Abstract
TiO2/Bi2O3 nanocomposites (Sonic-TiO2/Bi2O3) were synthesized via combination of thermohydrolysis and ultrasonic chemical precipitation techniques. For the sake of contrast, pure TiO2 nanoparticles (NPs) and conventional TiO2/Bi2O3 nanocomposites (Stir-TiO2/Bi2O3) were also prepared. XRD, FT-IR, FE-SEM, TEM, EDX and UV–Vis studies were adopted to determine the structural, chemical composition and optical properties of the as-prepared samples. The photocatalytic activities of the samples were evaluated by measuring the photo-degradation of Orange II in aqueous solution under visible light irradiation (λ ≥ 400 nm). The efficiencies towards OII degradation were determined to be 44.0, 81.8 and 94.7 % for pure TiO2 NPs, Stir-TiO2/Bi2O3 and Sonic-TiO2/Bi2O3, respectively. These results reveal that the loading of Bi2O3 can greatly improve visible light photocatalytic performance of TiO2, and the ultrasonic treatment can also improve photocatalytic performance of TiO2/Bi2O3 nanocomposites. Based on the experimental results, possible enhanced visible-light photocatalytic degradation mechanisms were also discussed. The present findings may provide a new approach to synthesize high efficiency TiO2/Bi2O3 nanocomposite photocatalysts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the development of the chemical industry, more and more chemicals have been synthesized and used on many occasions. As a result, more and more poisonous waste water containing organic pollutants has been produced [1]. In almost all countries of the world, the poisonous organic waste water needs to be treated to remove the organic pollutants before being discharged [2]. Various methods such as absorption [3], ultrasonic degradation [4], biodegradation [5] and photocatalysis [6] have been used to reach this goal. Among these methods, photocatalysis has been regarded as one of the most promising sewage treatment techniques due to its low cost and non-toxic and efficiency natures [7].
Up to the present, much research has proved that photocatalysis is an effective method of destroying organic aqueous waste streams [8]. Unfortunately, the photocatalysis technique still has many problems, which restrict its large-scale industrial application [9]. For example, most of the high efficiency photocatalysts like TiO2 (band gap = 3.2 eV), ZnO (band gap = 3.2 eV) and so on are wide gap semiconductors: they can only be activated by UV light, which is a very small proportion of sunlight [10]. In order to harness the cheap and clean sunlight power efficiently, it is necessary to develop new visible light (45 % of the solar spectrum)-activated photocatalysts with narrow band gap and high catalytic capability [11].
Bi2O3, a narrow band gap (band gap = 2.8 eV) semiconductor, has attracted much attention due to its unique optical and electrical properties [12]. Many research groups have investigated the photocatalytic performance of Bi2O3, and the results show that the Bi2O3 can exhibit photocatalytic activities [13]. Unfortunately, pure Bi2O3 cannot be used as an efficient visible light-driven photocatalyst because of its low photocatalytic performance. To solve this problem, three principal kinds of modification methods have been developed. The first method is the doping of new elements [14], the second is the loading of precious metals [15], and the last is modification with semiconductors; for example, when Bi2O3 was combined with TiO2, a wide band gap semiconductor, the as-prepared nanocomposite materials exhibited excellent photocatalytic performances under visible light irradiation, even better than pure TiO2 and Bi2O3 [16, 17]. Therefore, the TiO2/Bi2O3 nanocomposite materials can be regarded as a promising candidate for the new visible light-driven activated photocatalysts which we want.
Currently, several methods have been developed for the synthesis of TiO2/Bi2O3 nanocomposite materials. Huo et al. [18] have prepared ordered TiO2/Bi2O3 film by the sol–gel method in a presence template. Li et al. [19] have fabricated heterostructured TiO2/Bi2O3 composite fibers via combination of the solvothermal method and electrospinning technique. In addition, the electrochemical anode method [20], the evaporation-induced self-assembly method [21] and the blending method [22] have also been applied in the synthesis of TiO2/Bi2O3 nanocomposite materials. Although the preparation and photocatalytic performances of TiO2/Bi2O3 nanocomposite materials have been reported many times over the years, most of them employ traditional synthetic methods. Research on ultrasonic-assisted synthesis of these materials has seldom been reported. In this work, sonochemical synthesis, a new synthetic method compared to the general methods, has been applied in the preparation of TiO2/Bi2O3 nanocomposites, and we hope we can obtain a new highly efficient TiO2/Bi2O3 nanocomposite photocatalyst.
