Abstract
The quest to develop an environmentally friendly, sustainable protocol for immensely bioactive spirooxindole derivatives has encouraged to explore oxalic acid dihydrate: proline low transition temperature mixture as a new designer solvent for the said synthesis at room temperature. It has been successfully applied for the three component reaction of isatin, malononitrile or ethyl cyanoacetate and 1,3–dicarbonyl compounds for the first time. Moderate to good yield of the products, shorter reaction time, energy efficiency, chromatography-free purification process, recyclability and high atom economy are the captivating feature of this protocol which will find applications in multi-component, diversity-oriented synthesis.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the development of environmentally friendly synthetic routes for drug discovery processes, multi-component reactions (MCRs) have been recognized as a powerful tool during last ten years. These reactions comprises the formation of several bonds in a single operation with remarkable advantages such as operational simplicity, reduction of tedious workup, extraction and purification processes, a high degree of atom economy and a shorter reaction time [1–4]. Furthermore, the quest to develop an ecologically sustainable protocol for immensely bioactive molecules has provoked the scientific community to adopt a "green" chemistry principle. In this esteem, to meet the fundamental scientific challenges of protecting human health and environment, significant attention has been paid to MCRs for their use in eco-compatible reactions promoting a benign solvent rather than a toxic organic solvent.
In recent years, low transition temperature mixtures (LTTMs) or deep eutectic solvents (DESs) have been found to be powerful, greener and cleaner alternatives to conventional molecular solvents. Since inception [5], they have been considered as designer solvents, as their properties are solely dependent on the natures of the hydrogen bond donor and hydrogen bond acceptors. In comparison with ionic liquids, LTTMs can be prepared with 100 % atom economy from easily accessible chemicals. They are found to be non-toxic to the environment, biodegradable and inexpensive. As LTTMs consist mainly of ionic species, they have a strong ability to dissolve CO2 [6]. Recently, they have been successfully applied as extraction media for proteins and potential lubricants [7, 8]. Various organic transformations like acid-catalyzed reactions, base-catalyzed reactions, Suzuki coupling and Heck coupling have been reported using a DES or LTTM as a catalyst [9–13]. Additionally, numerous MCRs have been investigated using a DES or LTTM as a solvent [14, 15]. The use of LTTMs in MCRs has opened a new green avenue for synthetic organic chemistry. For this reason, MCRs in LTTMs are of having outstanding value in organic synthesis and green chemistry.
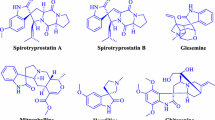
Among N-heterocycle skeletons, the indole moiety, being a “privileged scaffold”, is an important active feature and structural unit of variety of natural products [16]. Furthermore, an enhancement in biological activity was observed in spirooxindole derivatives due to the presence of a sterically constrained spiro structure at the C3 position [17–20]. For example, some cytostatic alkaloids, like spirotryprostatins and pteropodines, are effective inhibitors of microtubule activity and modulators of muscarinic serotonin receptor functions (Fig. 1). Spirooxindole compounds with substituted, fused 4H chromenes at the C3 position have immense importance because of their diuretic, anticoagulant, anticancer and spasmolitic activities [21–23].
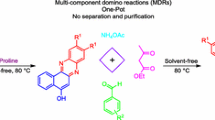
A variety of protocols [24–39] have been reported for the synthesis of spiro [4H-pyran-3,3-oxindole] derivatives. Most of the reported methods are associated with the use of either carcinogenic organic solvents, high temperatures, expensive and non-commercially available catalysts, or are lacking catalyst reusability. Therefore, there is still need for versatile, simple and environmentally friendly sustainable protocols. Considering the above subjects and our continuing efforts to develop new environmentally benign synthetic methodologies [40–44], herein, we explored the role of oxalic acid dihydrate: proline LTTM as a reaction-promoting medium for the first time for the synthesis of diverse spirooxindole derivatives at room temperature (Scheme 1).
