Abstract
A series of novel fused tetrazolylbenzo[h]chromenes 3a–f, 4a–f and 5a–f were prepared starting from 2-amino-benzo[h]chromene-3-carbonitrile 1a–f. All structures of the newly synthesized compounds were confirmed by IR, NMR, mass spectral studies, and elemental analyses. The newly synthesized compounds were screened for their antibacterial and antifungal activity. Some of the derivatives have exhibited promising biological activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromene annulated heterocyle derivatives represent an important class of oxygen-containing heterocycles, being the main components in many of naturally occurring products, and having a wide spectrum of biological and pharmacological properties, like antimicrobial [1], antiviral, antileishmanial [2], mutagenicity [3], antiproliferative [4], antitumor [5], anticancer [6], and central nervous system activity [7]. Some of them are widely employed as cosmetics, pigments [8], and potential biodegradable agrochemicals [9].
Tetrazoles constitute an interesting class of heterocyclic compounds that have a wide range of applications. These nitrogen-rich heterocycles have been used as a metabolically stable isosteric replacement for the carboxylic acid moiety [10], as a cis-peptide bond mimetic [11, 12], as a precursor to other heterocycles [13], in high-energy compounds [14], and as a coordinating group in directed ortho-metalation [15–17]. They are also resistant to metabolic degradation as well as towards chemical oxidants [18]. Recently, several biologically relevant substances incorporating a tetrazole moiety have been developed. For example, Losartan is an angiotensin II antagonist, which has been used to treat hypertension [19, 20]. Pentylenetetrazole has been extensively used in models for anxiety [21, 22]. Some tetrazoles show affinity to benzodiazepine receptors [23].
On the other hand, pyrimidine scaffolds have been potentially important pharmacophores of many bioactive molecules [25]. They are known to possess antitubercular, antimicrobial [24], antiplatelet [26], antiinflammatory [27], antigenotoxic [28], antiallergic [29], anti-proliferative, and apoptotic activity [30].
It is evident from the literature that diazepine derivatives have been found to exhibit various pharmacological activities. They are widely used as anticonvulsants, antianxiolitics, analgesics, sedatives, antidepressives, and hypnotic agents [31–35].
Prompted by these observations, and as part of our research program aimed at developing new biologically active heterocycles [36], in this paper, we designed and synthesized a series of novel condensed systems (benzopyran-containing tetrazoles fused with pyrimidine and diazepine) that combine these pharmacophores in a ring to give a compact and planar structure, which were evaluated for their antimicrobial activity.
Experimental
Melting points were recorded on a Stuart SMP30 melting point apparatus and were uncorrected. Column chromatography was performed using silica–gel (100–200 mesh size) purchased from Thomas Baker, and thin layer chromatography (TLC) was carried out using aluminium sheets pre-coated with silica gel 60F254 purchased from Merck. IR spectra (KBr) were obtained using a PerkinElmer Spectrum100 FT-IR Spectrometer. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Bruker WM-400 spectrometer in DMSO-d 6 with TMS as an internal standard. Mass spectra (ESI) were carried out on a JEOL SX-102 spectrometer. CHN analysis was done by Carlo Erba EA 1108 automatic elemental analyzer. The chemicals and solvents used were of commercial grade and were used without further purification unless otherwise stated.
General procedure for the synthesis of 4-aryl-3-(1H-tetrazol-5-yl)-4H-benzo[h]chromen-2-amines (2a–f)
To a mixture of compound 1a–f (10 mmol) in DMF (40 mL), sodium azide (12 mmol), and NH4Cl (12 mmol) were added and the reaction mixture was stirred at 120 °C for 7 h. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was cooled to room temperature, poured into ice-cold water (100 mL), and the solid separated was filtered, washed with water, dried, and purified by column chromatography on silica gel using hexane/ethyl acetate (7:3) as eluent to afford compound 2a–f.
4-Phenyl-3-(1H-tetrazol-5-yl)-4H-benzo[h]chromen-2-amine (2a)
Orange solid, yield: 68 %; mp: 191–194 °C. IR (KBr, cm−1): 3,471, 3,291, 3,222, 2,967, 1,610, 1,597, 1,517, 1,491, 1,402, 1,349, 1,326, 1,200, 1,076. 1H NMR (DMSO-d 6, 400 MHz): δ 4.90 (s, 1H, H-4), 7.10–7.34 (m, 8H, ArH + NH2), 7.55–7.67 (m, 3H, ArH), 7.89 (d, 1H, ArH), 8.24 (d, 1H, ArH), 10.51 (s, 1H, NH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 40.96, 89.30, 120.57, 120.73, 122.78, 123.90, 126.23, 126.67, 126.76, 126.93, 127.68, 128.71, 132.70, 142.76, 145.71, 158.93, 161.20 ppm. ESI–MS (m/z): 342 (M+1)+. Anal. Calcd for C20H15N5O: C, 70.37; H, 4.43; N, 20.52. Found: C, 70.49; H, 4.50; N, 20.41.
4-(4-Chlorophenyl)-3-(1H-tetrazol-5-yl)-4H-benzo[h]chromen-2-amine (2b)
Brown solid, yield: 64 %; mp: 162–164 °C. IR (KBr, cm−1): 3,432, 3,372, 3,242, 2,983, 1,600, 1,586, 1,508, 1,492, 1,392, 1,337, 1,311, 1,209, 1,072. 1H NMR (DMSO-d 6, 400 MHz): δ 4.86 (s, 1H, H-4), 7.01–7.20 (m, 3H, ArH + NH2), 7.39–7.53 (m, 7H, ArH), 7.81 (d, 1H, ArH), 8.19 (d, 1H, ArH), 10.46 (s, 1H, NH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 40.93, 89.76, 120.47, 120.78, 123.71, 124.82, 125.97, 126.82, 126.94, 127.80, 129.05, 129.48, 133.27, 133.38, 142.93, 145.30, 159.00, 161.32 ppm. ESI–MS (m/z): 376 (M+1)+. Anal. Calcd for C20H14ClN5O: C, 63.92; H, 3.75; N, 18.64. Found: C, 63.83; H, 3.69; N, 18.75.
