Abstract
Pyridine-4-carboxylic acid (PYCA) functionalized Fe3O4 nanoparticles as an organic–inorganic hybrid heterogeneous catalyst was fabricated and characterized by FT-IR, XRD, TGA, TEM, SEM, and VSM techniques. The catalytic activity of the magnetic catalyst was probed through one-pot synthesis of pyrano[3,2-b]pyranone derivatives from three component reactions of aromatic aldehydes, kojic acid, and ethyl cyanoacetate under solvent-free conditions. Some advantages of this protocol are its environmentally benign method, simple procedure, high yields, and short reaction time. The catalyst was readily separated using an external magnet and reusable without significant loss of its catalytic efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanomaterials have attracted a great deal of attention in organic synthesis owing to their high surface-to-volume ratio, which are responsible for the higher catalytic activity [1–3]. Surface functionalized iron oxide magnetic nanoparticles (MNPs) are novel functional materials, which have been widely used in biotechnology and catalysis [4–9]. Magnetic nanocatalysts can easily be separated and recycled from the products by an external magnet. Good biocompatibility and biodegradability, as well as basic magnetic characteristics could be denoted for functional organic materials grafted to MNPs [10–12].
Multi-component reactions (MCRs) have proven to be valuable in organic and medicinal chemistry [13–16]. Such protocols can be used for drug design and drug discovery because of their simplicity, efficiency, and high selectivity [17, 18]. MCRs can reduce the number of steps and present advantages, such as low energy consumption and little to no waste production, leading to desired environmentally friendly processes. Synthesis of bioactive and complex molecules should be facile, fast, and efficient with minimal workup in this methodology [17–19]. The synthesis of 4H-pyran derivatives has attracted great interest because many of them have useful biological and pharmacological properties, e.g., as anticoagulant, spasmolytic, anticancer, and antianaphylactic agents [20–22]. Some 2-amino-4H-pyrans can also be used as photoactive materials [23]. Considering the importance of these compounds, many methods have been reported for their synthesis [24–30]. The reported methods for the synthesis of 2-amino-4H-pyran derivatives often involve multistep reactions, expensive catalysts [31], organic solvents, and microwave irradiation [32]. It is, therefore, important to find facile, efficient, and environmentally benign synthetic procedures for the preparation of these compounds. Previously, we reported the three-component reactions (3-CRs) of arylaldehydes, kojic acid, and ethyl cyanoacetate in the presence of a catalytic amount of K2CO3in H2O (5 ml) that led to pyrano[3,2-b]pyranones [33]. In continuation of our research, in this work, we synthesized a magnetic catalyst—pyridine-4-carboxylic acid (PYCA) functionalized Fe3O4 nanoparticles (Fe3O4–PYCA)—and investigated its catalytic application for the three-component synthesis of pyrano[3,2-b]pyranone derivatives from arylaldehydes, kojic acid, and ethyl cyanoacetate under solvent-free conditions (Scheme 1).
Experimental
Chemicals and materials
Melting points were measured on an Electrothermal 9100 apparatus. The X-ray powder diffraction (XRD) of the catalyst was carried out on a Philips PW 1830 X-ray diffractometer with CuKα source (λ = 1.5418 Å) in a range of Bragg’s angle (10–80°) at room temperature. Scanning electron microscope (SEM) analyses were taken using VEGA//TESCAN KYKY-EM 3200 microscope (acceleration voltage 26 kV). Transmission electron microscopy (TEM) experiments were conducted on a Philips EM 208 electron microscope. Thermogravimetric analysis (TGA) was recorded on a Stanton Red craft STA-780 (London, UK). NMR spectra were recorded with a Bruker DRX-400 AVANCE instrument (400.1 MHz for 1H, 100.6 MHz for 13C). The spectra were measured in DMSO-d6 as solvent. IR spectra were recorded on an FT-IR Bruker vector 22 spectrophotometer. Magnetic measurements were performed using vibration sample magnetometer (VSM, MDK, and Model 7400) analysis.
