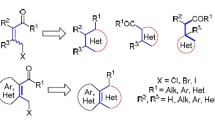
Abstract
Small poly-functionalized heterocycles are frequently found in pharmacophores and play important roles in drug discovery. Heterocyclic ketene aminals (HKAs) are versatile building blocks for the synthesis of a variety of heterocyclic compounds. In recent years, there has been significant progress in the chemistry of HKAs. All previous work focused on the developments of HKAs in reaction type. This review focused on the developments of HKA-based synthesis of various heterocyclic nuclei since 2002. We believe this will give some insights and help to bring about new ideas for further research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poly-functionalized heterocycles are frequently found in pharmacophores and play important roles in drug discovery. Heterocyclic ketene aminals (HKAs), also referred to as cyclic ketene N,N-acetals or cyclic 1,1-enediamines, are powerful and versatile building blocks in synthetic organic chemistry [1]. Due to the conjugation of the amino group and the electron-withdrawing group, the nucleophilicity of α-carbon is highly enhanced. As the amino group can serve as the second nucleophilic center, HKAs are often used to react with bis-electrophiles to construct various types of heterocyclic compounds. Bis-electrophiles, such as ethyl bromoacetate [2, 3], unsaturated carbonyl compounds [4–10], keto esters [11] and active carbonyl compounds [12], have been utilized successfully for fused heterocyclic preparation.
In recent years, there has been significant progress in the chemistry of HKAs. All previous work focused on the developments of HKAs in reaction type [1, 13]. This microreview focused on the developments of HKA-based synthesis of various heterocyclic nuclei since 2002. We believe this review will give some insights and help to bring about new ideas for further research.
Synthesis of pyridine- or pyridone-fused 1,3-diazaheterocycles
Bicyclic pyridine or pyridone motifs are of general interest in medicinal chemistry with therapeutic properties. When HKAs react with 1,3-biselectrophiles, such as unsaturated carboxylic acid esters, acrylonitrile, itaconic anhydride, etc., pyridine- or pyridone-fused 1,3-diazaheterocycles were usually produced as a result. In 2007, our group developed a simple method for the synthesis of polyfunctionalized pyridine-fused 1,3-diazaheterocycles 3 via reaction of HKAs 1 with bis(methylthio)methylene malononitrile 2 (Scheme 1) [14]. The reaction proceeded in a cascade way following a sequence of Michael addition, elimination and annulation by nucleophilic addition of the secondary amino group to the nitrile group. The yields of the reactions were largely dependent on the ring size of the HKAs. Six-membered HKAs usually gave good to excellent yields.
In 2008, a novel method for the synthesis of tetrahydropyridine-fused 1,3-diazaheterocycles 5 was developed by our group via reaction of HKAs 1 with Baylis–Hillman acetates 4 (Scheme 2) [15]. The reaction results were strongly dependent on the conditions. Product 5 was obtained as the sole product when the solvent was switched from polar tetrahydrofuran (THF) to nonpolar CH2Cl2 with the decrease of temperature to 0 °C.
2-[3-oxoisobenzofuran-1(3H)-ylidene]malononitrile 6 was an ideal 1,3-biselectrophile containing an exocyclic double bond for the synthesis of spiro compounds. An efficient route for the synthesis of polyfunctionalized spiro dihydropyridine-fused 1,3-diazaheterocycles 7 (Scheme 3) [16] was developed by the reaction of HKAs with compound 6.
With the emergence of high-throughput screening, multicomponent reactions (MCRs) are gaining importance in synthetic organic chemistry, especially in pharmaceutical chemistry. 1,3-Biselectrophiles may be in a clear form, or they can be created in situ. In 2006, our group first reported the one-pot synthesis of dihydropyridone-fused 1,3-diazaheterocycles 8 employing HKAs, Meldrum’s acid and aldehyde as components (Scheme 4) [17]. The reaction started with the condensation of Meldrum’s acid with the aldehyde to afford alkylidene Meldrum’s acid, which then reacted with HKAs via an aza–ene reaction, imine–enamine tautomerization, cyclocondensation and decarboxylation to afford the final product 8. The structures of the aldehydes had an obvious influence on the reactivity and yields.