4-(4-Fluorophenyl)-3-(1H-tetrazol-5-yl)-4H-benzo[h]chromen-2-amine (2c)
Yellow solid, yield: 66 %; mp: 181–183 °C. IR (KBr, cm−1): 3,446, 3,397, 3,221, 2,972, 1,609, 1,593, 1,509, 1,490, 1,400, 1,342, 1,322, 1,208, 1,071. 1H NMR (DMSO-d 6, 400 MHz): δ 4.87 (s, 1H, H-4), 7.03–7.24 (m, 3H, ArH + NH2), 7.44–7.58 (m, 7H, ArH), 7.81 (d, 1H, ArH), 8.11 (d, 1H, ArH), 10.37 (s, 1H, NH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 41.17, 89.68, 120.51, 120.83, 123.18, 124.74, 125.93, 126.77, 126.98, 127.71, 129.13, 129.56, 133.44, 133.67, 142.91, 145.21, 159.11, 161.41 ppm. ESI–MS (m/z): 360 (M+1)+. Anal. Calcd for C20H14FN5O: C, 66.85; H, 3.93; N, 19.49. Found: C, 66.97; H, 3.81; N, 19.56.
4-(4-Methylphenyl)-3-(1H-tetrazol-5-yl)-4H-benzo[h]chromen-2-amine (2d)
Yellow solid, yield: 64 %; mp: 170–172 °C. IR (KBr, cm−1): 3,391, 3,298, 3,202, 2,969, 1,602, 1,594, 1,519, 1,494, 1,410, 1,351, 1,328, 1,204, 1,078. 1H NMR (DMSO-d 6, 400 MHz): δ 2.32 (s, 3H, CH3), 4.84 (s, 1H, H-4), 7.04-7.37 (m, 7H, ArH + NH2), 7.48-7.60 (m, 3H, ArH), 7.80 (d, 1H, ArH), 8.16 (d, 1H, ArH), 10.48 (s, 1H, NH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 21.72, 41.31, 89.63, 120.01, 120.73, 123.35, 124.93, 126.71, 126.93, 127.10, 128.11, 128.41, 128.94, 130.38, 133.73, 137.44, 142.08, 159.04, 161.36 ppm. ESI–MS (m/z): 356 (M+1)+. Anal. Calcd for C21H17N5O: C, 70.97; H, 4.82; N, 19.71. Found: C, 70.88; H, 4.70; N, 19.83.
4-(4-Methoxyphenyl)-3-(1H-tetrazol-5-yl)-4H-benzo[h]chromen-2-amine (2e)
Orange solid, yield: 61 %; mp: 154–156 °C. IR (KBr, cm−1): 3,383, 3,277, 3,226, 2,982, 1,604, 1,595, 1,512, 1,493, 1,411, 1,341, 1,321, 1,203, 1,072. 1H NMR (DMSO-d 6, 400 MHz): δ 3.84 (s, 3H, OCH3), 4.88 (s, 1H, H-4), 7.03–7.35 (m, 7H, ArH + NH2), 7.50-7.59 (m, 3H, ArH), 7.81 (d, 1H, ArH), 8.19 (d, 1H, ArH), 10.32 (s, 1H, NH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 41.79, 55.48, 89.91, 114.74, 121.17, 123.62, 124.83, 126.62, 126.91, 127.23, 127.61, 128.54, 128.82, 129.67, 133.76, 144.82, 158.91, 159.25, 161.77 ppm. ESI–MS (m/z): 372 (M+1)+. Anal. Calcd for C21H17N5O2: C, 67.91; H, 4.61; N, 18.86. Found: C, 67.84; H, 4.70; N, 18.94.
4-(3-Nitrophenyl)-3-(1H-tetrazol-5-yl)-4H-benzo[h]chromen-2-amine (2f)
Brown solid, yield: 58 %; mp: 187–189 °C. IR (KBr, cm−1): 3,461, 3,394, 3,221, 2,977, 1,605, 1,591, 1,511, 1,492, 1,407, 1,343, 1,322, 1,209, 1,075. 1H NMR (DMSO-d 6, 400 MHz): δ 5.08 (s, 1H, H-4), 7.02 (d, 1H, ArH), 7.30–7.38 (m, 5H, ArH + NH2), 7.48 (t, 1H, ArH), 7.54 (d, 1H, ArH), 7.74 (d, 1H, ArH), 8.03 (d, 1H, ArH), 8.12 (s, 1H, ArH), 8.22 (d, 1H, ArH), 10.61 (s, 1H, NH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 42.11, 89.98, 119.72, 121.29, 122.67, 123.38, 123.94, 125.41, 125.86, 127.66, 127.84, 128.25, 130.41, 133.85, 134.81, 144.72, 146.88, 149.25, 159.43, 161.96 ppm. ESI–MS (m/z): 387 (M+1)+. Anal. Calcd for C20H14N6O3: C, 62.17; H, 3.65; N, 21.75. Found: C, 62.26; H, 3.71; N, 21.66.
General procedure for the synthesis of fused naphthopyrano[3,2-e]tetrazolo[2,3-c]pyrimidin-5-thiones (3a–f)
A mixture of compound 2a–f (2 mmol) and carbon disulfide (2 mmol) in pyridine (10 mL) were refluxed on a water bath for 16 h. After completion of the reaction (monitored by TLC), the reaction mixture was cooled to room temperature then poured into ice-cold water (20 mL) and neutralized with hydrochloric acid (1:1). The solid obtained was filtered, washed with water, dried, and recrystallized from ethanol to afford compound 3a–f.
14-Phenyl-5,6-dihydro-5H,14H-naphtho[1′,2′:5,6]pyrano[3,2-e][1–4]tetrazolo[2,3-c]pyrimidin-5-thione (3a)
White solid, yield: 64 %; mp: 175–177 °C. IR (KBr, cm−1): 3,411, 1,592, 1,546, 1,489, 1,468, 1,412, 1,178, 1,090. 1H NMR (DMSO-d 6, 400 MHz): δ 4.90 (s, 1H, H-4), 7.10–7.35 (m, 7H, ArH), 7.55–7.65 (m, 2H, ArH), 7.88 (d, 1H, ArH), 8.25 (d, 1H, ArH), 10.43 (s, 1H, NH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 40.91, 89.41, 120.47, 120.67, 122.74, 123.84, 126.17, 126.61, 126.71, 126.87, 127.62, 128.65, 132.65, 142.72, 145.65, 159.33, 161.92, 189.51 ppm. ESI–MS (m/z): 384 (M+1)+. Anal. Calcd for C21H13N5OS: C, 65.78; H, 3.42; N, 18.27. Found: C, 65.87; H, 3.51; N, 18.20.