General procedure
Preparation of pyridine-4-carboxylic acid functionalized Fe3O4 nanoparticles (Fe3O4–PYCA)
FeCl3.6H2O (4.865 g, 0.018 mol) and FeCl2·4H2O (1.789 g, 0.0089 mol) were added to 100 ml deionized water and sonicated until the salts dissolved completely. Then, 0.010 mol (0.123 g) of PYCA and NH4OH (10 ml) solution were added to the above mixture until the pH was raised to 11, at which point a black suspension was formed. This suspension was then refluxed at 100 °C for 6 h, with vigorous stirring. Fe3O4–PYCA nanoparticles (about 1.5 g) was separated from the aqueous solution by magnetic decantation, washed with distilled water several times, and then dried in an oven overnight (Scheme 2). Synthesis was done under N2 atmosphere.
General procedure for the synthesis of pyrano[3,2-b]pyranone derivatives under solvent-free conditions
A mixture of aromatic aldehydes (1 mmol), kojic acid (1 mmol, 0.14 g), and ethyl cyanoacetate (1 mmol, 0.11 g), with Fe3O4–PYCA nanoparticles (8 mol%) as a catalyst, was stirred under solvent-free conditions for 45–55 min at 70 °C. After completion of the reaction, which was monitored by TLC, the reaction mixture was cooled to room temperature. Then, the reaction mixture was dissolved in dichloromethane (10 ml), and subsequently, the Fe3O4–PYCA nanoparticle catalyst was separated by an external magnet for 5 min. The solution containing the product was evaporated, the residue solid was recrystallized using hexane/ethyl acetate (9:1), and the product obtained was a white powder. Finally, the isolated catalyst was washed several times with dried CH2Cl2, and dried under vacuum at 60 °C to give the pure Fe3O4–PYCA nanoparticle catalyst.
All of the desired products were characterized by comparison of their physical data with those of known compounds. Selected spectra for two known products are given below:
Ethyl 2-amino-6-(hydroxymethyl)-8-oxo-4-phenyl-4,8-dihydropyrano[3,2-b]pyran-3-carboxylate (Table 2 , entry 1)
White powder; mp = 193–194 °C. IR (KBr): ν max = 3460, 3383 (NH2), 3266 (OH), 1672 (C=O), 1621 (C=C), 1208 (Csp2–O), 1024 (Csp3–O) cm−1.; 1H NMR (400.13 MHz, DMSO-d6): δ = 1.00 (t, 3H, 3 J HH = 7.2 Hz, CH3), 3.93 (q, 2H, 3 J HH = 7.2 Hz, OCH2CH3), 4.20 (2 dd, 2H, 2 J HH = 15.6 Hz, 3 J HH = 5.6 Hz, CH2OH), 4.80 (s, 1H, CH), 5.69 (t, 1H, 3 J HH = 6.0 Hz, OH), 6.32 (s, 1H, CH), 7.21–7.25 (m, 3H, 3 Ar–H), 7.30–7.34 (m, 2H, 2 Ar–H), 7.84 (s, 2H, NH2) ppm; 13C NMR (100.6 MHz, DMSO d6): δ = 14.5 (CH3), 40.3 (CH), 59.4, 59.6 (2 OCH2), 75.5 (Cq), 111.8 (CH), 127.6 (CH), 128.0 (2 CH), 129.0 (2 CH), 136.4, 143.5, 152.2, 160.2, 168.0 (5 Cq), 168.6 (C=O, ester), 170.1 (C=O, pyrone) ppm.