Experimental
General
All chemicals and solvents were reagent grade. Analytical thin-layer chromatography (TLC) was performed using percolated silica gel 60-F254 plates. Infrared (IR) spectra on potassium bromide (KBr) disks were recorded on a Shimadzu IR-470 FT-IR spectrophotometer. Routine nuclear magnetic resonance (NMR) spectra were taken in DMSO d6 (dimethyl sulfoxide-d6) using a Bruker 300 and a 400-MHz spectrophotometer with tetramethylsilane (TMS) as an internal standard. Elemental analysis was done using an Euro elemental analyzer.
Typical procedure for preparation of an LTTM
The LTTM was synthesized according to the method reported in the literature [45]. The mixture of oxalic acid dihydrate (12.6 g, 100 mmol) and proline (11.5 g, 100 mmol) at a ratio of 1:1, when heated slowly at 80 °C for 1 h, resulted in the formation of a yellowish viscous liquid (i.e., LTTM) with 100 % atom economy (Scheme 2).
Typical procedure for an LTTM promoted reaction
A mixture of dimedone (0.140 g, 1 mmol), isatin (0.147 g, 1 mmol) and malononitrile (0.066 g, 1 mmol) was stirred in round bottom flask containing 5 mL of oxalic acid dihydrate: proline LTTM at room temperature for an appropriate time. After completion of the reaction, as indicated by TLC, 10 mL of water was added to the reaction mixture and the insoluble crude product was filtered off. The aqueous layer was evaporated under vacuum to recover the LTTM. The crude product was washed with distilled water (5 mL) and recrystallized from ethanol (10 mL).
Recyclability of an oxalic acid dihydrate: proline LTTM
Recyclability ofthe LTTM was studied using the reaction of 1 mmol of isatin, 1 mmol of dimedone, 1 mmol of malononitrile and the oxalic acid dihydrate: proline LTTM under optimized conditions. After completion of the reaction, as indicated by TLC, 10 mL of water was added to the reaction mixture and the crude product was separated by filtration. The LTTM was recovered by evaporating the water at 80 °C under vacuum. The recycled LTTM was reused for the next batch and recycled again. No observable differences were found between the 1H NMR spectra of fresh and recovered LTTM after four successive runs. Fresh LTTM: 1H NMR (300 MHz, DMSO d6): δ H (ppm) 1.84–1.99 (m, 3H), 2.17–2.23 (m, 1H), 3.16–3.24 (m, 2H), 4.11–4.16 (t, 1H), 7.60 (bs, 9H).
Recovered LTTM: 1H NMR (300 MHz, DMSO d6): δ H (ppm) 1.83–1.96 (m, 3H), 2.16–2.21 (m, 1H), 3.16–3.22 (m, 2H), 4.10–4.15 (t, 1H), 7.22 (bs, 9H).
Selected spectral data
2-amino-7,7-dimethyl-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indoline]-3-carbonitrile (4a)
Colorless Powder, M.P. 269–271 °C, IR (KBr) (Vmax/cm−1): 3399, 3322, 3196, 2202, 1706, 1672. 1H NMR (300 MHz, DMSO d6): δH (ppm) 1.01–1.04 (d, 6H, 2CH3), 2.12–2.05 (dd, 2H, CH2), 2.50–2.56 (d, 2H, CH2), 6.78–6.80 (d, 1H, Ar-H), 6.86–6.91 (t, 1H, Ar-H), 6.96–6.98 (t, 1H, Ar-H), 7.11–7.14 (1H, m, Ar-H), 7.17 (s, 2H, NH2), 10.35 (s, 1H, NH). 13C NMR (75 MHz, DMSO d6): d (ppm) 27.52, 28.04, 32.37, 47.32, 50.52, 58.13, 109.71, 111.29, 117.72, 122.14, 123.45, 128.61, 134.87, 142.54, 159.25, 164.58, 178.47, 195.29 Anal. Calcd for C19H17N3O3: C, 68.05; H, 5.11; N, 14.31 %. Found: C, 68.09; H, 5.08; N, 14.28 %.
