Abstract
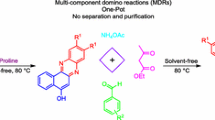
A green and eco-friendly synthesis of novel 2-(1H-indol-3-yl)-3-phenyl-2,3-dihydroquinazolin-4(1H)-one derivatives have been developed using L-proline as a simple catalyst. Two efficient methods have been described for the synthesis of dihydroquinazolin-4(1H)-one derivatives. In the first method, 1H-1,3-benzoxazine-2,4-dione reacted with substituted anilines and 1H-indole-3-carbaldehydes in water in the presence of L-proline as a catalyst, whereas in the second method, a similar reaction using 1H-indol-3-ylmethanol instead of indole-3-carbaldehyde was catalyzed by L-proline/K2CO3 at room temperature for 30 min to afford the same products. These two protocols involve eco-friendly and inexpensive catalysts and produce the target products in excellent yields with a sufficient purity, so that there was no need of column chromatography for their isolation or purification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Green chemistry concerns environmentally friendly construction of novel products and chemical processes [1] while minimizing the usage and generation of hazardous chemicals. Organic solvents are the key factor in environmental pollution, and many of them are carcinogenic and toxic, which makes reactions not requiring the use of organic solvents desirable. Water is an alternative solvent for many chemical reactions; it possesses many valuable properties such as ready availability, chemical stability, nontoxicity, recyclability, and easy handling. Moreover, in many reactions, water has been shown to significantly enhance the reaction rate in comparison to organic solvents due to hydrogen bonding, hydrophobic effect, high heat capacity, and high cohesive energy density [2].
Quinazolin-4-ones and their derivatives are core structural subunits that exist in a number of bioactive natural products [3]. Quinazolin-4-one derivatives exhibit a broad spectrum of biological and pharmaceutical activities, including antihypertensive [4], antidiabetic [5], anti-inflammatory [6], antibacterial [7], anticonvulsant, antitumor [8], central nervous system (CNS), depressant, and diuretic [9] activities. Quinazolinone moiety is a building block for approximately 150 naturally occurring alkaloids [10] such as glycosminine, luotonin, and deoxyvasicinone and drugs like methaqualone and piriqualone.
The construction of complex molecules through multicomponent reactions (MCRs) constitutes a very attractive strategy in organic synthesis [11]. In a multi-component reaction [12], three or more reactants are involved in a cascade of bond-forming individual steps to provide a complex molecule without isolation of intermediates or modification of the reaction conditions [13]. Attractive features of MCRs are simplicity of operation, reduction of isolation and purification steps, and minimization of costs, time, energy, solvents, and waste production [14]. Moreover, by employing an array of diverse reagents, molecular diversity is efficiently generated. In fact, MCRs have been extensively employed in combinatorial and diversity-oriented synthesis [15].
RESULTS AND DISCUSSION
Isatoic anhydride, 1H-indole-3-carbaldehyde, and aniline were chosen as model substrates to optimize the reaction conditions including the solvent and catalyst. As illustrated in Scheme 1, one pot three-component reaction of equimolar amounts of isatoic anhydride (1), aniline (2a), and 1H-indole-3-carbaldehyde (4a) (1 mmol each) was carried out by stirring the reactants in water as a green solvent in the presence of L-proline at room temperature for 10 min. The product, previously unknown 2-(1H-indol-3-yl)-3-phenyl-2,3-dihydroquinazolin-4(1H)-one (5a) was isolated in excellent yield after simple workup. The structure of 5a was confirmed by IR, NMR, and mass spectra.
The reaction of 1, 2a, and 4a was then optimized by performing a series of experiments using different solvents at different temperatures (Table 1). However, the one-pot reaction in water at room temperature for 10 min (entry no. 1) gave reasonably high yield (93%) of 5a compared to other solvents such as ethanol, ethylene glycol, PEG-600, glycerol, acetic acid, DMF, DMSO, or PPA. The reaction conditions were further optimized to find the best catalyst. For this purpose, various Lewis acid catalysts like L-proline, InCl3, SnCl2, ZnCl2, and FeCl3 and solvents such as ethanol, acetic acid, and aqueous HCl were tried. Nevertheless, the reaction in water in the presence of L-proline at room temperature for 10 min provided the best yield of 5a (Table 2, entry no. 1).
