NH2), 7.23- 7.34 (m, 5H). 13C NMR (100 MHz, CDCl3): δ (ppm) 200.1, 159.2, 155.0, 145.4, 127.6, 128.6, 126.0, 119.2, 112.5, 57.9, 50.8, 39.6, 37.9, 32.4, 27.1.
Results and discussion
According to the principles of green chemistry, we studied the developed synthesis of 2-amino-4H-chromenes using benzaldehyde (1), malononitrile (2), and enolizable C–H activated acidic compound (resorcinol (3a), phenol (4a), or dimedone (5a) at room temperature in the presence of potassium carbonate/cyanuric acid as a catalyst in water. First, 4-chlorobenzylaldehyde was selected as a model substrate for the investigated amounts of the catalyst (Tables 1, 2, 3). In all the reactions, different ratios and amounts of cyanuric acid and K2CO3 were examined. A longer reaction time was necessary and a lower yield was obtained when we used cyanuric acid or K2CO3 alone, while the best result was obtained for 10 mol% of catalyst with a mole ratio of 3:1 (K2CO3:cyanuric acid). For the next step, the reaction between malononitrile and an enolizable C–H activated acidic compound with various aldehydes, in the presence of a catalytic amount of K2CO3/cyanuric acid was investigated (Tables 4, 5, 6). Benzaldehydes with electron-withdrawing groups, such as chloro and nitro, had quicker reactions than those with electron-donating groups such as methoxy, methyl, etc. In addition, when using dimedone as an enolizable C–H activated acidic compound, this reaction occurs more slowly and less efficient. A suggested mechanism for the synthesis of 4H-chromene-3-carbonitrile is shown in Scheme 1.






Conclusion
We have reported a rapid, green, and highly efficient method for the synthesis of 2-amino-4H-chromene with cyano-functionality derivatives. The present work describes a simple and appropriate method for the one-pot reaction between various benzaldehyde, malononitrile and enolizable C–H activated acidic compounds. The reaction was performed in the presence of K2CO3/cyanuric acid as a mixture of organic and inorganic catalysts in aqueous solution. The main advantages of this approach are short reaction time, excellent yield, clean reaction media, simple work-up and easy purification.
References
M.G. Dekamin, M. Eslami, A. Maleki, Tetrahedron 69, 1074 (2013)
D. Kumar, V.B. Reddy, S. Sharad, U. Dube, S. Kapur, Eur. J. Med. Chem. 44, 3805 (2009)
R. Pratap, V. Ji Ram, Chem. Rev. 114, 10476 (2014)
S. Kanakaraju, G. Chandramouli, Res. Chem. Intermed. 41, 2809 (2015)
J. Mori, M. Iwashima, M. Takeuchi, H. Saito, Chem. Pharm. Bull. 54, 391 (2006)
S.T. Chung, W.H. Huang, C.K. Huang, F.C. Liu, R.Y. Huang, C.C. Wu, A.R. Lee, Res. Chem. Intermed. 42, 1195 (2016)
O.S. Patrusheva, V.V. Zarubaev, A.A. Shtro, Y.R. Orshanskaya, S.A. Boldyrev, I.V. Ilyina, SYu. Kurbakova, D.V. Korchagina, K.P. Volcho, N.F. Salakhutdinov, Bioorg. Med. Chem. 24, 5158 (2016)
S. Bondock, H. Gieman, Res. Chem. Intermed. 41, 8381 (2015)
M.P. Surpur, S. Kshirsagar, S.D. Samant, Tetrahedron. Lett. 50, 719 (2009)
R.L. Magar, P.B. Thorat, V.B. Jadhav, S.U. Tekale, S.A. Dake, B.R. Patil, R.P. Pawar, J. Mol. Catal. A-Chem. 374–375, 118 (2013)
J. Safari, M. Heydarian, Z. Zarnegar, Arab. J. Chem. (2013). doi:10.1016/j.arabjc.2013.11.038
M.E. Sedaghat, M. Rajabpour Booshehri, M.R. Nazarifar, F. Farhadi, Appl. Clay Sci. 95, 55 (2014)
N. Montazeri, T. Noghani, M. Ghorchibeigy, R. Zoghi, J. Chem. 2014, 1 (2014)
B. Amirheidari, M. Seifi, M. Abaszadeh, Res. Chem. Intermed. 42, 3413 (2016)
S. Shinde, G. Rashinkar, R. Salunkhe, J. Mol. Liq. 178, 122 (2013)
N.V. Shitole, K.F. Shelke, S.A. Sadaphal, B.B. Shingate, M.S. Shingare, Green Chem. Lett. Rev. 3, 83 (2010)
S. Verma, S.L. Jain, Tetrahedron Lett. 53, 6055 (2012)
M.A. Gouda, A.A. Abu-Hashem, Green Chem. Lett. Rev. 5, 203 (2012)
J. Albadi, A. Mansournezhad, M. Darvishi-Paduk, Chin. Chem. Lett. 24, 208 (2013)
M. Ghashang, S. Sheik Mansoor, K. Aswin, Res. Chem. Intermed. 41, 3117 (2015)
J. Hyang Park, Y. Rok Lee, S. Hong Kim, Tetrahedron, 69, 9682 (2013)
Z. Zarnegar, J. Safari, J. Mol. Struct. 53, 1072 (2014)
N. Geetmani Singh, R. Nongrum, C. Kathing, J. World Star Rani, R. Nongkhlaw, Green Chem. Lett. Rev. 7, 137 (2014)
M.T. Maghsoodlou, M. Safarzaei, M.R. Mousavi, N. Hazeri, J. Aboonajmi, M. Shirzaei, Iran. J. Org. Chem. 6, 1197 (2014)
Y. Li, X. Meng, G. Cai, B. Du, B. Zhao, Res. Chem. Intermed. 40, 699 (2014)
M.A. Ghasemzadeh, M.H. Abdollahi-Basir, M. Babaei, Green Chem. Lett. Rev. 8, 40 (2015)
M. Ghashang, Res. Chem. Intermed. 42, 4191 (2016)
A. Maleki, H. Movahed, P. Ravaghi, Carbohydr. Polym. 20, 259 (2017)
H. Kiyani, F. Ghorbani, J. Saudi Chem. Soc. 18, 689 (2014)
S. Makarem, A. A. Mohammadi, A. R. Fakhari, Tetrahedron Lett. 7194 (2008)
K. Gong, H.L. Wang, J. Luo, Z.L. Liu, Heterocycl. Chem. 46, 1145 (2009)
A. Mobinikhaledi, M.A. Bodaghi Fard, Acta. Chim. Slov. 57, 931 (2010)
S.J. Tu, H. Jiang, Q.Y. Zhuang, C.B. Miu, D.Q. Shi, X.S. Wang, Y. Gao, Chin. J. Org. Chem. 23, 488 (2003)
N. Hazeri, M.T. Maghsoodlou, F. Mir, M. Kangani, Chin. J. Catal. 35, 391 (2014)
L.M. Wang, J.H Shao H. Tian, Y.H. Wang, B. Liu, J. Fluo. Chem. 127, 97 (2006)
T.S. Jin, A.Q. Wang, H. Ma, J.S. Zhang, T.S. Li, Indian J. Chem. 45B, 470 (2006)
Acknowledgement
We gratefully acknowledge financial support from the Research Council of the University of Sistan and Baluchestan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heydari, R., Shahraki, R., Hossaini, M. et al. K2CO3/cyanuric acid catalyzed synthesis of 2-amino-4H-chromene derivatives in water. Res Chem Intermed 43, 4611–4622 (2017). https://doi.org/10.1007/s11164-017-2900-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-2900-0