TiO2/Bi2O3 nanocomposites (Sonic-TiO2/Bi2O3) were prepared via the combination of the hermohydrolysis method and the ultrasonic-assisted chemical precipitation technique. For comparison, pure TiO2 nanoparticles (NPs) and conventional TiO2/Bi2O3 nanocomposites (Stir-TiO2/Bi2O3) were also prepared. All the as-synthesized products were well characterized with the aid of X-ray diffraction (XRD), Fourier transform-infrared spectroscopy (FT-IR), field-emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), energy dispersive analysis of X-ray (EDX) and ultra-violet adsorption spectrum (UV–Vis). The photocatalytic activities of all the samples have also been investigated by the photocatalytic decolorization of Orange II (OII) in aqueous solution under visible light irradiation.
Experimental
Materials
Chemicals used in the experiment were titanium oxysulfate hydrate (TiOSO4·xH2O, CP), bismuth nitrate pentahydrate (Bi(NO3)3·5H2O, AR), hexamethylene-tetramine (HMT, C6H12N4, AR), ethylene glycol (EG, C2H6O2, AR) and orange II (OII, C16H11N2NaO4S·5H2O, AR). TiOSO4·xH2O was purchased from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, PR China), and the others were all purchased from Sinopharm Chemical Reagent (Shanghai, PR China). All the reagents were used without further purification. Deionized water used throughout the experiments was prepared from a laboratory ultra-pure water purifier.
Preparation of TiO2 nanoparticles with the thermohydrolysis method
The TiO2 NPs were synthesized via the thermohydrolysis technique [23]. Typically, 10 g of TiOSO4·xH2O were added to 45 mL of distilled water at room temperature under continuous stirring. After being stirred for more 30 min, the mixture was transferred into a Teflon-lined stainless-steel autoclave (100 mL capacity) and sealed. After being heated at 100 °C for 48 h, the autoclave was cooled to room temperature. The white precipitates were collected, washed, and then dried in an oven at 80 °C for 6 h. Next, the obtained white powders were annealed at 600 °C for 6 h, and then well-crystallized TiO2 NPs were obtained.
Preparation of TiO2/Bi2O3 nanocomposites with ultrasonic-assisted chemical precipitation method
In a typical case, 1.6 g of TiO2 NPs were dispersed in 50 mL Bi(NO3)3 EG solution (0.04 mol/L) to form a white suspension under vigorous stirring, and then 50 mL of the HMT EG solution (0.04 mol/L) were poured into the above suspension. The obtained mixture was transferred to a conical flask and heated at boiling temperature until all the EG had evaporated, while an ultrasonic bath (40 kHz, 120 W; NingBo Scientz Biotechnology, PR China) was applied to treat the mixture in this heating process. After that, the product was collected and calcined at 300 °C for 4 h. Finally, the TiO2/Bi2O3 nanocomposites (denoted by Sonic-TiO2/Bi2O3) were obtained, and the nominal weight fraction of Bi2O3 to Bi2O3 + TiO2 in the Sonic-TiO2/Bi2O3 was about 36.8 %.
Preparation of TiO2/Bi2O3 nanocomposites with conventional precipitation method
To investigate the influence of ultrasonic treatment on the chemical constitution, morphologies, optical properties and photocatalytic performances of Sonic-TiO2/Bi2O3 nanocomposites, another kind of TiO2/Bi2O3 nanocomposite materials were synthesized by a conventional precipitation method. In the conventional preparation process, the ultrasonic treatment was replaced by vigorous stirring, while the other procedures were all the same compared with the synthesis process of Sonic-TiO2/Bi2O3 nanocomposites. The as-obtained sample was called Stir-TiO2/Bi2O3, and the nominal weight fraction of Bi2O3 to Bi2O3 + TiO2 in the Stir-TiO2/Bi2O3 was the same as that in the Sonic-TiO2/Bi2O3.