The synthesis of 5 was also achieved through a stepwise method (Scheme 2). Initially, isatoic anhydride (1) was reacted with aniline (2) in presence of L-proline in water for 5 min to yield intermediate 3, and the latter reacted with 1H-indole-3-carbaldehyde (4a) in the presence of L-proline in water for 5–10 min to afford target compound 5a. To better understand the scope and generality of this simple procedure, the reaction of 1 with various substituted anilines 2a–2g containing electron-donating or electron-withdrawing groups and 1H-indole-3-carbaldehydes 4a and 4b was performed in one-pot under the optimized conditions to form compounds 5a–5n, and the results are summarized in Table 3.
In view of these results, we proposed the reaction mechanism shown in Scheme 3. The reaction starts with the condensation of 1 and aniline 2 in the presence of L-proline, followed by decarboxylation, to yield the corresponding 2-amino-N-phenylbenzamide (3). The condensation between the amino group of 3 and aldehyde group of 4 gives the corresponding Schiff base intermediate which undergoes nucleophilic cyclization to form final product 5.
Compound 5a could also be prepared via an alternative method using (1H-indol-3-yl)methanol (6a) instead of aldehyde 4a (Scheme 4). The condensation of 1, 2a, and 4a in the presence of L-proline/K2CO3 in water at room temperature for 30 min afforded 91% of 5a with a good purity. Likewise, other compounds 5 were synthesized in this way with high yields (Table 3). A plausible mechanism of this reaction is shown in Scheme 5.
EXPERIMENTAL
The melting points were determined in open capillary tubes using a sulfuric acid bath and are uncorrected. TLC was run on silica gel G, and visualization was done using iodine vapor or UV light. The IR spectra were recorded in KBr pellets on a Perkin Elmer 1000 spectrometer. The 1H NMR spectra were recorded in DMSO-d6 on a Varian 400 MHz spectrometer using TMS as internal standard. The mass spectra were recorded on an Agilent 1200 Series LC/MS instrument.
3-Aryl-2-(1H-indol-3-yl)-2,3-dihydroquinazolin-4(1H)-ones 5a–5n (general procedures). a. One-pot synthesis. A mixture of isatoic anhydride (1, 10 mmol), substituted aniline 2a–2g (10 mmol), 1H-indole-3-carbaldehyde 4a or 4b (10 mmol), L-proline (0.1 mmol), and water (20 mL) was stirred at room temperature for 15 min. The off-white solid separated from the mixture was collected by filtration, washed with hexane (10 mL), dried, and recrystallized from ethanol.
b. Stepwise synthesis. A mixture of 1 (10 mmol), 2 (10 mmol), L-proline (0.1 mmol), and water (20 mL) was stirred at room temperature for 5 min. The off-white solid separated from the mixture was collected by filtration, washed with hexane (10 mL), dried, and recrystallized from ethanol to obtain pure 2-amino-N-arylbenzamide 3. A mixture of 3 (10 mmol), 4 (10 mmol), L-proline (0.1 mmol), and water (20 mL) was stirred at room temperature for 5–10 min. The off-white solid separated from the mixture was collected by filtration, washed with hexane (10 mL), dried, and recrystallized from ethanol.
c. One-pot synthesis using alcohol 6 instead of aldehyde 4. A mixture of 1 (10 mmol), 2 (10 mmol), 6 (10 mmol), L-proline (0.1 mmol), K2CO3 (0.15 mmol), and water (20 mL) was stirred at room temperature for 30 min. The off-white solid separated from the mixture was collected by filtration, washed with water (5 mL) and hexane (10 mL), dried, and recrystallized from ethanol.