When acetone was used as a carbonyl component, compound 9 [18] was produced by employing l-proline as a catalyst (Scheme 5). In the tautomerization of amidine and enamine, most HKA derivatives adopt the enamine form according to their spectrum. Interestingly, due to a steric effect, compound 9 existed exclusively as the amidine form rather than the enamine form.
Following a similar strategy, heating a mixture of HKAs with 1,3-cyclohexanedione derivatives and salicylaldehyde derivatives in water afforded polycyclic 1,4-dihydropyridine derivatives 10 (Scheme 6) in high yield [19]. Alternatively, a four-component reaction of aromatic aldehydes, diamines, nitro ketene dithioacetal and cyclic 1,3-diones or malononitrile afforded octahydro-imidazo[1,2-a] quinolin-6-ones 11 (Scheme 7) [20] or polyfunctionalized 1,4-dihydropyridine-fused 1,3- diazaheterocycles 12 (Scheme 8) [21] in good yields. When HKAs were treated with aldehydes and 2-hydroxy-1,4-naphthoquinone under solvent-free conditions, benzo[g]imidazo[1,2-a]quinolinediones 13 were formed via Et3N-catalyzed annulation (Scheme 9) [22].
Thus, refluxing a mixture of different types of HKAs, isatins and ethyl trifluoroacetate (Scheme 10) [23] or indan-1,3-dione (Scheme 11) [24] catalyzed by piperidine or p-toluenesulfonic acid (p-TSA) afforded structurally diverse spirooxindoles. Alternatively, a four-component reaction of 1,n-diamines, nitro ketene dithioacetal, isatin derivatives and malononitrile in the presence of 10 mol% of piperidine under reflux in ethanol produced highly functionalized spirooxindole derivatives 16 (Scheme 12) [25].
2-(2-Chloroaroyl)methyleneimidazolidines 17 represent a class of polyfunctional scaffolds with 4 reactive sites. The halogen atom on the aromatic ring may act as potential leaving group subjected to an intramolecular SNAr reaction. When treated with 1 equiv of K2CO3 in dimethylformamide (DMF) at 100 °C, the three-component condensation products 18 or 19 were subject to intramolecular nucleophilic aryl substitution to afford tetracyclic benzo[b]imidazo[1,2,3-ij] [1, 8] naphthyridines (Scheme 13) [26, 27].
In 2011, Li’s group reported 2-(2-chloroaroyl)methyleneimidazolidines 22 could react with allenic esters 23 to afford imidazo(pyrido)[1,2-a]pyridines 24 [28] via 1,4-diazabicyclo[2.2.2] octane (DABCO)-catalyzed tandem annulation, and imidazo(pyrido)[3,2,1-ij] [1, 8]-naphthyridines 25 (Scheme 14) were formed when treating with 1 equiv of K2CO3 in DMF at 100 °C.
They also developed an efficient four-component protocol to synthesize imidazo[1,2-a]pyridines 27 and imidazo[1,2,3-ij] [1, 8] naphthyridine derivatives 28 (Scheme 15) from HKAs 26, aldehydes, diketene 29, and amines via cascade reactions [29]. Six sequential reactions including diketene ring-opening, Knoevenagel condensation, aza–ene reaction, imine–enamine tautomerization, cyclocondensation and intramolecular SNAr were involved in the one-pot preparation.
Ram [30] reported when HKAs 30 were treated with suitably functionalized 2H-pyran-2-one 31, almost equal amounts of imidazo[1,2-a]pyridine 32 and pyranone derivative 33 (Scheme 16) were obtained. However, when aroyl-substituted HKAs 1 were used, the bicyclic intermediate 34 underwent photocyclization to afford tetracyclic aza-anthracenones 35 (Scheme 17) [31].