Characterization
All the three prepared samples (TiO2 NPs, Sonic-TiO2/Bi2O3 and Stir-TiO2/Bi2O3) were analyzed for their crystallite phase with a X-ray diffractometer (XRD, X’Pert PRO; Philips, Netherlands) with Cu Kα radiation (λ = 1.5418 Å). Jade software was also used for the analysis of different peaks. The functional groups of the samples were examined through a Fourier transform-infrared spectroscopy (FT-IR, NEXUS-6700; Nicolet, USA) using a KBr pressed disk. The morphologies and element distribution of them were characterized by field emission scanning electron microscopy (FE-SEM, Nova 400 Nano; FEI, America) with energy-dispersive spectra (EDX, IE 350 Penta FET X-3; Oxford Inca, UK) and transmission electron microscopy (TEM, Tecnai G20; FEI). The optical absorption properties were performed using a UV–Visible absorption spectrometer (UV–Vis, U-3010; HITACHI, Japan). Furthermore, the absorbance of dye solution in photocatalytic experiments was measured with a visible spectrophotometer (V-1600; MAPADA, China).
Photocatalysis experiment
Photocatalytic activities of the samples were evaluated by the degradation of OII under visible light with a home-made photocatalytic reaction system. A 500-W halogen tungsten lamp (Philips) equipped with a cutoff filter (λ ≥ 400 nm) was employed as the visible light source, and a magnetic stirrer was also applied to agitate the suspension containing the photocatalysts and dye solution. To keep a uniform temperature, the suspension of photocatalysts and OII solution was cooled with a constant temperature water bath.
In a typical photocatalytic test, 0.15 g of Sonic-TiO2/Bi2O3 were suspended in 150 mL OII aqueous solution (5 mg/L) and stirred for 30 min in the dark to ensure that the OII molecules absorbed completely on the catalyst surface before irradiation. Then, the light was turned on and the photocatalytic reactions started. Under stirring, about 5 mL of suspension was taken out at regular intervals of 30 min, centrifuged, and analyzed using a visible light spectrophotometer. The photocatalytic efficiency (E) was evaluated in terms of the percentage of OII degraded using the following equation:
where C 0 (mg/L) is the initial concentration of OII, and C (mg/L) is the concentration after photocatalytic reaction at different time. The photocatalytic experiments of the pure TiO2 NPs and Stir-TiO2/Bi2O3 were also carried out in the same way.
Results and discussion
XRD analysis
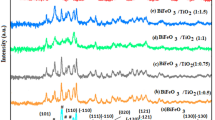
XRD technique was applied to determine the crystal structures of the three resulting samples, and the XRD patterns are presented in Fig. 1. The diffraction peaks of TiO2 are detected all over the three samples. Specifically, the peaks at 2θ = 25.3°, 36.9°, 37.8°, 38.6°, 48.0°, 53.9°, 55.1°, 62.7°, 68.8°, 70.3° and 75.0° correspond to the standard anatase-TiO2 diffraction peaks for the (1 0 1), (1 0 3), (0 0 4), (1 1 2), (2 0 0), (1 0 5), (2 1 1), (2 0 4), (1 1 6), (2 2 0) and (2 1 5) lattice planes, respectively. From the XRD curves of the two nanocomposite samples, we can observe that there are nine new diffraction peaks at 2θ = 27.9°, 31.8°, 32.7°, 46.2°, 46.9°, 54.3°, 55.5°, 57.8° and 74.5°, which are attributed to (2 0 1), (0 0 2), (2 2 0), (2 2 2), (4 0 0), (2 0 3), (4 2 1), (4 0 2) and (6 1 0) facets of β-Bi2O3, respectively. These results indicate that the two nanocomposite samples all contain TiO2 and Bi2O3. The three samples are all well crystallized, and no other impure diffraction peaks can be detected in the patterns. It is worth noting that the anatase-TiO2 diffraction peaks of the two nanocomposite samples are weaker than that of pure TiO2 NPs, which can be attributed to the generation of Bi2O3. Furthermore, the peaks of Bi2O3 become stronger and sharper after ultrasonic treatment, indicating that the Bi2O3 in Sonic-TiO2/Bi2O3 have higher crystallinity than that in Stir-TiO2/Bi2O3.