2-(1H-Indol-3-yl)-3-phenyl-2,3-dihydroquinazolin-4(1H)-one (5a). Yield 93%, white solid, mp 221–223°C. IR spectrum, ν, cm–1: 3351 br (N–H), 1657 s (C=O). 1H NMR spectrum, δ, ppm: 5.90 s (1H, CH), 7.26–8.59 m (14H, Harom), 9.79 s (1H, NH, D2O exchangeable). 13C NMR spectrum, δC, ppm: 110.00, 111.60, 113.55, 114.13, 114.21, 114.33, 117.79, 118.11, 122.55, 122.63, 124.78, 124.88, 127.55, 129.65, 131.17, 131.21, 138.39, 138.55, 164.92. Mass spectrum: m/z 340.15 [M + 1]+. Calculated for C22H17N3O: M 339.14.
3-(2-Chlorophenyl)-2-(1H-indol-3-yl)-2,3-dihydroquinazolin-4(1H)-one (5b). Yield 90%, white solid, mp 218–220°C. IR spectrum, ν, cm–1: 3420 br (N–H), 1660 s (C=O). 1H NMR spectrum, δ, ppm: 5.98 s (1H, CH), 7.31–8.67 m (13H, Harom), 10.20 s (1H, NH, D2O exchangeable). 13C NMR spectrum, δC, ppm: 110.15, 111.74, 113.39, 114.20, 114.64, 114.90, 117.35, 118.24, 122.40, 122.78, 124.36, 124.67, 127.13, 129.47, 131.34, 131.59, 138.50, 138.81, 164.71. Mass spectrum: m/z 374.23 [M + 1]+. Calculated for C22H16ClN3O: M 373.10.
3-(3-Chlorophenyl)-2-(1H-indol-3-yl)-2,3-dihydroquinazolin-4(1H)-one (5c). Yield 90%, white solid, mp 216–218°C. IR spectrum, ν, cm–1: 3411 br (N–H), 1624 s (C=O). 1H NMR spectrum, δ, ppm: 5.71 s (1H, CH), 7.03–8.55 m (13H, Harom), 10.01 s (1H, NH, D2O exchangeable). 13C NMR spectrum, δC, ppm: 101.28, 103.57, 105.08, 106.31, 108.01, 110.37, 112.17, 113.82, 113.93, 114.22, 116.40, 119.36, 120.58, 124.15, 125.17, 127.66, 129.39, 160.57. Mass spectrum: m/z 374.48 [M + 1]+. Calculated for C22H16ClN3O: M 373.10.
3-(4-Chlorophenyl)-2-(1H-indol-3-yl)-2,3-dihydroquinazolin-4(1H)-one (5d). Yield 89%, white solid, mp 220–222°C. IR spectrum, ν, cm–1: 3428 br (NH), 1639 s (C=O). 1H NMR spectrum, δ, ppm: 5.25 s (1H, CH), 7.13–8.40 m (13H, Harom), 9.69 s (1H, NH, D2O exchangeable). 13C NMR spectrum, δC, ppm: 101.10, 103.28, 105.00, 106.27, 108.13, 110.45, 112.05, 113.77, 113.80, 114.25, 116.39, 119.19, 120.67, 124.03, 125.44, 127.57, 129.20, 160.46. Mass spectrum: m/z 374.37 [M + 1]+. Calculated for C22H16ClN3O: M 373.10.
2-(1H-Indol-3-yl)-3-(2-nitrophenyl)-2,3-dihydroquinazolin-4(1H)-one (5e). Yield: 84%, white solid, mp 212–214°C. IR spectrum, ν, cm–1: 3480 br (N–H), 1652 s (C=O). 1H NMR spectrum, δ, ppm: 5.42 s (1H, CH), 7.29–8.68 m (13H, Harom), 9.51 s (1H, NH, D2O exchangeable). 13C NMR spectrum, δC, ppm: 101.38, 103.42, 105.14, 106.56, 108.71, 110.39, 112.62, 113.52, 113.79, 114.25, 116.51, 119.30, 120.58, 124.14, 125.36, 127.71, 129.12, 160.51. Mass spectrum: m/z 385.13 [M + 1]+. Calculated for C22H16N4O3: M 384.12.