In 2010, Xu and coworkers reported dissolution of compounds 36 in acetonitrile at room temperature led to the formation of two highly congested hexahydroimidazo[1,2-a]pyridine derivatives 37 and 38 (Scheme 18) formed by aza-Diels–Alder reaction [32].
Junjappa [33] reported heating a mixture of HKAs 39 with 1,3-biselectrophiles itaconic anhydride afforded functionalized bicyclic 1,2,3,4-tetrahydropyridones 40 (Scheme 19) in good yield. Alizadeh described an efficient synthesis of highly substituted pyrido[1,2-a]-fused 1,3-diazaheterocycles (Schemes 20, 21) via reaction between nitroketene aminals generated in situ from the addition of various diamines to nitroketene dithioacetal and itaconic anhydride [34] or dibenzylideneacetone [35]. Similar three-component reaction of diamines, nitroketene dithioacetal and alkyl prop-2-ynoates afforded 2-oxopyridine-fused 1,3-diazaheterocycles 41 (Scheme 22) [36].
An efficient synthesis of 1,4-dihydropyridine-fused 1,3-diazaheterocycles 42 (Scheme 23) was developed by reaction of nitroketene aminals generated in situ from the addition of various diamines to nitroketene dithioacetal and 2-iminochromenes in good yield [37].
β-Keto ester enol tosylates 43 reacted with HKAs 39 as a 1,3-biselectrophiles in the presence of a base to afford bicyclic pyridones 44 (Scheme 24) in excellent yields [38]. The reaction probably proceeded via a sequence of Michael addition, elimination, imine–enamine tautomerization and cyclocondensation.
Lin’s group developed an efficient synthesis of highly substituted bicyclic pyridines 45 (Scheme 25) by clocondensation of HKAs 39, triethoxymethane, and ethyl trifluoroacetate under solvent-free and catalyst-free conditions in excellent yields [39]. It was found HKA with various substituents and different ring sizes were all good substrates for the one-pot cyclocondensation reaction.
One-pot reaction of HKAs 1, triethoxymethane and nitroalkenes 46 in the absence of catalyst and solvent gave dihydropyridine-fused diazaheterocycles 47 (Scheme 26) in high yield [40].
In 2013, they also found HKAs 1 reacted with 4-arylmethylene-2-phenyloxazol-5(4H)-ones 48 in the presence of acetic acid to give bicyclic pyridone derivatives 49 (Scheme 27) [41]. Acid catalysts were essential for the reaction.
In 2014, an efficient method for synthesis of pyrrolo[3,4-e]pyridine derivatives 51 (Scheme 28) was developed by reaction of HKAs 1 with 2,3-dioxopyrrolidines 50 [42]. The reaction proceeded smoothly in a short time under catalyst-free conditions. A mechanism involving aza–ene, imine–enamine tautomerization followed by cyclization was proposed.
Synthesis of fused pyrrole derivatives
The pyrrole nucleus is featured in many natural products and drugs. When HKAs reacted with 1,2-bis-electrophiles, such as alkyl glyoxylate, N-alkyl maleimide, etc., multi-functional fused pyrroles were usually produced as a result. When HKAs 1 were treated with N-alkyl maleimide 52 in EtOH at room temperature, bicyclic pyrrolidinone 53 was formed via aza–ene and imine–enamine tautomerization followed by lactamization (Scheme 29) [43]. The reaction proceeded smoothly under catalyst-free conditions. It was interesting to note ring sizes had an effect on the outcome of the reaction and six-membered HKAs were proved to be the most reactive.
Lin’s group reported HKAs 1 reacted with arylglyoxal monohydrates 54 and cyclohexane-1,3-diones 55 in water–ethanol medium under catalyst-free conditions [44]. The kinetically controlled product 56 was synthesized within 1 h (Scheme 30), and would transform into thermodynamically controlled products 57 over an additional 5 h (Scheme 31).