FT-IR analysis
Fourier transform-infrared spectra of two nanocomposite samples and TiO2 NPs are shown in Fig. 2. The peaks at about 3,750 and 1,552 cm−1 are ascribed to unconjugated water [24, 25], while two bands located around 3,440 and 1,643 cm−1 can be reasonably assigned to the adsorbed water molecules in the three samples [26, 27]. The FT-IR band at 400–800 cm−1 is ascribed to the Ti–O–Ti stretching vibration mode in crystal TiO2 [28]. The absorption assigned to the SO4 2− has been observed at 1,037 cm−1, which is commonly observed for TiO2 prepared with titanium oxysulfate hydrate [29]. No significant differences can be observed in the spectra between the three samples, except that a red shift of the Ti–O–Ti bands occurs in the spectrum of Sonic-TiO2/Bi2O3, which might be due to the combination of TiO2 and Bi2O3. This further confirms that the Sonic-TiO2/Bi2O3 is a composite material.
Morphologies analysis
The morphologies of the samples were characterized to obtain the microstructural information. The FE-SEM images of the TiO2 NPs, Stir-TiO2/Bi2O3, Sonic-TiO2/Bi2O3 and TEM image of Sonic-TiO2/Bi2O3 nanocomposites are indicated in Fig. 3a–d, respectively. As shown in Fig. 3a, the TiO2 NPs sample is composed of siamesed massive aggregates which are accumulated from small NPs. Under close observation of these NPs, their diameter range is about 50–100 nm. From Fig. 3b–d, the morphologies of the two nanocomposite samples are also massive aggregates composed of NPs, but the aggregates sizes of them are larger than that of TiO2 NPs. The serious agglomeration phenomenon can be chalked up to the generation of Bi2O3. In comparison, the aggregates sizes of Sonic-TiO2/Bi2O3 are smaller than that of Stir-TiO2/Bi2O3, which indicates that ultrasonic treatment can reduce the agglomeration degree of the TiO2/Bi2O3 nanocomposites.
EDX analysis
The EDX analysis of the TiO2 NPs, Stir-TiO2/Bi2O3 and Sonic-TiO2/Bi2O3 nanocomposites are shown in Fig. 4a–c, and the elemental composition tables of the three samples are attached to the figures, respectively. According to the spectroscopies, the TiO2 NPs contain Ti and O, while two nanocomposite samples all contain Ti, O and Bi. And the two nanocomposite samples have almost the same content of the three elements. These results further testify that the two nanocomposite samples are composed of TiO2 and Bi2O3, which is consistent with the results of XRD and FT-IR analysis.
UV–Vis spectra analysis
The optical absorption properties of the as-obtained samples were measured by a UV–Vis spectrometer, and the UV–Vis absorption spectra for them are shown in Fig. 5. It can be seen that all TiO2/Bi2O3 nanocomposite samples show a spectral response in visible areas owing to the photosensitizing effect of Bi2O3. It is well known that photocatalytic reactions are initiated by light, and none of the photocatlysts could work if they cannot absorb light. However, when photocatalysts are illuminated by lights, only a few lights can be absorbed, and most of them have been reflected off. Therefore, the photo-absorption capacity can affect the photocatalytic performances of photocatalytsts [30]. In comparison to Stir-TiO2/Bi2O3, the Sonic-TiO2/Bi2O3 displays higher photo-absorption capacity in the visible region, which might increase its photocatalytic performance. Furthermore, the band gap energies are estimated as 3.19, 3.07 and 2.94 eV for TiO2 NPs, Stir-TiO2/Bi2O3 and Sonic-TiO2/Bi2O3, respectively, indicating that the Sonic-TiO2/Bi2O3 can be excited by visible light. The strong visible light absorption capacity and narrow band gap of Sonic-TiO2/Bi2O3 suggests that it should possess excellent visible light photocatalytic activity compared to that of Stir-TiO2/Bi2O3 and TiO2 NPs [31].