2-Amino-5-oxo-7,7-dimethyl-spiro[(4H)-5,6,7,8-tetrahydrochromene-4,3′-(3′H)-1′-methyl-indol]-(1′H)-2′-one-3-carbonitrile (4e)
Colorless Powder, M.P. 255–258 °C, IR (KBr) (Vmax/cm−1): 3472, 3315, 2189, 1715, 1654.1H NMR (400 MHz, DMSO d6): δ H (ppm) 0.99 (s, 3H, CH3), 1.05 (s, 3H, CH3), 2.14–2.02 (2H, AB system, 2JHH = 16.4 Hz), 2.43 (dd, 2H, CH2), 3.18 (s, 3H, N-CH3), 6.38 (s, 2H, NH2), 6.81–6.79 (1H, d, Ar), 6.95–6.94 (2H, d, Ar), 7.21–7.17 (1H, m, Ar). 13C NMR (100 MHz, DMSO d6): d (ppm) 26.66, 27.63, 28.41, 32.21, 46.85, 50.56, 108.17, 111.54, 117.20, 122.77, 122.95, 128.75, 133.38, 143.70, 159.17, 164.09, 176.95, 194.95. Anal. Calcd for C20H19N3O3: C, 68.75; H, 5.48; N, 12.03 %. Found: C, 68.69; H, 5.53; N, 12.08 %.
2-Amino-5-oxo-7,7-dimethyl-spiro[(4H)-5,6,7,8-tetrahydrochromene-4,3′-(3′H)-1′-phenyl-indol]-(1′H)-2′-one-3-carbonitrile (4f)
Colorless Powder, M.P. > 300 °C, IR (KBr) (Vmax/cm−1): 3595, 3376, 3294, 2192, 1682, 1602.1H NMR (300 MHz, DMSO d6): δ H (ppm) 1.03 (s, 3H, CH3), 1.05 (s, 3H, CH3), 2.26–2.12 (2H, AB system, 2JHH = 16.2 Hz), 2.62 (s, 2H, CH2), 6.67–6.65 (2H, d, NH2), 7.06–7.01 (2H, t, Ar), 7.22–7.14 (2H, m, Ar), 7.40–7.34 (2H, m, Ar), 7.50–7.45 (1H, m, Ar), 7.63–7.58 (2H, m, Ar). 13C NMR (100 MHz, DMSO d6): d (ppm) 27.53, 28.10, 32.50, 47.14, 50.35, 52.74, 109.04, 111.36, 117.62, 123.52, 123.87, 127.10, 128.56, 128.94, 130.12, 133.72, 135.27, 143.77, 159.32, 164.94, 176.59, 194.64. Anal. Calcd for C25H21N3O3: C, 72.98; H, 5.14; N, 10.21 %. Found: C, 73.03; H, 5.11; N, 10.18 %.
Ethyl-2-amino-5-oxo-spiro[(4H)-5,6,7,8-tetrahydrochromene-4,3′-(3′H)-5′-nitro-indol]-(1′H)-2′-one-3-carboxylate (4j)
Colorless powder, M.P. 277–279 °C, IR (KBr) (Vmax/cm−1): 3290, 3187, 2959, 1732, 1686, 1644. 1H NMR (300 MHz, DMSO d6): δ H (ppm) 0.845–0.798 (t, 3H, CH3), 0.97 (s, 3H, CH3), 1.01 (s, 3H, CH3), 2.11 (s, 2H, CH2), 2.65–2.50 (2H, AB system, 2JHH = 17.4 Hz), 3.77–3.72 (q, 2H, CH2), 6.885–6.91 (d, 2H, NH2), 7.75 (1H, s, Ar), 8.10–7.98 (m, 3H, Ar), 13C NMR (100 MHz, DMSO d6): d (ppm) 26.66, 27.63, 28.41, 32.21, 46.85, 50.56, 108.17, 111.54, 117.20, 122.77, 122.95, 128.75, 133.38, 143.70, 159.17, 164.09, 176.95, 194.95. Anal. Calcd for C21H21N3O7: C, 59.01 %; H, 4.95 %; N, 9.83 %. Found: C, 58.95 %; H, 4.93 %,; N, 9.88 %.