Similarly, HKAs 1 reacted with arylglyoxal monohydrates 54 and 1,3-diphenylpropane-1,3-dione under catalyst-free conditions [45] in ethanol to yield multi-functional fused pyrroles 58 in high yield (Scheme 32).
Thus, refluxing a mixture of HKAs 1, arylglyoxal monohydrate 54, and indoles 59 in ethanol in the presence of acetic acid led to the formation of highly functionalized bicyclic pyrrole derivatives 60 (Scheme 33) [46].
Yan discovered HKAs 1 reacted with acenaphthylene-1,2-dione 61 and ethyl trifluoroacetylacetate to afford polycyclic pyrroles 62 bearing four consecutive quaternary stereocenters (Scheme 34) [47]. Most of the products were generated with diastereoselectivity up to 99:1. An efficient synthesis of oxa-aza[3.3.3]propellanes 63 (Scheme 35) [48] were developed via one-pot four-component reaction involving ninhydrin, malononitrile, diamines and nitroketene dithioacetal. The reaction proceeded by an attack of nitroketene aminals generated in situ from the addition of various diamines to a Knoevenagel adduct of malononitrile with ninhydrin followed by sequential cyclization.
It was found that six- or seven-membered HKAs reacted with ethyl 2,3-diiodoacrylate or diethyl 2,3-diiodofumarate 64 to yield bicyclic pyrroles 65 catalyzed by PdCl2 in the presence of Cs2CO3 [49]. However, when five-membered HKAs were used as substrates, a series of bicyclic pyridones 66 were obtained under the same conditions as above in moderate yield (Scheme 36). This may be due to variations in the nucleophilicity of HKAs with different ring sizes. Usually, six-membered HKAs were more reactive than other HKAs.
Alizadeh reported three-component reaction of nitroketene dithioacetal with 1,n-diamines in the presence of diaroylacetylene 67 or acetylenedicarboxylate 68 afforded fully substituted 1H-pyrrolo[1,2-a]-fused 1,3-diazaheterocycles (Scheme 37) [50] or bicyclic pyrrolidinones 69 (Scheme 38) [51] in good to excellent yields. They also reported [52] three-component reaction of 1,n-diamines, nitroketene dithioacetal and ninhydrin in aqueous media gave indeno[2′,1′:4,5]pyrrolo [1,2-a]-fused 1,3-diazaheterocycles 70 in good yields (Scheme 39).
Synthesis of indole derivatives
The indole skeleton is one of the most abundant and relevant heterocycles in natural products and drugs. In 2010, Lin’s group developed an efficient synthesis of 1,3-diazaheterocycle-fused [1,2-a] indoles 72 (Scheme 40) by refluxing a reaction mixture of HKAs 1 and 1,4-benzoquinones 71 in the presence of acetic acid via a Nenitzescu strategy [53]. The reaction started with an attack of HKAs at the α-position of 1,4-benzoquinones 71, then the adduct underwent imine–enamine tautomerization, subsequent condensation and elimination of H2O to afford the target compound.
In 2014 it was found when HKAs 1 were treated with quinones 73 in ethanol at room temperature, indolone derivatives 74 were produced in 30 min via an unexpected anti-Nenitzescu strategy (Scheme 41) [54]. The reaction started with aza–ene reaction of HKAs onto carbonyl of 1,4-benzoquinones 73, then the adduct underwent imine–enamine tautomerization, Michael addition, keto–enol tautomerization and oxidation to afford the target compound. The origin of site selectivity was explained according to the computational results.
In 2015, they also found HKAs 1 could be treated with halogenated quinones 75 without a catalyst in acetone at room temperature to yield fused [1,2-a]indolone derivatives 76 via a Nenitzescu strategy (Scheme 42) [55]. It should be noted ring sizes and the electron-withdrawing property of the halides had an obvious effect on reaction yield. Six- and seven-membered HKAs were proved to be more reactive than five-membered HKAs. The halides with a stronger electron-withdrawing property usually gave higher yields.