Ethyl 2-amino-4-(4-cyanophenyl)-6-(hydroxymethyl)-8-oxo-4,8-dihydropyrano[3,2-b]pyran-3-carboxylate (Table 2 , entry 4)
White powder; mp = 194–196 °C. IR (KBr): ν max = 3355, 3262 (NH2, OH), 2227 (CN), 1671 (C=O), 1631 (C=C), 1210 (Csp2–O), 1033 (Csp3–O) cm−1; 1H NMR (400.13 MHz, DMSO-d6): δ = 0.97 (t, 3H, 3 J HH = 7.2 Hz, CH3), 3.88–3.96 (m, 2H, OCH2CH3), 4.19 (2 dd, 2H, 2 J HH = 16.0 Hz, 3 J HH = 6.0 Hz, CH2OH), 4.93 (s, 1H, CH), 5.69 (t, 1H, 3 J HH = 6.0 Hz, OH), 6.32 (s, 1H, CH), 7.46 (d, 2H, 3 J HH = 8.4 Hz, 2 Ar–H), 7.80 (d, 2H, 3JHH = 8.4 Hz, 2 Ar–H), 7.93 (s, 2H, NH2) ppm.; 13C NMR (100.6 MHz, DMSO d6): δ = 14.5 (CH3), 40.5 (CH), 59.5, 59.6 (2 OCH2), 74.6 (Cq), 110.5 (CH), 111.1 (CH), 119.9 (CN), 129.3 (2 CH), 133.0 (2 CH), 136.5, 149.0, 150.8, 160.1, 167.8 (5 Cq), 168.7 (C=O, ester), 170.0 (C=O, pyrone) ppm.
Results and discussion
Characterization of the prepared Fe3O4–PYCA nanoparticles
X-ray diffraction (XRD) analysis
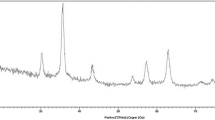
Phase investigation of the supported catalyst was performed by XRD and result presented in Fig. 1. The result shown in Fig. 1 was fitted for six observed peaks with the following Miller indices: (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1), and (4 4 0). The XRD pattern indicates that Fe3O4–PYCA nanoparticles is broadened owing to very small crystallite size, indicating that the PYCA have been successfully supported on Fe3O4 nanoparticle. The average MNPs core diameter was calculated to be about 11 nm from the XRD results by Scherer’s equation, D = k λ/β cosθ, where k is a constant (generally considered as 0.94), λ is the wavelength of Cu Ka (1.54 Å), β is the corrected diffraction line full-width at half-maximum (FWHM), and θ is Bragg’s angle [34, 35].
Fourier transform infrared (FT-IR)
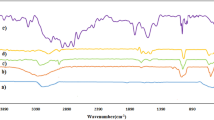
In The FT-IR data of Fe3O4–PYCA nanoparticles, the distinctive absorption bands of 1629, 1130, 2923, and 2853 cm−1 are attributed to the C=O, C–O and C–H stretching of pyridine-4-carboxylate unit. The characteristic absorbing peaks of Fe3O4 appear at 633 and 583 cm−1, which can be ascribed to the vibrations of Fe–O group. Therefore, the data obtained from FT-IR spectroscopy can confirm the existence of the nanomagnetic particle and heterocyclic moiety in the structure of Fe3O4–PYCA nanoparticles (Fig. 2).
Thermogravimetric analysis (TGA)
Thermogravimetric analysis (TGA) and differential thermalanalysis (DTG) were performed at the range of 25–900 °C, to determine the loading of organic groups coated on the surface of the magnetite (Fig. 3). The amount of adsorbed pyridine-4-carboxylic acid calculated by Eq. (1) is 0.4 mmol g−1.
TEM analysis
Morphology of synthesized of Fe3O4–PYCA nanoparticles were investigated by TEM, and are shown in Fig. 4 to be spherical. Average particle size is estimated to be about 13 nm from the TEM micrographs, which is in very good agreement with the crystallite size estimated from XRD at 11.5 nm. This is an indication of the nearly single-crystalline character of Fe3O4–PYCA nanoparticles.
Scanning electron microscope (SEM)
Scanning electron microscopy (SEM) is a primary tool for determining the size distribution, particle shape, surface morphology, and fundamental physical properties. Fig. 5 shows the SEM image of the Fe3O4–PYCA nanoparticle.