7-Amino-2,2′,4-trioxo-1,1′,2,2′,3,4-hexahydrospiro[indole-3′,5-pyrano[2,3-d] pyrimidine]-6-carbonitrile (4k)
Colorless powder, M.P. 274–276 °C, IR (KBr) (Vmax/cm−1): 3306, 3145, 2204, 1714, 1393.1H NMR (300 MHz, DMSO d6):δ H (ppm) 6.80–6.78 (1H, d, Ar), 6.94–6.91 (1H, dd, Ar), 7.18–7.11 (1H, m, Ar), 7.32 (s, NH2). 10.44 (s, NH), 11.08 (s, NH), 13C NMR (75 MHz, DMSO d6): δ c (ppm) 47.15, 58.49, 87.31, 109.73, 117.29, 122.22, 124.17, 128.86, 133.95, 142.61, 149.66, 153.79, 158.72, 161.83, 178.05. Anal. Calcd for C15H9N5O4: C, 55.73 %; H, 2.81 %; N, 21.66 %. Found: C, 55.69 %; H, 2.85 %; N, 21.60 %.
7-Amino-2,2′,4-trioxo-1,1′,2,2′,3,4-hexahydrospiro[5′nitroindole-3′,5-pyrano[2,3-d]pyrimidine]-6-carbonitrile (4n)
Colorless powder, M.P. > 300 °C, IR (KBr) (Vmax/cm−1): 3444, 3299, 3182, 2200, 1750, 1698 1H NMR (300 MHz, DMSO d6): δH (ppm) 7.00–7.02 (1H, d, Ar), 7.55 (2H, s, NH2), 8.14–8.18 (1H, dd, Ar), 8.24–8.25 (1H, d, Ar), 11.18 (1H, s, NH), 11.22 (1H, s, NH), 12.40 (1H, s, NH), 13C NMR (75 MHz, DMSO d6): δc (ppm) 56.70, 86.28, 109.88, 117.19, 120.45, 126.35, 135.09, 143.05, 149.10, 149.73, 154.36, 159.12, 162.11, 178.83. Anal. Calcd for C15H8N6O6: C, 48.92 %; H, 2.19 %; N, 22.82 %. Found: C, 48.85 %; H, 2.24 %; N, 22.80 %.
7-Amino-2,2′,4-trioxo-1,1′,2,2′,3,4-hexahydrospiro[5′iodoindole-3′,5-pyrano[2,3-d] pyrimidine]-6-carbonitrile (4o)
Reddish powder, M.P. 276–278 °C (dec.), IR (KBr) (Vmax/cm−1): 3291, 3146, 2198, 1689, 1643 1H NMR (300 MHz, DMSO d6): δH (ppm) 6.62–6.65 (1H, d, Ar), 7.46–7.51 (2H, m, Ar), 7.55 (2H, s, NH2), 10.62 (1H, s, NH), 11.17 (1H, s, NH), 12.33 (1H, s, NH),. 13C NMR (75 MHz, DMSO d6): δc (ppm) 47.09, 57.47, 85.22, 86.69, 112.29, 117.34, 132.60, 136.55, 137.48, 142.30, 149.70, 154.01, 158.84, 161.99, 177.72. Anal. Calcd for C15H8IN5O4: C, 40.11 %; H, 1.80 %; N, 15.59 %. Found: C, 40.05 %; H, 1.78 %; N, 15.66 %.