14-(4-Chlorophenyl)-5,6-dihydro-5H,14H-naphtho[1′,2′:5,6]pyrano[3,2-e][1–4]tetrazolo[2,3-c]pyrimidin-5-thione (3b)
White solid, yield: 61 %; mp: 198–200 °C. IR (KBr, cm−1): 3,387, 1,590, 1,534, 1,477, 1,464, 1,417, 1,172, 1,087. 1H NMR (DMSO-d 6, 400 MHz): δ 4.84 (s, 1H, H-4), 6.99 (d, 1H, ArH), 7.18–7.33 (m, 4H, ArH), 7.52–7.58 (m, 3H, ArH), 7.80 (d, 1H, ArH), 8.14 (d, 1H, ArH), 10.32 (s, 1H, NH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 41.04, 89.71, 120.75, 120.88, 123.13, 124.79, 125.81, 126.37, 126.87, 127.73, 129.32, 129.85, 133.32, 133.41, 142.84, 145.41, 159.48, 162.04, 189.72 ppm. ESI–MS (m/z): 418 (M+1)+. Anal. Calcd for C21H12ClN5OS: C, 60.36; H, 2.89; N, 16.76. Found: C, 60.43; H, 2.71; N, 16.83.
14-(4-Fluorophenyl)-5,6-dihydro-5H,14H-naphtho[1′,2′:5,6]pyrano[3,2-e][1–4]tetrazolo[2,3-c]pyrimidin-5-thione (3c)
White solid, yield: 62 %; mp: 212–214 °C. IR (KBr, cm−1): 3,394, 1,594, 1,532, 1,482, 1,461, 1,411, 1,175, 1,091. 1H NMR (DMSO-d 6, 400 MHz): δ 4.88 (s, 1H, H-4), 7.04 (d, 1H, ArH), 7.16–7.31 (m, 4H, ArH), 7.54–7.60 (m, 3H, ArH), 7.82 (d, 1H, ArH), 8.16 (d, 1H, ArH), 10.36 (s, 1H, NH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 41.32, 89.76, 120.58, 120.89, 123.32, 124.98, 125.34, 126.72, 126.91, 127.89, 129.24, 129.76, 133.24, 133.62, 142.76, 145.53, 159.63, 162.21, 189.83 ppm. ESI–MS (m/z): 402 (M+1)+. Anal. Calcd for C21H12FN5OS: C, 62.83; H, 3.01; N, 17.45. Found: C, 62.97; H, 3.12; N, 17.36.
14-(4-Methylphenyl)-5,6-dihydro-5H,14H-naphtho[1′,2′:5,6]pyrano[3,2-e][1–4]tetrazolo[2,3-c]pyrimidin-5-thione (3d)
White solid, yield: 60 %; mp: 162–164 °C. IR (KBr, cm−1): 3,389, 1,590, 1,549, 1,492, 1,463, 1,410, 1,180, 1,087. 1H NMR (DMSO-d 6, 400 MHz): δ 2.33 (s, 3H, CH3), 4.80 (s, 1H, H-4), 7.02 (d, 1H, ArH), 7.14–7.36 (m, 4H, ArH), 7.46–7.59 (m, 3H, ArH), 7.81 (d, 1H, ArH), 8.19 (d, 1H, ArH), 10.39 (s, 1H, NH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 21.84, 41.38, 89.71, 120.67, 121.13, 123.54, 124.81, 126.67, 126.89, 127.23, 128.17, 128.47, 128.84, 130.42, 133.86, 137.52, 142.13, 159.38, 162.38, 189.87 ppm. ESI–MS (m/z): 398 (M+1)+. Anal. Calcd for C22H15N5OS: C, 66.48; H, 3.80; N, 17.62. Found: C, 66.34; H, 3.71; N, 17.77.
14-(4-Methoxyphenyl)-5,6-dihydro-5H,14H-naphtho[1′,2′:5,6]pyrano[3,2-e][1–4]tetrazolo[2,3-c]pyrimidin-5-thione (3e)
White solid, yield: 54 %; mp: 144–146 °C. IR (KBr, cm−1): 3,403, 1,594, 1,532, 1,481, 1,460, 1,417, 1,167, 1,091. 1H NMR (DMSO-d 6, 400 MHz): δ 3.87 (s, 3H, OCH3), 4.96 (s, 1H, H-4), 7.00 (d, 1H, ArH), 7.23–7.45 (m, 4H, ArH), 7.52–7.60 (m, 3H, ArH), 7.83 (d, 1H, ArH), 8.14 (d, 1H, ArH), 10.41 (s, 1H, NH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 41.84, 55.56, 89.94, 114.82, 121.27, 123.54, 124.87, 126.73, 126.94, 127.36, 127.74, 128.41, 128.61, 129.64, 133.78, 144.80, 159.82, 160.02, 162.52, 189.97 ppm. ESI–MS (m/z): 414 (M+1)+. Anal. Calcd for C22H15N5O2S: C, 63.91; H, 3.66; N, 16.94. Found: C, 63.82; H, 3.78; N, 16.87.