In 2009, Zeng et al. [56] developed a convenient electrochemical approach for the synthesis of fused indole derivatives containing active hydroxyl groups from catechols 78 and HKAs 77 (Scheme 43).
Koca developed a convenient procedure for the preparation of isoindole derivatives 83 (Scheme 44). Heating a mixture of HKAs 81 with 2 equiv. of acetylenic esters 68 in the presence of 4-dimethylaminopyridine (DMAP) for 30 min led to the formation of a fused isoindole derivative 3 [57]. A possible reaction scenario was proposed.
Synthesis of coumarin derivatives
Coumarin derivatives are a structural framework in a large number of bioactive natural products. In 2010, Lin’s group reported HKAs 1 reacted with coumarin derivatives 84 catalyzed by potassium hexamethyldisilazane (KHMDS) in dioxane under microwave irradiation to yield a series of polycyclic coumarin derivatives (Scheme 45) [58]. A mechanism involving 1,4-Michael addition, imine–enamine tautomerization, cyclocondensation and aromatization was proposed.
A regioselective method for synthesis of fused coumarin derivatives 86 was developed by reaction of HKAs 1 with 4-chloro-3-formylcoumarin 85 (Scheme 46) [59]. The reaction proceeded smoothly in EtOH catalyzed by Et3N via aza–ene, imine–enamine tautomerization, cycloaddition and dehydration to afford the product in excellent yields.
Yan developed a facile approach for the synthesis of tetracycloisocoumarins 88 based on AcOH-catalyzed cyclocondensation and rearrangement of HKAs 1 with 2,2-dihydroxy-2H-indene-1,3-dione 87 (Scheme 47) [60].
Synthesis of miscellaneous heterocycles
Zhu’s group found that fluoroalkanesulfonyl azide 89 reacted readily with HKAs 1 at room temperature, and developed an quantitative synthesis of 1,3-diazaheterocycle-fused 1,2,3-triazoles 90 by 1,3-dipolar cycloaddition of HKAs with fluoroalkanesulfonyl azide 89 (Scheme 48) [61]. This method was applicable to various HKAs and fluoroalkanesulfonyl azides and is suitable for combinatorial and parallel synthesis in new drug discovery.
Xu reported five-membered HKAs 1 reacted with ethyl 2-(bromomethyl)benzoate 91 in refluxing acetonitrile to afford the C-benzylated products 92 which underwent intramolecular cyclization under alkaline conditions to produce fused benzazepinones 93 (Scheme 49) [62].
Lin’s group developed a concise and efficient route for the synthesis of highly substituted imidazopyrroloquinoline derivatives 96 by simply refluxing a reaction mixture of different types of isatins 94 and HKAs 95 under catalysis of acetic acid (Scheme 50) [63]. A library of highly substituted imidazopyrroloquinoline derivatives was rapidly constructed as a result. A mechanism of the cascade reaction was proposed.
In 2013, Zhang discovered heating a mixture of HKAs 1 with 2-chloroquinoline-3-carbaldehydes 97 under the catalysis of piperidine at 75 °C afforded 1,3-diazaheterocycle-fused[1,2-a] [1, 8] naphthyridine derivatives 98 (Scheme 51) [64]. The reaction was studied via a joint experimental–computational approach.
Yan reported HKAs 39 underwent substitution–cyclization reaction with polyhalo isophthalonitrile 99 in the presence of t-BuOK to afford 1,3-diazaheterocycle-fused [1,2-b]isoquinolin-1(2H)-imines 100, which could be hydrolyzed to give highly functional polyhalo 1,3-diazaheterocycle-fused [1,2-b]isoquinolin-1(2H)-ones 101 (Scheme 52) [65].