7′-Amino-1′,3′-dimethyl-2,2′,4′-trioxo-1,1′,2,2′,3′,4′-hexahydrospiro[5′bromoindole-3,5′-pyrano[2,3-d]pyrimidine]-6′-carbonitrile (4r)
Colorless powder, M.P. > 300 °C, IR (KBr) (Vmax/cm−1): 3375, 3305, 3202, 2197, 1683, 1653. 1H NMR (300 MHz, DMSO d6): δ H (ppm) 3.05 (3H, s, N-CH3), 3.38 (3H, s, N-CH3), 6.79–6.77 (1H, d, Ar), 7.39–7.36 (2H, d, J = 10.8 Hz), 7.61–7.52 (2H, bs, NH2), 10.61 (s, NH). 13C NMR (75 MHz, DMSO d6): δ c (ppm) 28.10, 29.80, 48.08, 57.67, 87.04, 117.63, 113.94, 117.09, 127.19, 131.64, 136.54, 141.98, 150.14, 152.72, 158.65, 159.98, 177.63. Anal. Calcd for C17H12N5O4: C, 47.46 %; H, 2.81 %; N, 16.28 %. Found: C, 47.50 %; H, 2.78 %; N, 16.22 %.
7′-Amino-1′,3′-dimethyl-2,2′,4′-trioxo-1,1′,2,2′,3′,4′-hexahydrospiro[5′iodoindole-3,5′-pyrano[2,3-d]pyrimidine]-6′-carbonitrile (4s)
Colorless powder, M.P. = 265–267 °C (dec.), IR (KBr) (Vmax/cm−1): 3369, 3301, 2196, 1705, 1679 1H NMR (300 MHz, DMSO d6): δ H (ppm) 3.37 (6H, s, 2 N-CH3), 6.64–6.67 (1H, d, Ar), 7.49–7.51 (2H, d, Ar), 7.61 (2H, s, NH2), 10.64 (1H, s, NH). 13C NMR (75 MHz, DMSO d6): δ c (ppm) 28.13, 29.81, 47.83, 57.43, 85.22, 86.94, 112.34, 117.20, 132.54, 136.70, 137.53, 142.28, 150.16, 152.72, 158.65, 160.02, 177.69. Anal. Calcd for C17H12IN5O4: C, 42.79 %; H, 2.53 %; N, 14.68 %. Found: C, 42.84 %; H, 2.50 %; N, 14.71 %.
7′-Amino-5-chloro-2,4′-dioxo-2′-thioxo-1′,2′,3′,4′-tetrahydrospiro[indoline-3,5′-pyrano [2,3-d] pyrimidine]-6′-carbonitrile (4u)
Colorless powder, M.P. 224–226 °C, IR (KBr) (Vmax/cm−1): 3359, 3282, 3148, 2197, 1686, 1575. 1H NMR (300 MHz, DMSO d6): δ H (ppm) 6.83–6.80 (1H, d, Ar), 7.21–7.24 (1H, d, Ar), 7.40 (1H, s, Ar), 7.53 (2H, s, NH2), 10.71 (1H, s, NH), 12.55 (1H, s, NH). 13C NMR (75 MHz, DMSO d6): δ c (ppm) 57.32, 91.48, 111.13, 117.14, 124.85, 126.37, 128.93, 135.47, 141.53, 153.46, 158.69, 159.68, 174.47, 177.51. Anal. Calcd for C15H11N5O3SCl: C, 48.20 %; H, 2.16 %; N, 18.74 %; S, 8.58 %. Found: C, 48.25 %; H, 2.18 %; N, 18.70 %; S, 8.55 %.
7′-Amino-1-methyl-2,4′-dioxo-2′-thioxo-1′,2′,3′,4′-tetrahydrospiro[indoline-3,5′-pyrano[2,3-d]pyrimidine]-6′-carbonitrile (4x)
Colorless powder, M.P. > 300 °C, IR (KBr) (Vmax/cm−1): 3344, 3175, 2848, 2206, 1682, 1647, 1593. 1H NMR (300 MHz, DMSO d6): δ H (ppm) 3.14 (3H, s, N-CH3), 6.98–7.03 (2H, t, Ar), 7.24–7.28 (2H, t, Ar J = 10.8 Hz), 7.315 (1H, s, NH), 7.46 (2H, s, NH2), 12.50 (1H, s, NH). 13C NMR (75 MHz, DMSO d6): δ c (ppm) 26.94, 56.65, 91.96, 108.55, 110.96, 123.11, 125.95, 129.31, 132.83, 138.16, 144.02, 158.70, 160.01, 161.27, 174.41, 176.16. Anal. Calcd for C16H11N5O3S: C, 54.38 %; H, 3.14 %; N, 19.82 %; S, 9.07 %. Found: C, 54.32 %; H, 3.11 %; N, 19.87 %; S, 9.11 %.