14-(3-Nitrophenyl)-5,6-dihydro-5H,14H-naphtho[1′,2′:5,6]pyrano[3,2-e][1–4]tetrazolo[2,3-c]pyrimidin-5-thione (3f)
White solid, yield: 52 %; mp: 223–226 °C. IR (KBr, cm−1): 3,413, 1,596, 1,537, 1,481, 1,462, 1,410, 1,181, 1,088. 1H NMR (DMSO-d 6, 400 MHz): δ 5.08 (s, 1H, H-4), 6.98 (d, 1H, ArH), 7.34–7.48 (m, 3H, ArH), 7.51 (t, 1H, ArH), 7.58 (d, 1H, ArH), 7.81 (d, 1H, ArH), 8.04 (d, 1H, ArH), 8.14 (s, 1H, ArH), 8.23 (d, 1H, ArH), 10.52 (s, 1H, NH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 41.98, 90.08, 119.81, 121.42, 122.78, 123.84, 123.51, 125.63, 125.94, 127.69, 127.81, 128.43, 130.48, 133.91, 134.93, 144.77, 146.93, 149.41, 159.94, 163.19, 190.14 ppm. ESI–MS (m/z): 429 (M+1)+. Anal. Calcd for C21H12N6O3S: C, 58.87; H, 2.82; N, 19.62. Found: C, 58.94; H, 2.74; N, 19.56.
General procedure for the synthesis of fused naphthopyrano[3,2-e]tetrazolo[2,3-c]pyrimidines (4a–f)
To a mixture of compound 2a–f (2 mmol) and benzaldehyde (2 mmol) in methanol (10 mL), conc. HCl (0.5 mL) was added and the reaction mixture was refluxed for 20 h. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was cooled to room temperature and neutralized with saturated sodium bicarbonate solution, and the solid separated was filtered, washed with water, dried, and recrystallized from ethanol to afford compound 4a–f.
5,14-Diphenyl-6-hydro-5H,14H-naphtho[1′,2′:5,6]pyrano[3,2-e][1–4]tetrazolo[2,3-c]pyrimidine (4a)
White solid, yield: 66 %; mp: 275–278 °C. IR (KBr, cm−1): 3,413, 1,617, 1,537, 1,493, 1,453, 1,379, 1,260, 1,154. 1H NMR (DMSO-d 6, 400 MHz): δ 5.30 (s, 1H, H-4), 5.81 (s, 1H, pyrimidine CH), 6.93 (d, 1H, ArH), 7.36–7.68 (m, 12H, ArH), 7.84–7.97 (m, 2H, ArH), 8.30 (d, 1H, ArH), 10.24 (s, 1H, NH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 41.10, 64.12, 88.32, 120.32, 120.70, 122.71, 123.35, 123.84, 125.56, 125.65, 125.73, 126.22, 126.68, 127.63, 128.77, 130.81, 132.70, 133.75, 139.17, 142.70, 160.02, 162.73 ppm. ESI–MS (m/z): 430 (M+1)+. Anal. Calcd for C27H19N5O: C, 75.51; H, 4.46; N, 16.31. Found: C, 75.63; H, 4.53; N, 16.20.
14-(4-Chlorophenyl)-5-phenyl-6-hydro-5H,14H-naphtho[1′,2′:5,6]pyrano[3,2-e][1–4]tetrazolo[2,3-c]pyrimidine (4b)
White solid, yield: 63 %; mp: 214–216 °C. IR (KBr, cm−1): 3,389, 1,600, 1,532, 1,495, 1,431, 1,372, 1,263, 1,142. 1H NMR (DMSO-d 6, 400 MHz): δ 5.19 (s, 1H, pyran CH), 5.88 (s, 1H, pyrimidine CH), 7.02–7.21 (m, 5H, ArH), 7.38–7.60 (m, 8H, ArH), 7.82 (d, 1H, ArH), 8.17 (d, 1H, ArH), 10.32 (s, 1H, NH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 41.32, 64.41, 88.38, 120.63, 120.76, 123.24, 124.86, 125.72, 126.41, 126.53, 127.12, 127.71, 128.47, 128.72, 129.43, 129.82, 133.24, 133.61, 139.13, 142.71, 145.84, 159.78, 162.42 ppm. ESI–MS (m/z): 464 (M+1)+. Anal. Calcd for C27H18ClN5O: C, 69.90; H, 3.91; N, 15.10. Found: C, 69.83; H, 3.82; N, 15.23.
14-(4-Fluorophenyl)-5-phenyl-6-hydro-5H,14H-naphtho[1′,2′:5,6]pyrano[3,2-e][1–4]tetrazolo[2,3-c]pyrimidine (4c)
White solid, yield: 60 %; mp: 172–175 °C. IR (KBr, cm−1): 3,383, 1,591, 1,529, 1,490, 1,431, 1,368, 1,264, 1,153. 1H NMR (DMSO-d 6, 400 MHz): δ 5.14 (s, 1H, pyran CH), 5.92 (s, 1H, pyrimidine CH), 7.01–7.26 (m, 5H, ArH), 7.47–7.62 (m, 8H, ArH), 7.81 (d, 1H, ArH), 8.20 (d, 1H, ArH), 10.34 (s, 1H, NH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 41.47, 64.72, 88.41, 120.47, 120.94, 123.24, 124.90, 125.44, 126.28, 126.69, 127.32, 127.78, 128.31, 128.86, 129.35, 129.68, 133.42, 133.87, 139.17, 142.58, 145.47, 159.72, 162.31 ppm. ESI–MS (m/z): 448 (M+1)+. Anal. Calcd for C27H18FN5O: C, 72.47; H, 4.05; N, 15.65. Found: C, 72.53; H, 4.13; N, 15.56.
14-(4-Methylphenyl)-5-phenyl-6-hydro-5H,14H-naphtho[1′,2′:5,6]pyrano[3,2-e][1–4]tetrazolo[2,3-c]pyrimidine (4d)
White solid, yield: 61 %; mp: 185–188 °C. IR (KBr, cm−1): 3,397, 1,588, 1,531, 1,484, 1,437, 1,382, 1,263, 1,150. 1H NMR (DMSO-d 6, 400 MHz): δ 2.35 (s, 3H, CH3), 5.17 (s, 1H, pyran CH), 5.90 (s, 1H, pyrimidine CH), 6.96 (d, 1H, ArH), 7.12–7.31 (m, 4H, ArH), 7.44–7.61 (m, 8H, ArH), 7.80 (d, 1H, ArH), 8.16 (d, 1H, ArH), 10.30 (s, 1H, NH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 21.66, 41.38, 64.61, 89.07, 120.61, 121.18, 123.54, 124.47, 126.53, 126.81, 126.94, 127.23, 127.74, 128.19, 128.53, 128.87, 129.62, 130.31, 133.83, 137.41, 138.53, 142.14, 159.81, 162.38 ppm. ESI–MS (m/z): 444 (M+1)+. Anal. Calcd for C28H21N5O: C, 75.83; H, 4.77; N, 15.79. Found: C, 75.74; H, 4.71; N, 15.87.