Yaqub [66] developed a novel method for the synthesis of tetracyclic fused-ring heterocycles 103 (Scheme 53), which are closely related to circumdatin alkaloids, via the reaction of substituted 3-formylchromone 102 with HKAs. The solvent polarity was found to play an important role on the yield of tetracyclic fused-ring heterocycles.
Alizadeh [67] developed a concise and efficient method for the synthesis of pyrimido[1,6-a]pyrimidine and imidazo[1,2-c]pyrimidine derivatives 105 by simply refluxing a reaction mixture of HKAs 1, or generated HKAs in situ from the addition of various diamines to nitroketene dithioacetal and N,N′-bis(arylmethylidene)arylmethane 104 (Scheme 54).
Yan developed an efficient one-pot synthesis of novel 1H-pyrazol-5(4H)-one-based heterocyclic ketene aminal 107 by refluxing a mixture of HKAs 1, 1-phenyl-1H-pyrazol-5(4H)-ones 106 and triethoxymethane under solvent-free and catalyst-free conditions (Scheme 55) [68].
A series of 2-benzenesulfonothiol-HKAs 109 (Scheme 56) were prepared via a silver(I)-mediated direct sulfenylation of HKAs with benzenesulfonic thioanhydride 108 [69]. The preparation method was efficient and convenient.
Conclusions
Possessing three reactive sites including α-carbon, nitrogen and oxygen in one molecule, HKAs could react with a variety of biselectrophiles, even 1,3-dipoles, to produce novel heterocyclic compounds hardly accessible by other methods. Recent developments in the preparation of various heterocyclic nuclei by reactions of HKAs were reviewed. From a chemist's point of view, MCRs closely approach the concept of ideal synthesis. Considering the importance of chirality, MCRs and synthesis of chiral HKAs and their asymmetric reactions will draw the attention from more and more chemists; on the other hand, as people are more aware of environmental protection, green HKA chemistry will have a bright future.
References
Z.T. Huang, M.X. Wang, Prog. Nat. Sci. 12, 249 (2002)
Z.T. Huang, Z.R. Liu, Chem. Ber. 122, 95 (1989)
L.B. Wang, C.Y. Yu, Z.T. Huang, Synthesis 26, 1441 (1994)
Z.T. Huang, L.H. Tzai, Chem. Ber. 119, 2208 (1986)
Z.T. Huang, Z.R. Liu, Heterocycles 24, 2247 (1986)
Z.T. Huang, M.X. Wang, J. Chem. Soc. Perkin Trans. 1, 1085 (1993)
R.C.F. Jones, P. Patel, S.C. Hirst, M.J. Smallridge, Tetrahedron 54, 6191 (1998)
R.C.F. Jones, P. Patel, S.C. Hirst, Tetrahedron Lett. 30, 5361 (1989)
J.H. Zhang, M.X. Wang, Z.T. Huang, Tetrahedron Lett. 39, 9237 (1998)
J.H. Zhang, M.X. Wang, Z.T. Huang, J. Chem. Soc. Perkin Trans. 1, 2087 (1999)