7′-Amino-5-iodo-2,4′-dioxo-2′-thioxo-1′,2′,3′,4′-tetrahydrospiro[indoline-3,5′-pyrano[2,3-d] pyrimidine]-6′-carbonitrile (4y)
Colorless powder, M.P. = 250–253 °C (dec.), IR (KBr) (Vmax/cm−1): 3275, 3156, 2194, 1681, 1615, 1570. 1H NMR (300 MHz, DMSO d6): δ H (ppm) 6.63–6.66 (1H, d, Ar), 7.49–7.52 (3H, m, 2Ar and 1NH), 7.63 (2H, s, NH2), 10.67 (1H, s, NH), 12.54 (1H, s, NH). 13C NMR (75 MHz, DMSO d6): δ c (ppm) 46.95, 57.23, 85.33, 112.31, 132.89, 135.96, 137.65, 142.33, 153.37, 158.69, 159.71, 174.32, 177.27. Anal. Calcd for C15H8IN5O3S: C, 38.73 %; H, 1.73 %; N, 15.05 %; S, 6.89 %. Found: C, 38.78 %; H, 1.69 %; N, 15.01 %; S, 6.95 %.
Result and discussion
In view of the important applications of bioactive spirooxindole derivatives, our current focus has been targeted on the facile synthesis of these moieties at room temperature. As a part of the endeavor, the reaction of isatin (1 mmol), malononitrile (1 mmol) and dimedone (1 mmol) was selected as a template reaction. The model reaction was carried out in different DESs or LTTMs at room temperature (Table 1, entries 1–8). In the absence of solvent, the reaction afforded traces of the product (Table 1, entry 1). A comparison of efficiency of the LTTM revealed that oxalic acid dihydrate: proline LTTM (Table 1, entry 6) as a superior solvent.
With these interesting results in our hand, we then explored the scope of the present method with various isatins, malononitrile or ethyl cyanoacetate, and various 1,3-dicarbonyl compounds; results are depicted in Table 2. As shown in Table 2, it was observed that this protocol works well with a wide array of substrates. A series of 5-substituted and N-substituted isatins and various dicarbonyl compounds were used in the reaction. Although the reaction of isatin and dimedone with malononitrile or ethyl cyanoacetate proceeded smoothly, the reaction time with ethyl cyanoacetate was longer than that with malononitrile (Table 2, entry 7–10), which is possibly due to lower reactivity of ethyl cyanoacetate.
The selectivity of an LTTM is due to the hydrogen bonding nature of the LTTM enhancing the electrophilicity of carbonyl groups which substantially promotes the reaction. Though the role of an LTTM is yet to be confirmed, we explore the probable mechanism of LTTM promoted one-pot synthesis of spirooxindole involving initial formation of a Knoevenagel adduct with subsequent Michael addition and cyclization via hydrogen bonding, as depicted in Scheme 3.
Next, recyclability is an essential aspect in view of green chemistry. The possibility of recyclability of LTTM was examined by using the reaction of isatin, malononitrile and dimedone as model substrates under optimized conditions. After completion of the reaction, water (10 mL) was added to the reaction mixture and crude product was separated by filtration. Finally, the LTTM was recovered by evaporating the water at 80 °C under vacuum and was reused for the next batch and recycled again. It was found that the LTTM could be used for four successive runs without a noticeable decrease in its activity (Table 3, Fig. 2). In addition, a group-assistant-purification (GAP) chemistry process [46] was used for purification of crude product. All the products were obtained as pure by just washing the solid crude products with ethanol, avoiding traditional chromatography and recrystallization purification processes. The products were characterized by spectroscopic analysis (1HNMR, 13CNMR, CHNS) and confirmed by comparison of the spectroscopic data and melting points with authentic literature data. Moreover, the sustainability and greenness of the protocol was proven by its atom economy. Higher atom economy values were found to support the greenness of the methodology (Fig. 3).