14-(4-Methoxyphenyl)-5-phenyl-6-hydro-5H,14H-naphtho[1′,2′:5,6]pyrano[3,2-e][1–4]tetrazolo[2,3-c]pyrimidine (4e)
White solid, yield: 57 %; mp: 196–198 °C. IR (KBr, cm−1): 3,389, 1,588, 1,523, 1,484, 1,451, 1,370, 1,257, 1,148. 1H NMR (DMSO-d 6, 400 MHz): δ 3.89 (s, 3H, OCH3), 5.20 (s, 1H, pyran CH), 6.02 (s, 1H, pyrimidine CH), 7.05 (d, 1H, ArH), 7.21–7.63 (m, 12H, ArH), 7.62 (d, 1H, ArH), 8.21 (d, 1H, ArH), 10.39 (s, 1H, NH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 41.72, 55.59, 65.19, 89.18, 114.79, 121.36, 123.71, 124.88, 126.32, 126.67, 126.89, 127.34, 127.68, 127.93, 128.37, 128.74, 128.89, 129.71, 133.63, 138.92, 144.86, 159.87, 160.23, 162.68 ppm. ESI–MS (m/z): 460 (M+1)+. Anal. Calcd for C28H21N5O2: C, 73.19; H, 4.61; N, 15.24. Found: C, 73.28; H, 4.54; N, 15.35.
14-(3-Nitrophenyl)-5-phenyl-6-hydro-5H,14H-naphtho[1′,2′:5,6]pyrano[3,2-e][1–4]tetrazolo[2,3-c]pyrimidine (4f)
White solid, yield: 52 %; mp: 242–244 °C. IR (KBr, cm−1): 3,419, 1,598, 1,532, 1,481, 1,453, 1,372, 1,261, 1,153. 1H NMR (DMSO-d 6, 400 MHz): δ 5.34 (s, 1H, pyran CH), 6.13 (s, 1H, pyrimidine CH), 7.01 (d, 1H, ArH), 7.36–7.47 (m, 8H, ArH), 7.50 (t, 1H, ArH), 7.56 (d, 1H, ArH), 7.77 (d, 1H, ArH), 8.01 (d, 1H, ArH), 8.10 (s, 1H, ArH), 8.27 (d, 1H, ArH), 10.47 (s, 1H, NH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 42.07, 65.31, 89.36, 119.84, 121.17, 122.59, 123.22, 123.87, 124.53, 125.94, 126.37, 127.42, 127.74, 127.93, 128.29, 128.67, 130.52, 130.84, 133.79, 134.66, 144.76, 146.81, 149.32, 160.08, 162.83 ppm. ESI–MS (m/z): 475 (M+1)+. Anal. Calcd for C27H18N6O3: C, 68.35; H, 3.82; N, 17.71. Found: C, 68.48; H, 3.91; N, 17.63.
General procedure for the synthesis of fused naphthopyrano[3,2-e]tetrazolo[2,3-c][1, 4]diazepines (5a–f)
A mixture of compound 2a–f (2 mmol), 4-methoxyphenacyl bromide (2 mmol) and sodium acetate (2.4 mmol) in ethanol (10 mL) were refluxed for 24 h. After completion of the reaction (monitored by TLC), the reaction mixture was cooled to room temperature then poured into water (20 mL) and then extracted with ethyl acetate (2 × 30 mL). The organic extract was separated, dried over anhydrous Na2SO4 and the solvent was removed under reduced pressure. The residue was then purified by column chromatography on silica gel using hexane/ethyl acetate (6:4) as eluent to obtain compound 5a–f.
15-Phenyl)-5-(4-methoxphenyl-15H-naphtho[1′,2′:5,6]pyrano[3,2-e][1–4]tetrazolo[2,3-c][1, 4]diazepine (5a)
Orange solid, yield: 56 %; mp: 216–218 °C. IR (KBr, cm−1): 1,616, 1,591, 1,511, 1,402, 1,374, 1,219, 1,091. 1H NMR (DMSO-d 6, 400 MHz): δ 3.84 (s, 3H, OCH3), 5.03 (s, 1H, pyran CH), 6.14 (s, 2H, CH2), 7.11–7.17 (m, 4H, ArH), 7.27 (d, 2H, ArH), 7.57–7.89 (m, 8H, ArH), 8.26 (d, 1H, ArH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 40.98, 48.07, 55.14, 105.84, 118.77, 120.51, 120.72, 122.75, 123.82, 125.75, 126.28, 126.62, 126.72, 127.50, 127.64, 128.24, 129.21, 132.68, 133.37, 140.56, 142.71, 157.24, 159.23, 162.11, 164.05 ppm. ESI–MS (m/z): 472 (M+1)+. Anal. Calcd for C29H21N5O2: C, 73.87; H, 4.49; N, 14.85. Found: C, 73.97; H, 4.37; N, 14.96.
15-(4-Chlorophenyl)-5-(4-methoxphenyl-15H-naphtho[1′,2′:5,6]pyrano[3,2-e][1–4]tetrazolo[2,3-c][1, 4]diazepine (5b)
Yellow solid, yield: 53 %; mp: 188–190 °C. IR (KBr, cm−1): 1,610, 1,594, 1,517, 1,411, 1,372, 1,218, 1,088. 1H NMR (DMSO-d 6, 400 MHz): δ 3.86 (s, 3H, OCH3), 5.08 (s, 1H, pyran CH), 6.18 (s, 2H, CH2), 6.98–7.22 (m, 4H, ArH), 7.33–7.59 (m, 8H, ArH), 7.81 (d, 1H, ArH), 8.17 (d, 1H, ArH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 41.11, 48.23, 55.38, 105.67, 118.62, 120.61, 120.83, 123.42, 124.76, 125.81, 126.52, 126.71, 126.88, 127.73, 129.23, 129.43, 131.24, 133.32, 133.84, 142.86, 145.42, 157.31, 159.17, 162.29, 164.31 ppm. ESI–MS (m/z): 506 (M+1)+. Anal. Calcd for C29H20ClN5O2: C, 68.84; H, 3.98; N, 13.84. Found: C, 68.76; H, 3.89; N, 13.92.