2-(1H-Indol-3-yl)-3-(3-nitrophenyl)-2,3-dihydroquinazolin-4(1H)-one (5f). Yield 85%, white solid, mp 215–217°C. IR spectrum, ν, cm–1: 3428 br (N–H), 1637 s (C=O). 1H NMR spectrum, δ, ppm: 5.17 s (1H, CH), 7.05–8.18 m (13H, Harom), 9.85 s (1H, NH, D2O exchangeable). 13C NMR spectrum, δC, ppm: 101.27, 103.33, 105.04, 106.47, 108.31, 110.22, 112.54, 113.31, 113.80, 114.19, 116.50, 119.17, 120.43, 124.27, 125.19, 127.49, 129.37, 160.28. Mass spectrum: m/z 385.26 [M + 1]+. Calculated for C22H16N4O3: M 384.12.
2-(1H-Indol-3-yl)-3-(4-methoxyphenyl)-2,3-dihydroquinazolin-4(1H)-one (5g). Yield 92%, white solid, mp 224–226°C. IR spectrum, ν, cm–1: 3410 br (N–H), 1667 s (C=O). 1H NMR spectrum, δ, ppm: 3.89 s (3H, OCH3), 5.26 s (1H, CH), 7.25–8.72 m (13H, Harom), 9.68 s (1H, NH, D2O exchangeable). 13C NMR spectrum, δC, ppm: 101.38, 103.45, 105.38, 106.67, 108.44, 110.35, 112.29, 113.57, 113.72, 114.25, 116.37, 119.40, 120.59, 124.88, 125.35, 127.12, 129.46, 160.49. Mass spectrum: m/z 370.13 [M + 1]+. Calculated for C23H19N3O2: M 369.15.
2-(5-Chloro-1H-indol-3-yl)-3-phenyl-2,3-dihydroquinazolin-4(1H)-one (5h). Yield 90%, white solid, mp 217–219°C. IR spectrum, ν, cm–1: 3461 br (N–H), 1649 s (C=O). 1H NMR spectrum, δ, ppm: 5.68 s (1H, CH), 7.35–8.81 m (13H, Harom), 9.90 s (1H, NH, D2O exchangeable). 13C NMR spectrum, δC, ppm: 101.31, 103.49, 105.63, 106.09, 108.15, 110.44, 112.37, 113.27, 113.84, 114.51, 116.14, 119.50, 120.46, 124.80, 125.77, 127.63, 129.09, 160.59. Mass spectrum: m/z 374.34 [M + 1]+. Calculated for C22H16ClN3O: M 373.10.
2-(5-Chloro-1H-indol-3-yl)-3-(2-chlorophenyl)-2,3-dihydroquinazolin-4(1H)-one (5i). Yield 88%, white solid, mp 213–215°C. IR spectrum, ν, cm–1: 3490 br (N–H), 1677 s (C=O). 1H NMR spectrum, δ, ppm: 5.75 s (1H, CH), 7.43–8.96 m (12H, Harom), 9.87 s (1H, NH, D2O exchangeable). 13C NMR spectrum, δC, ppm: 101.43, 103.56, 105.71, 106.16, 108.22, 110.59, 112.43, 113.37, 113.92, 114.60, 116.37, 119.74, 120.55, 124.31, 125.83, 127.79, 129.25, 160.63. Mass spectrum: m/z 408.41 [M + 1]+. Calculated for C22H15Cl2N3O: M 407.06.