Vibrating sample magnetometer (VSM)
The room temperature magnetization curves proved that the Fe3O4–PYCA nanoparticle is super paramagnetic. Saturation magnetization of MNPs was 92.13 emu/g and saturation magnetization of Fe3O4–PYCA nanoparticles was 76.89 emu/g. Compared with the uncoated Fe3O4 nanoparticle, the saturation magnetization of the Fe3O4–PYCA nanoparticle obviously decreased because the diamagnetic contribution of the organic group resulted in a low mass fraction of the Fe3O4 magnetic substance. Even with this reduction in the saturation magnetization, the solid could still be efficiently separated from solution with a permanent magnet (Fig. 6).
Catalytic application of Fe3O4–PYCA nanoparticles
First, to find optimization conditions, the reaction of benzaldehyde, kojic acid, and ethyl cyanoacetate in the presence of the Fe3O4–PYCA nanoparticles as catalyst was selected as model reaction. The reaction was carried out with different amounts of Fe3O4–PYCA nanoparticles as catalyst (4, 8, 12 mol%) in different temperatures (25, 50, 70 °C). The obtained results in Table 1 showed that optimal conditions were 8 mol% of Fe3O4–PYCA nanoparticles at 70 °C (Table 1, entry 8).
Next, various aromatic aldehydes were used in the reactions that led to the corresponding products in high to excellent yields (Table 2). As shown in Table 2, reactions with aromatic aldehydes, including electron-donating or electron-withdrawing substituents, afforded the desired products in high to excellent yields.
The suggested mechanism for the formation of the products, according to the literature survey [33], is shown in Scheme 3.
We also investigated recycling of the Fe3O4–PYCA nanoparticles as catalyst under solvent-free at 70 °C conditions using the model reaction of benzaldehyde, kojic acid, and ethyl cyanoacetate. The recovered catalyst was reused for four runs without any loss of its activities (Fig. 7, 8). However, the XRD spectrum of recycled Fe3O4–PYCA (after recycling four times) was provided and compared with fresh catalyst (Fig. 9). Analysis of these two spectra showed no difference.
The results show that the reactions in the presence of Fe3O4–PYCA nanoparticles were carried out in short times (45–55 min) and with high yield relative to the reported inorganic catalyst (K2CO3) by us [alimi] [33].
Conclusions
We synthesized Fe3O4–PYCA nanoparticles as an organic–inorganic hybrid heterogeneous catalyst and characterized them by FT-IR, XRD, TGA, TEM, SEM, and VSM techniques. Size evaluation via various techniques revealed the size of Fe3O4–PYCA nanoparticles to be around 11–13 nm with nearly single-crystalline character. The most interesting features of the present work include durability as well as efficient catalytic activity for synthesis of pyrano[3,2-b]pyranone derivatives via the reaction of aromatic aldehydes, kojic acid, and ethyl cyanoacetate under solvent-free conditions at 70 °C. The attractive features of this protocol are its simple procedure, inexpensive workup, ease of handling, high yields of products, and use of reusable nanomagnetic catalyst.
References
A. Tiwari, A.K. Mishra, H. Kobayashi, A.P.F. Turner, Intelligent Nanomaterials: Processes, Properties, and Applications (John Wiley & Sons, Inc., New Jersey, 2012)