R.C.F. Jones, P. Patel, S.C. Hirst, I. Turne, Tetrahedron 53, 11781 (1997)
J.H. Zhang, M.X. Wang, Z.T. Huang, J. Chem. Soc. Perkin Trans. 1, 321 (1999)
K.M. Wang, S.J. Yan, J. Lin, Eur. J. Org. Chem. 79, 1129–1145 (2014)
J.P. Liao, T. Zhang, C.Y. Yu, Z.T. Huang, Synlett 18, 761–764 (2007)
M. Yaqub, C.Y. Yu, Y.M. Jia, Z.T. Huang, Synlett 19, 1357–1360 (2008)
W.Y. Xu, Y.M. Jia, J.K. Yang, Z.T. Huang, C.Y. Yu, Synlett 21, 1835–1840 (2010)
C.Y. Yu, P.H. Yang, M.X. Zhao, Z.T. Huang, Synlett 17, 1835–1840 (2006)
P.H. Yang, W.Z. Wang, Y.S. Duan, C.T. Qu, Q.Z. Zhang, Heterocycl. Commun. 19(3), 167–169 (2013)
Y.L. Ma, K.M. Wang, X.R. Lin, S.J. Yan, J. Lin, Tetrahedron 70, 6578–6584 (2014)
A. Alizadeh, A. Rezvanian, C. R. Chim. 17, 103–107 (2014)
A. Alizadeh, T. Firuzyar, A. Mikaeili, J. Heterocycl. Chem. 50, 676–679 (2013)
L.R. Wen, Q.C. Sun, H.L. Zhang, M. Li, Org. Biol. Chem. 11(42), 7276–7288 (2013)
F.C. Yu, R. Huang, H.C. Ni, J. Fan, S.J. Yan, J. Lin, Green Chem. 15, 453–462 (2013)
X.B. Chen, X.M. Liu, R. Huang, S.J. Yan, J. Lin, Eur. J. Org. Chem. 78, 4607–4613 (2013)
A. Alizadeh, T. Firuzyar, A. Mikaeili, Synthesis 42, 3913–3917 (2010)
L.R. Wen, C.Y. Jiang, M. Li, L.J. Wang, Tetrahedron 67, 293–302 (2011)
L.R. Wen, C. Liu, M. Li, L.J. Wang, J. Org. Chem. 75, 7605–7614 (2010)
M. Li, Z.M. Zhou, L.R. Wen, Z.X. Qiu, J. Org. Chem. 76, 3054–3063 (2011)
M. Li, P. Shao, S.W. Wang, W. Kong, L.R. Wen, J. Org. Chem. 77, 8956–8967 (2012)
V.J. Ram, N. Agarwal, A. Sharon, P.R. Maulik, Tetrahedron Lett. 43, 307–310 (2002)
A. Sharon, R. Prantap, P.R. Maulik, V.J. Ram, Tetrahedron 61, 3781–3787 (2005)
X.S. Shao, Z.P. Xu, X.F. Zhao, X.Y. Xu, L.M. Tao, Z. Li, X.H. Qian, J. Agric. Food Chem. 58, 2690–2695 (2010)
S. Chakrabarti, K. Panda, N.C. Misra, H. Iia, H. Junjappa, Synlett 16, 1437–1441 (2005)
A. Alizadeh, A. Rezvanian, Helv. Chim. Acta 95, 152–156 (2012)
A. Alizadeh, A. Rezvanian, Synlett 22, 1105–1108 (2011)
A. Alizadeh, A. Mikaeili, T. Firuzyar, M. Ahmadi, Helv. Chim. Acta 94, 1343–1346 (2011)
A. Alizadeh, R. Ghanbaripour, L.G. Zhu, Synth. Commun. 43, 2575–2582 (2013)
S.J. Yan, Y.F. Niu, R. Huang, J. Lin, Synlett 20, 2821–2824 (2009)
S.J. Yan, Y.L. Chen, L. Liu, N.Q. He, J. Lin, Green Chem. 12, 2043–2052 (2010)
F.C. Yu, S.J. Yan, R. Huang, Y.J. Tang, J. Lin, RSC Adv. 1, 596–601 (2011)
X.B. Chen, D.D. Zhu, X.Y. Wang, S.Y. Yan, J. Lin, Tetrahedron 69, 9224–9236 (2013)
X.B. Chen, L. Zhu, L. Fang, S.J. Yan, J. Lin, RSC Adv. 4, 9926–9934 (2014)
J. Liu, H.R. Zhang, X.R. Lin, S.J. Yan, J. Lin, RSC Adv. 4, 27582–27590 (2014)