15-(4-Fluorohenyl)-5-(4-methoxphenyl-15H-naphtho[1′,2′:5,6]pyrano[3,2-e][1–4]tetrazolo[2,3-c][1,4]diazepine (5c)
Brown solid, yield: 55 %; mp: 170–172 °C. IR (KBr, cm−1): 1,613, 1,594, 1,532, 1,414, 1,379, 1,228, 1,087. 1H NMR (DMSO-d 6, 400 MHz): δ 3.85 (s, 3H, OCH3), 5.10 (s, 1H, pyran CH), 6.15 (s, 2H, CH2), 7.02–7.23 (m, 4H, ArH), 7.31–7.60 (m, 8H, ArH), 7.84 (d, 1H, ArH), 8.19 (d, 1H, ArH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 41.17, 48.26, 55.47, 105.54, 118.47, 120.28, 120.74, 123.67, 124.71, 125.83, 126.14, 126.65, 126.79, 127.62, 129.24, 129.63, 131.41, 133.53, 133.71, 142.79, 145.38, 157.26, 159.21, 162.13, 164.09 ppm. ESI–MS (m/z): 490 (M+1)+. Anal. Calcd for C29H20FN5O2: C, 71.16; H, 4.12; N, 14.31. Found: C, 71.27; H, 4.21; N, 14.23.
15-(4-Methylphenyl)-5-(4-methoxphenyl-15H-naphtho[1′,2′:5,6]pyrano[3,2-e][1–4]tetrazolo[2,3-c][1, 4]diazepine (5d)
Orange solid, yield: 50 %; mp: 176–178 °C. IR (KBr, cm−1): 1,602, 1,596, 1,527, 1,434, 1,377, 1,216, 1,090. 1H NMR (DMSO-d 6, 400 MHz): δ 2.28 (s, 3H, CH3), 3.87 (s, 3H, OCH3), 5.12 (s, 1H, pyran CH), 6.22 (s, 2H, CH2), 6.96–7.27 (m, 4H, ArH), 7.36–7.61 (m, 8H, ArH), 7.83 (d, 1H, ArH), 8.20 (d, 1H, ArH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 22.02, 41.24, 48.34, 55.57, 105.67, 118.37, 120.62, 123.38, 124.71, 125.83, 126.31, 126.68, 126.87, 127.24, 128.17, 128.43, 128.87, 130.29, 130.54, 133.67, 137.62, 142.14, 157.63, 159.34, 162.19, 164.23 ppm. ESI–MS (m/z): 486 (M+1)+. Anal. Calcd for C30H23N5O2: C, 74.21; H, 4.77; N, 14.42. Found: C, 74.13; H, 4.69; N, 14.53.
5,15-Di-(4-Methoxyphenyl)-15H-naphtho[1′,2′:5,6]pyrano[3,2-e][1–4]tetrazolo[2,3-c][1, 4]diazepine (5e)
Brown solid, yield: 47 %; mp: 204–206 °C. IR (KBr, cm−1): 1,610, 1,592, 1,536, 1,422, 1,377, 1,211, 1,087. 1H NMR (DMSO-d 6, 400 MHz): δ 3.85 (s, 3H, OCH3), 3.88 (s, 3H, OCH3), 5.17 (s, 1H, pyran CH), 6.24 (s, 2H, CH2), 7.00–7.24 (m, 4H, ArH), 7.42–7.61 (m, 8H, ArH), 7.82 (d, 1H, ArH), 8.23 (d, 1H, ArH) ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 41.37, 49.17, 55.88, 55.94, 105.12, 114.90, 118.86, 120.11, 121.17, 123.58, 124.74, 126.18, 126.87, 127.34, 127.87, 128.39, 128.76, 129.53, 130.34, 133.72, 144.86, 157.32, 158.21, 159.73, 162.52, 164.47 ppm. ESI–MS (m/z): 502 (M+1)+. Anal. Calcd for C30H23N5O3: C, 71.84; H, 4.62; N, 13.96. Found: C, 71.76; H, 4.71; N, 13.88.
15-(3-Nitrophenyl)-5-(4-methoxphenyl-15H-naphtho[1′,2′:5,6]pyrano[3,2-e][1–4]tetrazolo[2,3-c][1, 4]diazepine (5f)
Brown solid, yield: 43 %; mp: 241–244 °C. IR (KBr, cm−1): 1,619, 1,590, 1,541, 1,422, 1,375, 1,218, 1,087. 1H NMR (DMSO-d 6, 400 MHz): δ 3.89 (s, 3H, OCH3), 5.21 (s, 1H, pyran CH), 6.27 (s, 2H, CH2), 7.04–7.47 (m, 8H, ArH), 7.53 (t, 1H, ArH), 7.59 (d, 1H, ArH), 7.84 (d, 1H, ArH), 8.06 (d, 1H, ArH), 8.16 (s, 1H, ArH), 8.27 (d, 1H, ArH), ppm. 13C NMR (DMSO-d 6, 100 MHz): δ 41.42, 49.34, 55.96, 105.43, 118.26, 119.84, 121.37, 122.54, 123.45, 123.86, 125.47, 125.72, 126.39, 127.54, 127.76, 128.37, 130.43, 130.67, 133.70, 134.53, 144.85, 146.73, 149.32, 157.42, 159.21, 162.78, 164.69 ppm. ESI–MS (m/z): 517 (M+1)+. Anal. Calcd for C29H20N6O4: C, 67.44; H, 3.90; N, 16.27. Found: C, 67.51; H, 3.98; N, 16.19.