Photocatalytic activities
The photocatalytic activities of TiO2 NPs, Stir-TiO2/Bi2O3 and Sonic-TiO2/Bi2O3 were evaluated by measuring the photo-degradation of OII aqueous solution (5 mg/L) under visible light irradiation. Figure 6 shows the comparison of the photocatalytic activities between those prepared samples at the same initial condition. After 3 h of visible light irradiation, OII is degraded with different catalysts, but the photocatalytic efficiencies of them are not the same. The TiO2 NPs exhibit the poorest activity under visible light with only 44.0 % degradation, while the two nanocomposite samples show much higher photocatalytic activities. The photocatalytic efficiency could reach 94.7 % when Sonic-TiO2/Bi2O3 was employed, especially. Furthermore, the blank test shows that the OII degradation is so tiny in the absence of photocatalysts that the self-degradation of OII can be ignored.
The UV–Vis absorption spectra of OII solution after degradation for different times over Sonic-TiO2/Bi2O3 under visible light irradiation were recorded, and are shown in Fig. 7. It can be seen that all of the absorption peaks decrease with the extension of the irradiation time, indicating that the OII molecules have been destroyed in the photocatalytic reaction process [32]. The maximum absorption peak (λ = 485 nm) decreases gradually and almost completely disappears after 180 min, which suggests that the Sonic-TiO2/Bi2O3 exhibits excellent photocatalytic activity in the decolonization of OII.
In order to further study the photocatalytic abilities of the prepared samples and the reaction kinetics of OII degradation, the Langmuir–Hinshelwood model [33] has been used here:
where k (min−1) is the rate constant value, and t (min) is the reaction time. Figure 8 shows the plot depicting the linear relationship between −ln(C/C 0) and time for all the samples, while the rate constant values (k) and standard deviation values (R) are also given in the figure. The results show that −ln(C/C 0) exhibits a good linear relationship with the irradiation time (R 1 = 0.993, R 2 = 0.999, R 3 = 0.989), indicating that the photocatalytic degradation of OII over the three prepared samples follows a first-order kinetic reaction. The rate constant values are determined to be 0.0148 and 0.0092 min−1 for Sonic-TiO2/Bi2O3 and Stir-TiO2/Bi2O3, respectively, which are distinctly higher than that of the TiO2 NPs (k 3 = 0.0029 min−1). This fact indicates that the modification with Bi2O3 can obviously enhance the photocatalytic activity of TiO2 under visible light. Furthermore, ultrasonic treatment can even promote the degradation activities of nanocomposite photocatalysts.
Tentative photocatalytic mechanisms
As we all know, TiO2 is a wide gap (band gap = 3.2 eV) semiconductor, which cannot be activated by visible light, and OII theoretically cannot be decomposed in this system [34]. But the TiO2 NPs still display 44.0 % degradation yield of OII dye within 180 min, possibly owing to self-sensitization of the OII. As shown in Fig. 9a, the OII molecules adsorbed on the surface of TiO2 NPs can absorb visible light to produce a singlet and/or triplet state of OII molecules (OII*). The oxidation potential of OII* is more negative than the potential of the conduction band (CB) of TiO2 NPs, which involves the possibility that electrons would inject into the CB of TiO2 from OII*. Therefore, the OII is converted to the cationic dye radical, and the injected electrons can take part in the degradation reactions of the pollutants. As a result, the OII molecules have been decomposed in this TiO2–OII system [35].