X.B. Chen, Z.C. Liu, L.F. Yang, S.J. Yan, J. Lin, ACS Sustain. Chem. Eng 2, 1155–1163 (2014)
X.B. Chen, S.J. Yan, A. Su, W. Liu, J. Lin, Tetrahedron 71, 4745–4751 (2015)
X.B. Chen, X.Y. Wang, D.D. Zhu, S.J. Yan, J. Lin, Tetrahedron 70, 1047–1054 (2014)
X.B. Chen, Z.C. Liu, X.R. Lin, R. Huang, S.J. Yan, J. Lin, ACS Sustain. Chem. Eng. 2, 2391–2398 (2014)
A. Rezvanian, A. Alizadeh, Tetrahedron 68, 10164–10168 (2012)
L. Hu, K.M. Wang, M. Zhao, X.R. Lin, H.Y. Zhu, S.J. Yan, J. Lin, Tetrahedron 70, 4478–4484 (2014)
A. Alizadeh, A. Rezvanian, Y. Deng, Tetrahedron 66, 9933–9937 (2010)
A. Alizadeh, A. Mikaeili, J. Heterocycl. Chem. 51, 527–531 (2014)
A. Alizadeh, A. Zarei, A. Rezvanian, Synthesis 43, 497–501 (2011)
L.J. Yang, S.J. Yan, W. Chen, J. Lin, Synthesis 42, 3536–3544 (2010)
B. Zhou, Z.C. Liu, W.W. Qu, R. Yang, X.R. Lin, S.J. Yan, J. Lin, Green Chem. 16, 4359–4370 (2014)
F.C. Yu, X.P. Hao, X.R. Lin, S.J. Yan, J. Lin, Tetrahedron 71, 4084–4089 (2015)
C.C. Zeng, F.J. Liu, D.W. Ping, L.M. Hu, Y.L. Cai, R.G. Zhong, J. Org. Chem. 74, 6386–6389 (2009)
S.H. Űngoren, İ. Koca, F. Yilmaz, Tetrahedron 67, 5409–5414 (2011)
S.J. Yan, J. Lin, Chin. J. Org. Chem. 30(3), 465–468 (2010)
F.C. Yu, Z.Q. Chen, X.P. Hao, S.J. Yan, R. Huang, J. Lin, RSC Adv. 4, 6110–6115 (2014)
S.J. Yan, Y.L. Chen, L. Liu, Y.J. Tang, J. Lin, Tetrahedron Lett. 52, 465–467 (2011)
J.W. Gu, W.T. Xiong, Z.H. Zhang, S.Z. Zhu, Synthesis 43, 1717–1722 (2011)
Z.H. Xu, Y.F. Jie, M.X. Wang, Z.T. Huang, Synthesis 34, 523–527 (2002)
F.C. Yu, S.Y. Yan, L. Hu, Y.C. Wang, J. Lin, Org. Lett. 13(18), 4782–4785 (2011)
Y.C. Zhang, Z.C. Liu, R. Yang, J.H. Zhang, S.J. Yan, J. Lin, Org. Biomol. Chem. 11, 7276–7288 (2013)
S.Y. Yan, C. Huang, C.X. Su, Y.F. Ni, J. Lin, J. Comb. Chem. 12, 91–94 (2010)
M. Yaqub, R. Perveen, Z. Shafiq, H. Pervez, M.N. Tahirb, Synlett 23, 1357–1360 (2012)
A. Alizadeh, J. Mokhtari, M. Ahmadi, Tetrahedron 68, 319–322 (2012)
F.C. Yu, Z.Q. Chen, X.P. Hao, X.Y. Jiang, S.Y. Yan, J. Lin, RSC Adv. 3, 13183–13192 (2013)
T.T. Liu, X.R. Lin, R. Huang, L.F. Yang, S.J. Yan, J. Lin, Tetrahedron 70, 8858–8862 (2014)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 21575112) and funding from the Education Department of Shaanxi Province (15JS091).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, P.H. Recent developments in the heterocyclic ketene aminal-based synthesis of heterocycles. Res Chem Intermed 42, 5617–5637 (2016). https://doi.org/10.1007/s11164-015-2391-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2391-9