Biological protocol
Antimicrobial activity
All the newly synthesized compounds 2a–f, 3a–f, 4a–f, and 5a–f were screened for their antibacterial activity against Gram-positive bacteria (B. subtilis, S. aureus) and Gram-negative bacteria (P. aeuroginosa, E. coli). The antifungal activity of the compounds was assayed against C. albicans and A. niger. The MICs of the compound assays were carried out using the microdilution susceptibility method. Ciprofloxacin was used as reference antibacterial agent. Fluconazole was used as reference antifungal agent. The test compounds, ciprofloxacin and fluconazole, were dissolved in DMSO at a concentration of 800 μg/mL, then diluted in culture medium (nutrient agar for bacteria and potato dextrose agar for fungi), and two-fold serial dilutions of the solution was prepared (400, 200, 100, 50, 25, 12.5 and 6.25 μg/mL). The tubes were incubated at 36 °C for 24 h and 48 h for bacteria and fungi, respectively. The minimum inhibitory concentrations (MIC, μg/mL) of the compounds were recorded as the lowest concentration of each chemical compounds in the tubes with no turbidity (i.e. no growth) of inoculated bacteria/fungi.
Results and discussion
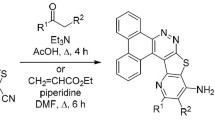
The reaction sequences employed for the synthesis of compounds 2a–f, 3a–f, 4a–f, and 5a–f are shown in Scheme 1. The starting materials used in the present study, namely 2-amino-4-aryl-benzo[h]chromene-3-carbonitriles 1a–f, were prepared by a known procedure [37]. The compound 1a–f on reaction with sodium azide in presence of dimethylformamide gave corresponding tetrazole derivatives 2a–f. The six- and seven-membered fused heterocycles 3a–f, 4a–f, and 5a–f were prepared by cyclization of 2a–f with carbon disulfide, benzaldehyde and 4-methoxyphenacyl bromide, respectively. The physical data of synthesized compounds are given in the “Experimental” section.
The structures of all the newly synthesized compounds were established on the basis of their spectral and elemental data. Spectral data of compounds were in full agreement with proposed structures. The formation of tetrazolylbenzo[h]chromene 2a–f from 2-amino-benzo[h]chromeno-3-carbonitrile 1a–f was confirmed by its IR, NMR and mass spectral data. In IR spectra, the presence of a –NH band at 3,383–3,471 cm−1 and absence of a sharp absorption band (CN) around 2,200 cm−1 showed the formation of tetrazole, while 1H NMR showed a singlet at δ 10.32–10.61 ppm due to the –NH proton. In 13C NMR, the tetrazole carbon was observed at δ 161.20–161.96 ppm. Further, the cyclization of 2a–f and 3a–f was supported by its IR, NMR and mass spectra. In its IR spectrum, NH stretching vibrations appeared around 3,387–3,413 cm−1, and a band near 1,167–1,181 cm−1 confirms the C=S group in the synthesized compounds. The 1H NMR spectrum of 3a–f exhibited a singlet at δ 10.32–10.52 ppm due to the proton of –NH. The formation of 3a–f was further confirmed by its 13C NMR, in which the signal due to C=S was observed around δ 189.51–190.14 ppm. Similarly, the formation of 4a–f and 5a–f were also confirmed by their IR, NMR, and mass spectral data. The 1H NMR of 4a–f, demonstrated a singlet at δ 5.81–6.13 ppm due to the –CH proton of the pyrimidine ring and its 13C NMR showed a signal at δ 64.12–65.31 ppm. In the IR spectrum of 5a–f, the disappearance of bands around 3,202–3,471 cm−1 due to –NH2 and –NH groups clearly confirms the formation of fused tetrazolodiazepine ring. In its 1H NMR and 13C NMR, peaks due to the –CH2 group were observed at δ 6.14–6.27 ppm and δ 48.07–49.34 ppm, respectively, which clearly indicate the smooth cyclization. In all the synthesized compounds the pyran proton was observed in the range of δ 4.80–5.34 ppm and all other aromatic and aliphatic protons were observed at the expected regions. The mass spectra detected the expected molecular ion signals corresponding to respective molecular formula of the synthesized compounds. The elemental analyses values were in good agreement with the theoretical data. The detailed spectral data are given in the “Experimental” section..
Antimicrobial activity
The antibacterial activity of the synthesized compounds 2a–f, 3a–f, 4a–f, and 5a–f was screened against Gram-positive bacteria such as Bacillus subtilis, Staphylococcus aureus, and Gram-negative bacteria, i.e. Pseudomonas aeruginosa, Escherichia coli, using nutrient agar medium. The antifungal activity of the compounds was tested against Candia albicans and Aspergillus niger using potato dextrose agar medium. The minimum inhibitory concentration (MIC) was carried out using the microdilution susceptibility method [38]. Ciprofloxacin was used as a standard antibacterial drug and fluconazole was used as standard antifungal drug. The observed data on the antimicrobial activity of compounds and control drugs are given in Table 1.
The results of antimicrobial activity data of tested compounds revealed that, among the screened compounds, only compounds 3d and 4e were found to be active against B. subtilis. Compound 3d displayed good activity towards S. aureus, whereas compound 4e showed good activity against E. coli. The remaining compounds showed moderate to poor activity towards both bacteria.
Amongst the tested compounds, only compounds 2c and 4e were found to be potent against both fungal strains. Compound 3d exhibited good activity towards A. niger. Except 2c, 3d, and 4e , the remaining compounds showed moderate to poor activity against fungi. The structure–activity relationship of the synthesized compounds revealed that the compounds having a diazepine ring showed least activity compared to other compounds.
Conclusion
In conclusion, we have designed and synthesized a new series of fused naphthopyranotetrazolopyrimidines, thiopyrimidines, and diazepines. Various chemical and spectral data supported the structures of the newly synthesized compounds. The synthesized compounds were screened for their in vitro antibacterial and antifungal activity. Among the tested compounds, 3d and 4e displayed good activity against both bacterial and fungal strains, whereas compound 2c exhibited good activity towards fungi.