When TiO2 was modified with Bi2O3, the photocatalytic mechanisms become much more complex, which can be classified into two reasonable paths. As shown in Fig. 9b, one is the OII dye sensitization process, which is similar to the photocatalytic process of pure TiO2 NPs [35]. Another path is the Bi2O3-assisted photocatalytic process. As the band gap of Bi2O3 is 2.8 eV, it can be easily excited by visible light to form photogenerated electron–hole pairs, while the electrons and holes could induce a series of reactions to decompose OII [14, 15]. In addition, the CB potential of TiO2 is more negative than that of Bi2O3, while the valence band (VB) of Bi2O3 is more positive than that of TiO2 [36]. Hence, the electrons in the CB of TiO2 could transfer to the CB of Bi2O3, and the holes in the VB of Bi2O3 could transfer to the VB of TiO2. These heterojunctions between Bi2O3 and TiO2 could promote separation of photogenerated charge carriers and could also enhance photocatalytic activity [37].
An ultrasonic wave is a kind of sound wave with high energy [38]. When it was applied in the synthesis of TiO2/Bi2O3 nanocomposites, the high energy could promote the crystallization of the NPs, responsible for the further crystallization process [39]. With the help of ultrasonic treatment, the crystallinity of Bi2O3 NPs growing on the surface of TiO2 would be higher, which is beneficial to the charge transfer. As a result, the Sonic-TiO2/Bi2O3 nanocomposite catalyst exhibits the highest photocatalytic activity among the three samples.
Conclusions
In summary, we have demonstrated a new ultrasonic-assisted chemical precipitation method to prepare TiO2/Bi2O3 nanocomposites (Sonic-TiO2/Bi2O3). XRD, FT-IR, FE-SEM, TEM and EDX results indicate that the Sonic-TiO2/Bi2O3 nanocomposites have been successfully synthesized. Optical absorption property analysis shows that this Sonic-TiO2/Bi2O3 possesses the highest visible light absorption capacity. Furthermore, such Sonic-TiO2/Bi2O3 exhibits higher photocatalytic activity for the degradation of OII dye solution under visible light irradiation than either the pure TiO2 NPs or the TiO2/Bi2O3 obtained by a conventional precipitation method.
References
C.A. Martinez-Huitle, S. Ferro, Chem. Soc. Rev. 35, 1324–1340 (2006)
N. Hudson, A. Baker, D. Reynolds, River Res. Appl. 23, 631–649 (2007)
A. Ahmad, A. Idris, B. Hameed, Desalin. Water Treat. 51, 2554–2563 (2013)
V.O. Abramov, A.V. Abramova, P.P. Keremetin, M.S. Mullakaev, G.B. Vexler, T.J. Mason, Ultrason. Sonochem. 21, 812–818 (2014)
L.M. Nieto, G. Hodaifa, S. Rodríguez, J.A. Giménez, J. Ochando, Chem. Eng. J. 173, 503–510 (2011)
T. Mousanejad, M. Khosravi, S. Tabatabaii, A. Khataee, K. Zare, Res. Chem. Intermed. 40, 711–722 (2014)
T. Zhu, J.S. Chen, X.W. Lou, J. Phys. Chem. C 116, 6873–6878 (2012)
A. Petrella, M. Petrella, G. Boghetich, P. Mastrorilli, V. Petruzzelli, E. Ranieri, D. Petruzzelli, Ind. Eng. Chem. Res. 52, 2201–2208 (2013)
Y.H. Zhao, Appl. Mech. Mater. 416, 1652–1656 (2013)