The proficiency of this protocol has been compared with some of the previously reported methods and results are compiled in Table 4. It is clear from Table 4 that oxalic acid dihydrate: proline is an environmentally benign and efficient reaction promoting medium for the synthesis of a series of spirooxindole derivatives.
Conclusion
An efficient and simple method for the synthesis of spirooxindole derivatives was developed via one-pot, three component reaction of isatin, malononitrile or ethyl cyanoacetate and 1,3-dicarbonyl compounds. Most of the reported methods involve an expensive catalyst which requires tedious preparation, involve harmful organic solvents and lack recyclability. The present method offers several advantages, such as simple LTTM preparation with 100 % atom efficiency, good product yield, shorter reaction times, LTTM recyclability enabling avoidance of hazardous organic solvents, and a GAP chemistry procedure. Owing to the advantages of this protocol over other methods, we expect that it will find extensive application in combinatorial chemistry as well as multi-component green synthesis as it involves green, three component, LTTM prompted reaction that afford different types of spirooxindole fused heterocycles.
References
J. Zhu, H. Bienayme, Multi-component Reactions (Wiley-VCH, Weinheim Germany, 2005)
R.A. Sheldon, Pure Appl. Chem. 72, 1233–1246 (2000)
B.M. Trost, Science 254, 1471–1477 (1991)
M.S. Singh, S. Chowdhury, RSC Adv. 2, 4547–4592 (2012)
A.P. Abbott, G. Capper, D.L. Davies, R.K. Rasheed, V. Tambyrajah, Chem. Commun. 70–71 (2003)
X. Li, M. Hou, B. Han, X. Wang, L. Zou, J. Chem. Eng. Data 53, 548–550 (2008)
Q. Zeng, Y. Wang, Y. Huang, X. Ding, J. Chen, K. Xu, Analyst 139, 2565 (2014)
A.P. Abbott, E.I. Ahmed, R.C. Harris, K.S. Ryder, Green Chem. 16, 4156 (2014)
N. Azizi, S. Dezfooli, M.M. Hashemi, J. Mol. Liq. 194, 62–67 (2014)
D.R. Chandam, A.G. Mulik, P.P. Patil, S.D. Jagdale, D.R. Patil, S.A. Sankpal, M.B. Deshmukh, J. Mol. Liq. 207, 14–20 (2015)
S.B. Phadtare, G.S. Shankarling, Green Chem. 12, 458–462 (2010)
G. Imperato, S. Hoger, D. Lenoir, B. Konig, Green Chem. 8, 1051–1055 (2006)
F. Ilgen, B. Konig, Green Chem. 11, 848–854 (2009)
S. Gore, S. Baskaran, B. Konig, Green Chem. 13, 1009–1013 (2011)
Z.-H. Zhang, X.-N. Zhang, L.-P. Mo, Y.-X. Li, F.P. Ma, Green Chem. 14, 1502–1506 (2012)
W.J. Houlihan, W.A. Remers, R.K. Brown, Indoles, Part I (Wiley, New York, 1992)
T.H. Kang, K. Matsumoto, Y. Murakami, H. Takayama, M. Kitajima, N. Aimi, H. Watanabe, Eur. J. Pharmacol. 444, 39–45 (2002)
J. Ma, S.M. Hecht, Chem. Commun. 1190–1191 (2004)
M.M. Khafagy, A.H. El-Wahas, F.A. Eid, A.M. El, Farmaco 57, 715–722 (2002)
J. Skommer, D. Wlodkowic, M.M. Matto, P. Eray, J. Leuk. Res. 30, 322–331 (2006)