References
M.M. Khafagy, A.H.F.A. El-Wahas, F.A. Eid, A.M. El-Agrody, Farmaco 57, 715 (2002)
X. Fan, D. Feng, Y. Qua, X. Zhang, J. Wang, P.M. Loiseau, G. Andrei, R. Snoeck, E. De Clercq, Bioorg. Med. Chem. Lett. 20, 809 (2010)
K. Hiramoto, A. Nasuhara, K. Michiloshi, T. Kato, K. Kikugawa, Mutat. Res. 395, 47 (1997)
C.P. Dell, C.W. Smith, Chem. Abstr. 119, 139102d (1993)
S.J. Mohr, M.A. Chirigos, F.S. Fuhrman, J.W. Pryor, Cancer Res. 35, 3750 (1975)
S.A. Patil, J. Wang, X.S. Li, J. Chen, T.S. Jones, A.H. Ahmed, R. Patil, W.L. Seibel, W. Li, D.D. Miller, Bioorg. Med. Chem. Lett. 22, 4458 (2012)
F. Eiden, F. Denk, Arch. Pharm. (Weinheim) 324, 353 (1991)
G.P. Ellis, in The chemistry of heterocyclic compounds: chromenes, chromanes and chromones, ed. by A. Weissberger, E.C. Taylor (John Wiley, New York, 1977), p. 11
E.A. Hafez, M.H. Elnagdi, A.G.A. Elagemey, F.M.A.A. El-Taweel, Heterocycles 26, 903 (1987)
R.J. Herr, Bioorg. Med. Chem. 10, 3379 (2002)
J. Zabrocki, G.D. Smith, J.B. Dunbar Jr, H. Iijima, G.R. Marshall, J. Am. Chem. Soc. 110, 5875 (1988)
J. Zabrocki, G.R. Marshall, Methods Mol. Med. 23, 417 (1999)
D. Moderhack, J. Prakt, Chem/Chem.-Ztg. 340, 687 (1998)
V.A. Ostrovskii, M.S. Pevzner, T.P. Kofman, M.B. Shcherbinin, I.V. Tselinskii, Targets Heterocycl. Syst. 3, 467 (1999)
F. Ek, L.-G. Wistrand, T. Frejd, Tetrahedron 59, 6759 (2003)
L.A. Flippin, Tetrahedron Lett. 32, 6857 (1991)
P. Rhonnstad, D. Wensbo, Tetrahedron Lett. 43, 3137 (2002)
T. Eicher, S. Hauptmann, The Chemistry of Heterocycles (Thieme, NewYork, 1995), p. 212
J.V. Duncia, D.J. Carini, A.T. Chiu, A.L. Johnson, W.A. Price, P.C. Wong, R.R. Wexler, P.B.M.W.M. Timmermans, Med. Res. Rev. 12, 149 (1992)
R.D. Smith, J.V. Duncia, R.J. Lee, D.D. Christ, A.T. Chiu, D.J. Carini, W.F. Herblin, P.B.M.W.M. Timmermans, R.R. Wexler, P.C. Wong, Methods Neurosci. 13, 258 (1993)
R.-Q. Huang, C.L. Bell-Horner, M.I. Dibas, D.F. Covey, J.A. Drewe, G.H. Dillon, J. Pharmacol. Exp. Ther. 298, 986 (2001)
M.E. Jung, H. Lal, M.B. Gatch, Neurosci. Biobehav. Rev. 26, 429 (2002)
S. Daya, P.T. Kaye, M. Mphahlele, J. Med. Sci. Res. 24, 137 (1996)
N.R. Kamdar, D.D. Haveliwala, P.T. Mistry, S.K. Patel, Eur. J. Med. Chem. 45, 5056 (2010)
N.A. Hassan, KhM Abu-Zeid, A.B. EI-Gazzar, Heterocycl. Commun. 12, 73 (2006)
O. Bruno, C. Brullo, A. Ranise, S. Schenone, F. Bondavalli, E. Barocelli, V. Ballabeni, M. Chiavarini, M. Tognolini, M. Impicciatore, Bioorg. Med. Chem. Lett. 11, 1397 (2001)
E.P.S. da Falcao, S.J. de Melo, R.M. Srivastava, M.T.J.A. de Catanho, S.C. Do Nascimento, Eur. J. Med. Chem. 41, 276 (2006)
F. Chabchoub, M. Messaad, H.B. Mansour, L. Chekir-Ghedira, M. Salem, Eur. J. Med. Chem. 42, 715 (2007)
J.P. Yevich, D.L. Temple Jr, R.R. Covington, D.A. Owens, R.J. Seidehamel, K.W. Dungan, J. Med. Chem. 25, 864 (1982)
A.M. Hussein, O.M. Ahmed, Bioorg. Med. Chem. 18, 2639 (2010)
L. Costantino, D. Barlocco, Curr. Med. Chem. 13, 65 (2006)
H. Schutz, Benzodiazepines (Springer, Heidelberg, 1982)
J.K. Landquist, in Comprehensive Heterocyclic Chemistry, vol. 1, ed. by A.R. Katritzky, C.W. Rees (Pergamon, Oxford, 1984), pp. 166–170
R.I. Fryer, Bicyclic Diazepines, in Comprehensive Heterocyclic Chemistry, ed. By E.C. Taylor (Wiley, New York, USA, 1991), Vol. 50, Chapter II
L.O. Randall, B. Kappel, in Benzodiazepines, ed. by S. Garattini, E. Musini, L.O. Randall (Raven Press, New York, 1973), p. 27
S. Kanakaraju, B. Prasanna, S. Basavoju, G.V.P. Chandramouli, J. Mol. Struct. 1017, 60 (2012)
A.M. Shestopalov, Y.M. Emelianova, V.N. Nesterov, Russ. Chem. Bull. Int. Ed. 51, 2238 (2002)
P.R. Murray, E.J. Baron, M.A. Pfaller, F.C. Tenover, R.H. Yolken, in Manual of Clinical Microbiology, ed. by G.L. Wood, J.A. Washington (Am. Soc. Microbiol., Washington D.C, 1995)
Acknowledgment
The authors are thankful to the Director, National Institute of Technology, Warangal, for providing research facilities and financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanakaraju, S., Chandramouli, G.V.P. Synthesis and antimicrobial studies of some novel series of fused naphthopyranotetrazole derivatives. Res Chem Intermed 41, 2809–2822 (2015). https://doi.org/10.1007/s11164-013-1390-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1390-y