S.G. Kumar, L.G. Devi, J. Phys. Chem. A 115, 13211–13241 (2011)
Y. Lu, Y. Lin, D. Wang, L. Wang, T. Xie, T. Jiang, Nano Res 4, 1144–1152 (2011)
M. Muruganandham, R. Amutha, G.J. Lee, S.H. Hsieh, J.J. Wu, M. Sillanpää, J. Phys. Chem. C 116, 12906–12915 (2012)
X. Liu, L. Pan, J. Li, K. Yu, Z. Sun, J. Nanosci. Nanotechnol. 13, 5044–5047 (2013)
H.Y. Jiang, J.J. Liu, K. Cheng, W.B. Sun, J. Lin, J. Phys. Chem. C 117, 20029–20036 (2013)
H.Y. Jiang, K. Cheng, J. Lin, Phys. Chem. Chem. Phys. 14, 12114–12121 (2012)
Z.F. Bian, J. Zhu, S.H. Wang, Y. Cao, X.F. Qian, H.X. Li, J. Phys. Chem. C 112, 6258–6262 (2008)
D. Li, Y. Zhang, X. Zhou, S. Guo, J. Hazard. Mater. 258, 42–49 (2013)
Y.N. Huo, X.F. Chen, J. Zhang, G.F. Pan, J.P. Jia, H.X. Li, Appl. Catal. B: Environ. 148, 550–556 (2014)
Y.J. Li, T.P. Cao, C.L. Shao, C.H. Wang, J. Inorg. Mater. 27, 687–692 (2012)
X. Zhao, H.J. Liu, J.H. Qu, Appl. Surf. Sci. 257, 4621–4624 (2011)
Z.F. Bian, J. Zhu, S.H. Wang, Y. Cao, X.F. Qian, H.X. Li, J. Phys. Chem. C 112, 6258–6262 (2008)
A.K. Chakraborty, M.E. Hossain, M.M. Rhaman, K.M.A. Sobahan, J. Environ. Sci. 26, 458–465 (2014)
A. Di Paola, M. Bellardita, L. Palmisano, R. Amadelli, L. Samiolo, Catal. Lett. 143, 844–852 (2013)
K. Chakarova, K. Hadjiivanov, Micropor. Mesopor. Mater. 143, 180–188 (2011)
Y. Cheng, L. An, J. Lan, F. Gao, R.Q. Tan, X.M. Li, G.H. Wang, Mater. Res. Bull. 48, 4287–4293 (2013)
V. Pilla, S.R. de Lima, A.A. Andrade, A.C.A. Silva, N.O. Dantas, Chem. Phys. Lett. 580, 130–134 (2013)
M. Tortorelli, G. Landi, L. Lisi, G. Russo, Micropor. Mesopor. Mater. 200, 216–224 (2014)
G.Z. Liao, S. Chen, X. Quan, Y.B. Zhang, H.M. Zhao, Appl. Catal. B: Environ. 102, 126–131 (2011)
L.L. Chen, Y. Zhai, H.Y. Ding, G.H. Zhou, Y.F. Zhu, D. Hui, Compos. Part B-Eng. 45, 111–116 (2013)
J. Zhu, S. Wang, J. Wang, D. Zhang, H. Li, Appl. Catal. B: Environ. 102, 120–125 (2011)
P. Malathy, K. Vignesh, M. Rajarajan, A. Suganthi, Ceram. Int. 40, 101–107 (2014)
R. Chauhan, A. Kumar, R. Chaudhary, Res. Chem. Intermed. 39, 645–657 (2013)
Z. Liu, B. Wu, Y. Zhao, J. Niu, Y. Zhu, Ceram. Int. 40, 5597–5603 (2014)
Y. Zhang, S. Liu, Z. Xiu, Q. Lu, H. Sun, G. Liu, J. Nanopart. Res. 16, 1–9 (2014)
Y. Bessekhouad, N. Chaoui, M. Trzpit, N. Ghazzal, D. Robert, J.V. Weber, J. Photochem. Photobiol. A: Chem. 183, 218–224 (2006)
J. Hou, C. Yang, Z. Wang, S. Jiao, H. Zhu, Appl. Catal. B: Environ. 129, 333–341 (2013)
J. Chen, S. Qin, Y. Liu, F. Xin, X. Yin, Res. Chem. Intermed. 40, 637–648 (2014)
K. Ullah, L. Zhu, Z.D. Meng, S. Ye, S. Sarkar, W.C. Oh, J. Mater. Sci. 49, 4139–4147 (2014)
L. Zhou, W. Wang, L. Zhang, J. Mol. Catal. A: Chem. 268, 195–200 (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
An, L., Wang, G., Cheng, Y. et al. Ultrasonic-assisted synthesis of visible-light-driven TiO2/Bi2O3 nanocomposite photocatalysts: characterization, properties and azo dye removal application. Res Chem Intermed 41, 7449–7461 (2015). https://doi.org/10.1007/s11164-014-1836-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1836-x