B. Hu, J. Pan, H.L. Yu, J.W. Liu, J.H. Xu, Process Biochem. 44, 1019–1024 (2009)
K.J. Klabunde, R. Mulukutla, Chemical and Catalytic Aspects of Nanocrystals. Nanoscale Materials in Chemistry (Wiley Interscience, NewYork, 2001)
C.N.R. Rao, A. Müller, K. Anthony, Nanomaterials Chemistry: Recent Developments and New Directions (John Wiley & Sons, Inc., New Jersey, 2007)
A.H. Lu, E.L. Salabas, F. Schuth, Angew. Chem. Int. Ed. 46, 1222–1244 (2007)
C.S. Gill, B.A. Price, C.W. Jones, J. Catal. 251, 145–152 (2007)
A. Taher, J.B. Kim, J.Y. Jung, W.S. Ahn, M.J. Jin, Synlett 15, 2477–2482 (2009)
O.C. Dalaigh, A.S. Corr, Y. Gunko, J.S. Connon, Angew. Chem. Int. Ed. 46, 4329–4332 (2007)
Y. Zhang, Y. Zhao, C. Xia, J. Mol. Catal. A: Chem. 306, 107–112 (2009)
D. Wang, D. Astruc, Chem. Rev. 114, 6949–6985 (2014)
Q.M. Kainz, O. Reiser, Acc. Chem. Res. 47, 667–677 (2014)
R.B. Nasir Baig, M.N. Nadagouda, R.S. Varma, Coord. Chem. Rev. 287, 137–156 (2014)
J. Zhu, H. Bienayme, Multicomponent Reactions (Wiley-VCH, Weinheim, 2005)
A. Dömling, Chem. Rev. 106, 17–89 (2006)
S. Jimenez-Alonso, H. Chavez, A. Estevez-Braan, A. Ravelo, G. Feresin, A. Tapia, Tetrahedron 64, 8938–8942 (2008)
D.F. Tejedor, G. Tellado, Chem. Soc. Rev. 36, 484–491 (2007)
A. Nefzi, J.M. Ostresh, R.A. Houghten, Chem. Rev. 97, 449–472 (1997)
L.A. Thompson, Curr. Opin. Chem. Biol. 4, 324–337 (2000)
A. Dömling, Curr. Opin. Chem. Biol. 6, 306–313 (2002)
L. Bonsignore, G. Loy, D. Secci, A. Calignano, Eur. J. Med. Chem. 28, 517–520 (1993)
F.M. Uckun, C. Mao, A.O. Vassilev, H. Huang, S.T. Jan, Bioorg. Med. Chem. Lett. 10, 541–545 (2000)
R. Gonzalez, N. Martin, C. Seoane, J.L. Marco, A. Albert, F.H. Cano, Tetrahedron Lett. 33, 3809–3812 (1992)
D. Armesto, W.M. Horspool, N. Martin, A. Ramos, C. Seaone, J. Org. Chem. 54, 3069–3072 (1989)
S. Asghari, M. Ahmadipour, Acta. Chem. Slov. 57, 953–956 (2010)
M.B. Miya, Y.J. Prakash Raob, G.L.D. Krupadanam, Heterocycl. Lett. 2, 214–217 (2012)
S. Paul, P. Bhattacharyya, A.R. Das, Tetrahedron Lett. 52, 4636–4641 (2011)
L.M. Wang, N. Jiao, J. Qiu, J.J. Yu, J.Q. Liu, F.L. Guo, Y. Liu, Tetrahedron 66, 339–343 (2010)
H.J. Wang, J. Lu, Z.H. Zhang, Monatsh. Chem. 141, 1107–1112 (2010)
M. Saeedi, M.M. Heravi, Y.S. Beheshtiha, H.A. Oskooie, Tetrahedron 66, 5345–5348 (2010)
U.R. Pratap, D.V. Jawale, P.D. Netankar, R.A. Mane, Tetrahedron Lett. 52, 5817–5819 (2011)
A. Samadi, D. Silva, M. Chioua, L. Infantes, E. Soriano, J. Marco-Contelles, Mol. Divers. 19, 103–122 (2015)
Y. Peng, G. Song, Catal. Commun. 8, 111–114 (2007)
S. Asghari, R. Baharfar, M. Alimi, M. Ahmadipour, M. Mohseni, Monatsh. Chem. 145, 1337–1342 (2014)
T. Wejrzanowski, R. Pielaszek, A. Opalińska, H. Matysiak, W. Lojkowski, K.J. Kurzydlowski, Appl. Surf. Sci. 253, 204–208 (2006)
R. Pielaszek, J. Appl. Crystallogr. 1, 43–50 (2003)
Acknowledgments
This research was supported by the Research Council of the University of Mazandaran in Iran.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Asghari, S., Mohammadnia, M. Synthesis and characterization of pyridine-4-carboxylic acid functionalized Fe3O4 nanoparticles as a magnetic catalyst for synthesis of pyrano[3,2-b]pyranone derivatives under solvent-free conditions. Res Chem Intermed 42, 1899–1911 (2016). https://doi.org/10.1007/s11164-015-2124-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2124-0