2-(5-Chloro-1H-indol-3-yl)-3-(3-chlorophenyl)-2,3-dihydroquinazolin-4(1H)-one (5j). Yield 88%, white solid, mp 215–217°C. IR spectrum, ν, cm–1: 3484 br (N–H), 1660 s (C=O). 1H NMR spectrum, δ, ppm: 5.69 s (1H, CH), 7.26–8.82 m (12H, Harom), 9.83 s (1H, NH, D2O exchangeable). 13C NMR spectrum, δC, ppm: 101.23, 103.47, 105.62, 106.12, 108.17, 110.40, 112.39, 113.24, 113.71, 114.10, 116.21, 119.05, 120.43, 124.58, 125.77, 127.80, 129.38, 160.80. Mass spectrum: m/z 408.31 [M + H]+. Calculated for C22H15Cl2N3O: M 407.06.
2-(5-Chloro-1H-indol-3-yl)-3-(4-chlorophenyl)-2,3-dihydroquinazolin-4(1H)-one (5k). Yield 82%, white solid, mp 218–220°C. IR spectrum, ν, cm–1: 3470 br (N–H), 1656 s (C=O). 1H NMR spectrum, δ, ppm: 5.55 s (1H, CH), 7.18–8.70 m (12H, Harom), 10.06 s (1H, NH, D2O exchangeable). 13C NMR spectrum, δC, ppm: 101.14, 103.34, 105.41, 106.05, 108.11, 110.35, 112.49, 113.39, 113.60, 114.14, 116.30, 119.00, 120.32, 124.44, 125.52, 127.71, 129.23, 160.65. Mass spectrum: m/z 408.20 [M + H]+. Calculated for C22H15Cl2N3O: M 407. 06.
2-(5-Chloro-1H-indol-3-yl)-3-(2-nitrophenyl)-2,3-dihydroquinazolin-4(1H)-one (5l). Yield 84%, white solid, mp 211–213°C. IR spectrum, ν, cm–1: 3486 br (N–H), 1686 s (C=O). 1H NMR spectrum, δ, ppm: 5.90 s (1H, CH), 7.30–8.90 m (12H, Harom), 10.00 s (1H, NH, D2O exchangeable). 13C NMR spectrum, δC, ppm: 101.29, 103.48, 105.57, 106.15, 108.37, 110.58, 112.67, 113.45, 113.75, 114.22, 116.38, 119.05, 120.47, 124.63, 125.82, 127.41, 129.67, 160.79. Mass spectrum: m/z 419.30 [M + H]+. Calculated for C22H15ClN4O3: M 418.08.
2-(5-Chloro-1H-indol-3-yl)-3-(3-nitrophenyl)-2,3-dihydroquinazolin-4(1H)-one (5m). Yield 84%, white solid, mp 213–215°C. IR spectrum, ν, cm–1: 3480 br (N–H), 1679 s (C=O). 1H NMR spectrum, δ, ppm: 5.86 s (1H, CH), 7.25–8.87 m (12H, Harom), 10.12 s (1H, NH, D2O exchangeable). 13C NMR spectrum, δC, ppm: 101.14, 103.37, 105.46, 106.08, 108.27, 110.24, 112.52, 113.31, 113.64, 114.15, 116.47, 119.12, 120.43, 124.55, 125.70, 127.38, 129.50, 160.66. Mass spectrum: m/z 419.15 [M + H]+. Calculated for C22H15ClN4O3: M 418.08.
2-(5-Chloro-1H-indol-3-yl)-3-(4-methoxyphenyl)-2,3-dihydroquinazolin-4(1H)-one (5n). Yield 91%, white solid, mp 220–222°C. IR spectrum, ν, cm–1: 3471 br (N–H), 1653 s (C=O). 1H NMR spectrum, δ, ppm: 3.89 s (3H, OCH3), 5.69 s (1H, CH), 7.25–8.78 m (12H, Harom), 10.05 s (1H, NH, D2O exchangeable). 13C NMR spectrum, δC, ppm: 101.23, 103.45, 105.57, 106.18, 108.35, 110.36, 112.60, 113.20, 113.74, 114.26, 116.50, 119.16, 120.57, 124.62, 125.81, 127.40, 129.63, 160.71. Mass spectrum: m/z 404.10 [M + H]+. Calculated for C23H18ClN3O2: M 403.11.