W.P. Smith, L.S. Sollis, D.P. Howes, C.P. Cherry, D.I. Starkey, N.K. Cobley, J. Med. Chem. 41, 787–797 (1998)
L. Bonsignore, G. Loy, D. Secci, A. Calignano, Eur. J. Med. Chem. 28, 517 (1993)
L. Andreani, E. Lapi, Bol. Chim. Farm. 99, 583 (1960)
M. Seyed, T. Mahmood, F. Maryam, C. R. Chimie 17, 1160–1164 (2014)
D.S. Raghuvanshi, K.N. Singh, J. Heterocycl. Chem. 47, 1323 (2010)
B. Karmakar, A. Nayak, J. Banerji, Tetrahedron Lett. 53, 5004–5007 (2012)
S.P. Satasia, P.N. Kalaria, J.R. Avalani, D.K. Raval, Tetrahedron 70, 5763–5767 (2014)
Y. Li, H. Chen, C. Shi, D. Shi, S. Ji, J. Comb. Chem. 12, 231 (2010)
K. Niknam, P. Abolpour, Monatsh. Chem. 4, 683–690 (2015)
G.S. Hari, Y.R. Lee, Synthesis 3, 453 (2010)
S.J. Chai, Y.F. Lai, J.C. Zu, H. Zheng, Q. Zhu, P.F. Zhang, Adv. Synth. Catal. 353, 371 (2011)
R.M. Kurosh, Y.M. Leila, Tetrahedron 67, 5693 (2011)
M. Dabiri, M. Bahramnejad, M. Baghbanzadeh, Tetrahedron 65, 9443–9447 (2009)
S. Gao, C.H. Tsai, C. Tseng, C. Yao, Tetrahedron 64, 9143 (2008)
H.M. Meshram, D.A. Kumar, B.R.V. Prasad, P.V. Goud, Helv. Chim. Acta 93, 648 (2010)
S.L. Zhu, S.J. Ji, Y. Zhang, Tetrahedron 63, 9365 (2007)
G. Shanthi, G. Subbulakshmi, P.T. Perumal, Tetrahedron 63, 2057 (2007)
L. Jalili-Baleh, M. Narges, K. Mehdi, L. Mamani, A. Foroumadi, A. Shafiee, Helv. Chim. Acta 96, 1601 (2013)
Y.L. Gu, Green Chem. 14, 2091–2128 (2012)
D.R. Chandam, A.G. Mulik, P.P. Patil, S.D. Jagdale, D.R. Patil, M.B. Deshmukh, Res. Chem. Intermed. 41, 761–771 (2015)
A.G. Mulik, D.R. Chandam, P.P. Patil, S.D. Jagdale, D.R. Patil, M.B. Deshmukh, J. Mol. Liq. 179, 104–109 (2013)
D.R. Patil, D.R. Chandam, A.G. Mulik, P.P. Patil, S.D. Jagdale, M.B. Deshmukh, Res. Chem. Intermed (2014). doi:10.1007/s11164-014-1782-7
D.R. Patil, D.R. Chandam, A.G. Mulik, P.P. Patil, S.D. Jagdale, R. Kant, V. Gupta, M.B. Deshmukh, Catal. Lett. 144, 949–958 (2014)
A.A. Patravale, A.H. Gore, D.R. Patil, G.B. Kolekar, M.B. Deshmukh, P.V. Anbhule, Ind. Eng. Chem. Res. 53, 16568–16578 (2014)
M. Francisco, M.C. Kroon, Angew. Chem. Int. Ed. 52, 3074–3085 (2013)
P. Kaur, S. Pindi, W. Wever, T. Rajale, G. Li, Chem. Commun. 46, 4330–4332 (2010)
Acknowledgments
We are thankful to Department of Chemistry, Shivaji University, Kolhapur for providing spectral analysis. We are grateful to the UGC New Delhi [F.No.41-211/2012(SR)] for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chandam, D.R., Mulik, A.G., Patil, D.R. et al. Oxalic acid dihydrate: proline as a new recyclable designer solvent: a sustainable, green avenue for the synthesis of spirooxindole. Res Chem Intermed 42, 1411–1423 (2016). https://doi.org/10.1007/s11164-015-2093-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2093-3