CONCLUSIONS
The syntheses of novel 2,3-dihydroquinazolin-4(1H)-one derivatives of potential synthetic and pharmacological interest via one-pot three-component and alternative methods using L-proline and L-propline/K2CO3 as catalyst in water have been described. The desired products have been obtained in high yields without any unwanted by-products. The new protocol features operational simplicity, high atom economy, and broad substrate scope. Further application of this protocol to total synthesis of biologically important natural products and more detailed mechanistic studies for this transformation are currently underway in our laboratory.
REFERENCES
Gupta, P. and Mahajan, A., RSC Adv., 2015, vol. 5, p. 26686. https://doi.org/10.1039/C5RA00358J
Otto, S. and Engberts, J.B.F.N., Org. Biomol. Chem., 2003, vol. 1, p. 2809. https://doi.org/10.1039/B305672D
Ashis Kumar, N., Subarna, G., and Chakraborty, R., Molecules, 2007, vol. 12, p. 2413. https://doi.org/10.3390/12102413
Pathak, S.R., Malhotra, V., and Shanker, R.N., Cent. Nerv. Syst. Agents Med. Chem., 2014, vol. 14, p. 34. https://doi.org/10.2174/1871524914666140825144729
Jangam, S.S. and Wankhede, S.B., Russ. J. Gen. Chem., 2019, vol. 89, p. 1029. https://doi.org/10.1134/S1070363219050256
Yadav, M.R., Shirude, S.T., Parmar, A., Balaraman, R., and Giridhar, R., Chem. Heterocycl. Compd., 2006, vol. 42, p. 1038. https://doi.org/10.1007/s10593-006-0201-4
Zayed, M.F. and Hassan, M.H., Saudi Pharm. J., 2014, vol. 22, p. 157. https://doi.org/10.1016/j.jsps.2013.03.004
El Azab, A.S. and El Tahir, K.E.H., Bioorg. Med. Chem. Lett., 2012, vol. 22, p. 327. https://doi.org/10.1016/j.bmcl.2011.11.007
Cantarelli, G., Farmaco, Ed. Sci., 1970, vol. 25, p. 761. PMID: 5484777
Mhaske, S.B. and Argade, N.P., Tetrahedron, 2006, vol. 62, p. 9787. https://doi.org/10.1016/j.tet.2006.07.098
Weber, L., Drug Discovery Today, 2002, vol. 7, p. 143. https://doi.org/10.1016/s1359-6446(01)02090-6
Sunderhaus, J.D., Dockendorff, C., and Martin, S.F., Org. Lett., 2007, vol. 9, p. 4223. https://doi.org/10.1021/ol7018357
Preeti and Singh, K.N., Org. Biomol. Chem., 2018, vol. 16, p. 9084. https://doi.org/10.1039/C8OB01872C
Cioc, R.C., Ruijter, E., and Orru, R.V.A., Green Chem., 2014, vol. 16, p. 2958. https://doi.org/10.1039/C4GC00013G
Biggs-Houck, J.E., Younai, A., and Shaw, J.T., Curr. Opin. Chem. Eng., 2010, vol. 14, p. 371. https://doi.org/10.1016/j.cbpa.2010.03.003
ACKNOWLEDGMENTS
The authors are grateful to the management of Dr. Reddy’s Laboratories Ltd. for supporting this work and highly appreciate the co-operation from all the supporting function colleagues.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest.
Rights and permissions
About this article
Cite this article
Hari Krishna Reddy, V., Prashanth Reddy, G., Krishna Mohan, T. et al. L-Proline/K2CO3-Catalyzed Eco-friendly Synthesis of Novel 2-(1H-Indol-3-yl)-3-phenyl-2,3-dihydroquinazolin-4(1H)-one Derivatives. Russ J Org Chem 57, 1678–1684 (2021). https://doi.org/10.1134/S1070428021100171
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428021100171